Special Issue Article Open Access
Impact of Sediment from St. Jones River, Delaware, USA on Microbial Functional Stability in Two Local Soils
| Qiquan Wang1*, Esosa Iriowen1, Shoujun Yuan1,2 and Donald L. Sparks3 | |
| 1Chemistry Department, Delaware State University, Dover, DE 19901, USA | |
| 2College of Civil Engineering, Hefei University of Technology, Hefei, Anhui 230009, China | |
| 3Department of Plant and Soil Sciences, University of Delaware, Newark, DE 19901, USA | |
| Corresponding Author : | Qiquan Wang Department of chemistry Delaware State University Dover, DE 19901, USA Tel: +001(302)857-6547 Fax: +001(302)857- 6539 E-mail: qwang@desu.edu |
| Received: October 24, 2011; Accepted: December 21, 2011; Published: December 23, 2011 | |
| Citation: Wang Q, Iriowen E, Yuan S, Sparks DL (2011) Impact of Sediment from St. Jones River, Delaware, USA on Microbial Functional Stability in Two Local Soils. J Bioremed Biodegrad S1:005. doi:10.4172/2155-6199.S1-005 | |
| Copyright: © 2011 Wang Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
For the purposes of navigation, flooding prevention, and/or environmental protection, aquatic sediment may need to be dredged and disposed. The impact of sediment on soil microbial communities is often neglected. In this study, two fresh sediment samples (A & B) from St. Jones River, Delaware, USA were amended into two different local soils, an agricultural soil and a forest soil. Eight microbial and enzymatic activities were selected to represent soil microbial functions and the impact of sediment amendment on soil microbial functional stability was determined. Based on the values of average resistance, which is the average of the absolute value of resistance in each activity, no significant difference was observed between the impacts of those two sediments on the microbial functional resistance in the agricultural soil. The average resistance values of the forest soil to the amendment of sediment B at 2 and 10% were at least 0.3 times lower than that in the agricultural soil, indicating that the forest soil was more resistant than the agricultural soil to sediment amendment. The forest soil appeared more sensitive to sediment amendment in the resistance to heat than the agricultural soil, but exhibited a bigger capacity tolerating the sediment amendment at the high percentage. Both soils were weakened in resilience from the heat disturbance by sediment amendment at 10%. Hence, microbial functional stability in these two local soils was markedly impacted by sediment amendment. The nutrient cycling of N, P, and S in two local soils was greatly depressed and easily-available organic-C was promptly consumed with sediment amendment. Heavy metal remediation and/or protective storage might need to be considered for sediment from St. Jones River when it is dredged in the future.
| Keywords |
| Sediment; Soil microbial function; Functional stability; Resistance; Resilience |
| Introduction |
| For the need of navigation, flooding prevention, and/or environmental protection, large volumes of costal and inland aquatic sediments have to be scooped periodically [1,2]. It was estimated that 100~200 Mm3 of sediment are dredged in Europe and 600 Mm3 worldwide annually [3,4]. Applying sediment as fertilizer in agricultural lands [5] and using it as constructional material in public works [4] have been proposed. Disposal to storage sites or nearby embankments without further treatment or precaution was also reported [6]. However, proper disposal or remediation is required when contaminated sediments, especially those from historically contaminated areas, are dredged [1]. The management of dredged sediment greatly depends on its potential risks to the environment, which are commonly assessed by chemical analysis of heavy metals and various toxic organic contaminants [7]. The impact of dredged sediment on soil microbial communities is often neglected. |
| Soil is a fascinating biological system with inhabiting microorganisms, which are of paramount importance for soil functioning in nutrient cycling, substance decomposition, and energy flow. About 80-90% of the processes in soil are mediated by microbes [8,9]. However, anthropogenic activities, such as cultivation and pollution, may alter sensitive soil microbial communities and result in changes of soil functional activities [10]. Thus a great attention has been paid to the changes of soil microbial diversity caused by various disturbances [11,12]. Meanwhile, soil microbial community functions, such as soil microbial and enzymatic activities/potentials, have also been investigated as affected by various pollutants, including heavy metals [13,14], veterinary antibiotics [15], pesticides [16], organic wastes [17], and petroleum [18]. A growing amount of experimental evidences indicates no consistent relationship between microbial diversity and soil functions [19-21]. |
| The impact of various perturbations on soil microbial functions is generally assessed based on the measurement of soil functional stability, including resistance and resilience [12,22]. It has been found that soil functional resistance greatly depends on the nature and concentration of contaminants, the nature of soil, and the species of enzymes [13,23,24]. When the impact of a primary disturbance/stress on soil functional stability is difficult to observe, its impact can also be assessed by exerting a secondary standard disturbance, such as heat shock and drying-rewetting cycle [25]. The soil received the primary disturbance may or may not appear less stable to the secondary disturbance than the soil without the primary disturbance/stress. For example, mercurycontaminated showed to have reduced resistance to heat compared the non-contaminated soil [26]. |
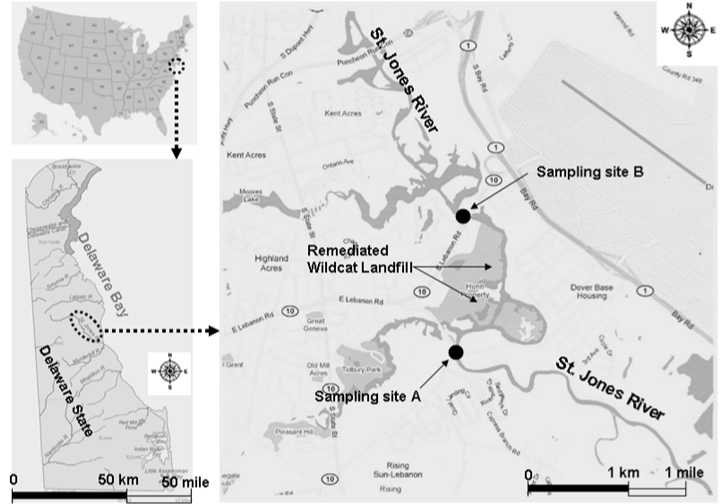
| This study aims at a preliminary understanding of the impact of sediment from St. Jones River on the microbial functional stability in two local soils. St. Jones River is a tidal river located in Central Delaware State, USA (Figure 1). It begins at the dam of the Silver Lake in Dover, the state capital city, and flows southeastwardly to Delaware Bay on the West Atlantic Coast with a length of 17 km and a draining area of 93 km2. It served as a major commercial navigation channel from Dover to Delaware Bay in early 1900s. Due to the rapid expansion of railways and roads in the area, however, it quickly lost its commercial value after 1940s and became gradually silted up. The accumulated sediment and the high nutrient load in St. Jones River have resulted in high turbidity and low dissolved oxygen in river water. The odor smell emitting from the anoxic sediment is bothering communities along the river. Additionally, St. Jones River was found to be seriously contaminated by Wildcat Landfill, which was located immediately beside the river (location shown in Figure 1) and was operated from 1962 to 1973. Wildcat Landfill was listed as a superfund site by USEPA in 1982 and removed from the list in 2003 after remediation [27]. For purposes of environmental quality improvement and flooding prevention, sediment dredging in part or the whole range of St. Jones River and subsequent disposal/storage may be needed in the near future. |
| In this study, two fresh sediment samples from St. Jones River were amended into two different local soils, an agricultural soil and a forest soil. Eight microbial and enzymatic activities were selected to represent soil microbial functions and the impact of sediment amendment on those functions was determined. The objectives of this study are, (i) to compare the impact of two sediment samples on soil functional resistance; (ii) to identify the difference of resistance between these two soils to the sediment amendment; (iii) to study the impact of sediment amendment on soil resistance to a standard heat, a secondary disturbance; and (iv) to investigate the impact of sediment amendment on the resilience of two soils from the standard heat disturbance. |
| Materials and Methods |
| Sediment and soil samples |
| Surface sediment samples (0-30 cm) was taken at two sites in middle St. Jones River using a 50 cm (length)×4.5 cm (i.d.) core sampler (Wildco® Instrument, Buffalo, NY, USA) on Jan. 04, 2010. Sampling site A and B are shown in Figure 1. In each sampling site, at least five grabs were made at different points and about 1 L of sediment were obtained. Sediment samples from the same sampling site were collected in the same 2-L glass beaker and thoroughly mixed using a wooden stick. After being covered with plastic foil, sediment samples were immediately transported to the lab for this study, which is less than 5 miles away from sampling site B. Fresh sediment samples were filtered through 4-mm plastic sieve in the laboratory and stored at 4Ã?Â?C for less than 2 days before experiments were performed. |
| Both agricultural and forest soil samples were obtained from Blackbird Creek area in Central Delaware. The agricultural soil sample (0-15 cm, topsoil) was obtained from a piece of agricultural land which had been cultivated and planted for about 30 years before it was purchased by the State and merged into Blackbird Creek Reserve 2 years ago. The forest soil sample (0-15 cm, topsoil) was obtained from Tybout Block in Blackbird Creek State Forest. Both sites are about 15 miles away from the lab. At each sampling site, 5 kg of fresh soil as a mix sample from different points in the field were collected. Soil samples were contained in plastic bucks and transported to the lab immediately after sampling. They were also sieved to 4mm and then stored at 4Ã?Â?C till being used in experiments. |
| About 50 g of each sediment and soil samples were oven-dried at 35Ã?Â?C and then sent to Cornell University Nutrient Analysis Laboratory (Ithaca, NY, USA) for heavy metal and soil texture analysis. Selected physical and chemical properties of soil and sediment samples are listed in Table 1. Heavy metal contents in sediment and soil samples are listed in Table 2. Based on USDA soil texture classification, the forest soil was a sandy loam soil, while the agricultural soil was a silt loam soil. |
| Amendment of sediment into soil |
| Fresh sediment was amended into fresh soil at different ratios by thoroughly mixing in a big volume food chopper. Sediment A and B were mixed with the agricultural soil at sediment content of 0, 2, and 10% (in dry mass), respectively, for the comparison study of the impact of two sediments on soil functional resistance. For the identification of any difference of resistance between these two soils to the sediment amendment and for the study of the impact of sediment amendment on soil resistance to a standard heat disturbance, sediment B was mixed with both the agricultural soil and the forest soil at 0, 2, and 10% (in dry mass), respectively. When investigating the impact of sediment amendment on the resilience of two soils from the heat disturbance, sediment B was mixed with both soils at 0, and 10% (in dry mass), respectively. The total dry equivalent mass of each prepared sedimentsoil mixture ranged from 500 to 1200 g, depending on the amount needed in the experiment. |
| Incubation and standard heat disturbance |
| Corresponding to the objective of this study, samples were treated differently for the assessment of sediment amendment impact on microbial functional activities, resistance to a standard heat, and resilience from heat disturbance. Samples were divided into 1 to 4 portions and each was contained in 1-L beaker. Sample treatments were summarized in Table 3. During incubations at 25Ã?Â?C, beakers were covered with pierced aluminum foil and moisture loss was compensated by adding non-sterile deionized water at every 5 days. During the oven heat at 50Ã?Â?C, beakers were covered with non-pierced aluminum foil and no moisture loss was compensated. |
| Microbial and enzymatic activity analysis |
| Glucose-induced soil respiration rate was determined using NaOHHCl titration method [28]. Each soil sample was thoroughly mixed with glucose solution in a small volume chopper and then weighed into a 500-ml flask for 24 h incubation in at 25Ã?Â?C. The produced CO2 was blown out at the end of incubation using N2 and adsorbed by standardized NaOH solution. Dehydrogenase activity was determined base on the transformation rate of 2,3,5-triphenyltetrazolium chloride (TTC) into triphenyl formazan (TPF) in soil slurry [29]. Each soil sample was weighed and mixed with CaCO3. After the addition of TTC solution, sample was incubated at 37Ã?Â?C for 24 h. The generated TPF was quantified by its absorbance at 485 nm. b-Glucosidase activity was quantified based on the generation rate of p-nitrophenol (PNP) from the decomposition rate of p-nitrophenyl-b-glucosidase in soil slurry [29]. Modified universal buffer at pH=6 was used and incubation was conducted at 37Ã?Â?C for 1 h. The generated PNP was quantified colorimetrically at 410 nm. Alkaline phosphatase activity was assayed based on the generation rate of PNP from the decomposition rate of p-disodium nitrophenyl phosphate in alkaline soil slurry [30]. Modified universal buffer at pH=11 was used and incubation was conducted at 37Ã?Â?C for 1 h. Arylsulfatase activity was determined based on the generation rate of PNP from the decomposition rate of potassium p-nitrophenyl sulfate in soil slurry [31]. Acetate buffer at pH=5.8 was used and incubation was conducted at 37Ã?Â?C for 1 h. Urease activity was determined based on the generation rate of NH4+-N from the decomposition of urea in soil slurry [29]. The soil sample was mixed with urea solution and incubated at 37Ã?Â?C for 2 h. The generated NH4+ was converted into NH3 in Kjeldahl distillation apparatus and absorbed by H3BO3 solution for HCl titration. Catalase activity was assayed based on the decomposition rate of H2O2 in soil slurry [28]. The soil was mixed with H2O2 solution and shaken at 25Ã?Â?C for 10 min. After centrifugation the remaining H2O2 in the supernatant was titrated by KMnO4 at acidic condition. Fluorescein diacetate (FDA) hydrolysis activity was determined based on the generation rate of fluorescein from the hydrolysis of FDA. The soil sample was mixed with sodium phosphate buffer at pH=7.6 and FDA solution and then shaken at 25Ã?Â?C for 2h. Fluorescein concentration was analyzed colorimetrically at 490 nm [28]. |
| Triplicates were performed for each activity analysis of each sample. |
| Calculation of resistance/resilience |
| Resistance/resilience was calculated based on Equation (1) [17,26], |
| where xtreated,i and xcontrol,i are values of No. i microbial functional activity of treated soil and the control, respectively, and Resi is resistance or resilience of the soil in No.i microbial functional activity. |
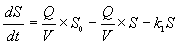 (1) (1) |
| Statistic analysis |
| The average resistance/resilience of a soil, , was calculated based on Equation (2), |
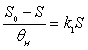 (2) (2) |
| where n is the total number of investigated microbial activities. The standard deviation, σRes , of the average resistance/resilience was calculated using Equation (3), |
 (3) (3) |
| where σi is the standard deviation of No. i microbial functional activity. |
| In significance analysis, F and t tests were used. If no significant difference between the precisions of two Res values was found in F test, t test was subsequently applied to identify any significant difference between the two Res values. All statistical analysis was conducted at 95% confidence level. |
| Results and Discussion |
| Heavy metal contamination in soil and sediment samples |
| By comparing with Delaware default background remediation standards [32] listed in Table 2, heavy metal contents in both forest and agricultural soils meet the criteria except for V content in both soils and Zn in the agricultural soil. The content of V in the forest soil and the agricultural soil was 1.9 and 6.1 times higher than the criteria, respectively. Zn content in the agricultural soil was 1.9 times higher than the standard. For the total content of heavy metals analyzed, the agricultural soil demonstrated to have a higher content than the forest soil. |
| More heavy metals in sediment samples were observed to exceed the standard than in soil samples. The contents of Mn, Pb, V, and Zn in both sediments exceeded the standard by 0.55 to 18.75 times. In addition, Cu content in sediment B exceeded the standard 0.17 times. For each heavy metal except for the content of Pb, sediment B displayed to be 0.42-1.04 times higher than sediment A. The content of Pb in sediment A was 701 mg kg-1, which was 9.14 times higher than that in sediment B. The observed high content of Pb and Cu in St. Jones River sediment is in consistence with the result from a previous study [33]. |
| The impact of sediment amendment on soil microbial functional activities |
| Values of investigated microbial functional activities of the nonamended and unheated agricultural and forest soil were listed in Table 4. The forest soil exhibited to be 1.4, 2.0, 0.4, 0.9, 0.3, and 0.4 times higher than the agricultural soil in soil respiration rate, dehydrogenase, b-glucosidase, urease, catalase, and FDA hydrolysis, respectively. Only in alkaline phosphatase and arylsulfatase activity the forest soil showed slightly lower (0.2 and 0.3 times lower, respectively) than the agricultural soil. The higher microbial activities, especially soil respiration rate and dehydrogenase, implied a higher biomass and overall microbial activity in the forest soil than in the agricultural soil [29]. |
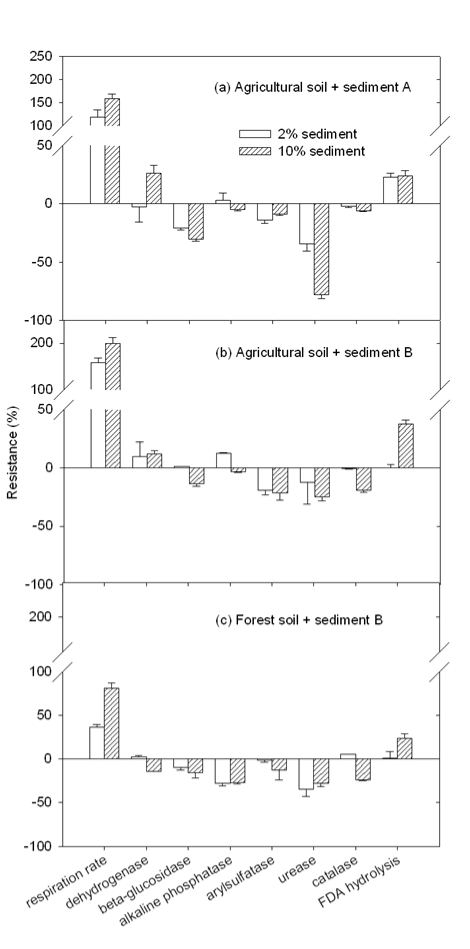
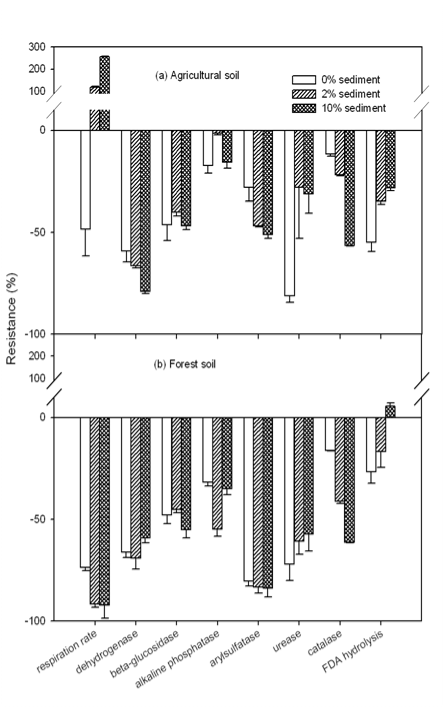
| Non-amended unheated soils were taken as blanks and resistance values were obtained by comparing activities of amended soils with the corresponding blank. With the amendment of sediment into soil, soil activities were altered differently (shown in Figure 2). For the amendment of sediment A and B in the agricultural soil, respiration rate was greatly stimulated with an increase of 119, 159, 159, and 199% for the amendment of 2 and 10% sediment A and 2 and 10% sediment B, respectively, compared to the blank. The higher amendment ratio of sediment, the higher increase was observed, indicating that the aerobic activity in amended soil was enhanced. It also implied the enhanced consumption of easily-available organic-C in the soil. This aerobic activity increase may directly result from the high content of biomass in sediment and/or the high organic matter content in sediment. It has been reported that soil respiration and biomass were significantly increased with the addition of organic waste [28,34]. Slight to moderate increase was observed in dehydrogenase and FDA activity, which are frequently taken as overall activity of soil microbes [35-37]. However, b-glucosidase, urease, alkaline phosphatase, arylsulfatase and catalase activities were found depressed with the amendment of sediment. Especially for the amendment of sediment A, 34 and 78% of urease activity in agricultural soil was inhibited with 2 and 10% sediment amendment, respectively. In addition, the inhibition on b-glucosidase, alkaline phosphatase, and catalase was enhanced with the increasing sediment amendment percentage. |
| The high contents of heavy metals, especially Ni, Cu, Pb, and Zn, in sediments, which are more than 18 times higher than those in agricultural soil, may be the major reason for the observed activity inhibitory effect. The addition of sediment greatly increased heavy content in amended soil. Particularly, soil pH was about 1 to 2 units lower than that of the sediment. The acidic soil environment may dissolve those insoluble heavy metals from the sediment, making them bioavailable in the amended soil. The addition of Cu2+ and Zn2+ salt in soil was reported to significantly inhibit phosphatase, arylsulfatase, and b-glucosidase [38]. In another study, arylsulfatse, phosphatase, protease, and urease in a forest soil were reported to be inhibited by either Cd2+, Cu2+, or Pb2+ salt [39]. Hence, the amendment of sediment A and B in agricultural soil may greatly enhanced aerobic microbial activity [28], but the nutrient cycling of N, P, and S [17] in soil was markedly depressed. |
| The effect of sediment A and B on agricultural soil microbial activities was similar, but some difference in the effect was identified. Compared with sediment A, sediment B amendment appeared to have noticeably more stimulation in soil respiration rate and FDA activity, which may be attributed mainly to the higher content of organic matter and/or biomass in sediment B than in sediment A. The amendment of sediment B also showed to have remarkably less inhibition on urease and b-glucosidase. Since Pb is the only heavy metal which content in sediment B is lower than in A among all analyzed heavy metals, it is very likely that the less inhabitation on above two activities might result from the pronouncedly low content of Pb in sediment B. |
| As demonstrated in Figure 2, sediment B appeared to exert less inhibitory effect than sediment A on agricultural soil microbial function, but it exerted more stimulation in soil respiration rate. Based on the overall changes of 8 activities determined in this study, the average resistance of the agricultural soil to each of the two sediment amendments was calculated. To the amendment of sediment A at 2 and 10%, the average resistance was 27.3 ±2.8 and 42.1 ±1.7%, respectively. To the amendment of sediment B at 2 and 10%, the average resistance was 26.8 ±3.1 and 41.3 ±2.0%, respectively. Though the resistance of individual activity to the amendments of two sediment samples was different, no significant difference (a=95%) was observed between the average resistance of the agricultural soil to the amendment sediment A and B at the same amendment ratio. Statistic analysis also showed significant difference (a=95%) in average resistance between two amendment ratios for both sediments. The higher amendment ratio, the less resistant the amended soil exhibited. |
| It can be argued that the resistance calculation might overestimate activity increase and underestimate activity decrease since the decrease is limited within 100% while the increase is allowed to exceed 100%. Additionally, the higher the absolute value of Res , the less resistant the soil is. This contrary assignment between the resistance value and the implication may cause confusion for understanding. However, the calculation used in this study is the only way which has been widely accepted for calculating resistance [17,26]. A more appropriate method producing values directly proportional to resistance and possessing no discrimination on activity decrease for resistance calculation might be needed. |
| Some differences in the microbial functional resistance between the forest soil and the agricultural soil to the amendment of sediment B were observed as well. Compared with the agricultural soil, the forest soil exhibited more resistant in soil respiration rate, FDA, and arylsulfatase activity. The same as discussed above, the higher resistance in respiration rate and FDA may be attributed to the higher content of organic matter in the forest soil than in the agricultural soil. It should be noted that Pb content in the forest soil was much higher than in the agricultural soil (shown in Table 2), which implied that the forest soil was more stressed by Pb than the agricultural. It could be very reasonable that Pb-tolerance mechanisms have already developed in the forest soil [26,38]. Hence, when both soils were exposed to Pb from sediment B, the forest soil may exhibit more resistant in relative activities. This may explain why a higher resistance in arylsulfatase was observed in the forest soil than in the agricultural soil. |
| However, compared with the agricultural soil, the forest soil showed less resistant in alkaline phosphatase activity. In the forest soil 28.1±2.3% and 27.2±1.6% inhibition was observed with the amendment of 2 and 10% of sediment B, respectively. Meanwhile only 12.5 ±0.8% increase and 3.1 ±0.8% inhibition was observed in the agricultural soil. Similarly, this may be attributed to the higher contents of heavy metals other than Pb in the agricultural soil than in the forest soil (shown in Table 2). The agricultural soil was much more stressed by heavy metals other than Pb than the forest soil, thus may appear more resistant in another group of activities, which include alkaline phosphatase activity. It has been reported that phosphatase could be inhibited by Cu, Zn, Cd, and As [34,38,40]. The same reason might be responsible for the lower resistance in dehydrogenase in the forest soil than in the agricultural soil. |
| The average resistance of the forest soil to the amendment of sediment B at 2 and 10% was calculated to be 14.8 ±1.6 and 28.3 ±2.0%, respectively, which is more than 0.3 times lower than that of the agricultural soil, correspondingly. Statistic analysis showed that the forest soil was significantly (a=95%) more resistant to the amendment of sediment B than the agricultural soil. Similar to the agricultural soil, the forest soil demonstrated to be significantly (a=95%) less resistant with the increasing amendment ratio of sediment B. |
| The impact of sediment amendment on soil microbial functional resistance to a heat disturbance |
| When investigating the soil microbial functional resistance to a standard heat disturbance, non-amended unheated soils served again as blanks. (Activity values in blanks are listed in Table 4) After receiving the standard heat, both the non-amended agricultural and forest soils were inhibited in all activities to various extents compared to the relative blank (shown in Figure 3). In the agricultural soil, urease activity was the most seriously affected and 81.2 ±3.1% inhibition was observed, while catalase activity was the most slightly affected with a inhibition of 11.6±1.6%. In the forest soil, arylsulfatase is the most seriously inhibited activity with an inhibition of 80.4 ±2.3% and catalase showed as the most slightly inhibited activity with an inhibition of 16.3 ±0.3%. The average resistance to heat for the non-amended agricultural and forest soil was 43.4 ±2.3 and 51.9 ±1.5%, respectively. Statistic analysis indicated that the non-amended forest soil appeared to be significantly (a=95%) less resistant to heat than the non-amended agricultural soil. This phenomenon may result from the mild environment to which the forest soil was exposed. Unlike agricultural soil which is normally exposed under sunlight, forest soil is usually covered by leaf debris and under shadows of trees. Mechanisms to tolerate high temperature might be not well established in forest soil. |
| Compared with the non-amended agricultural soil, the amendment of sediment B in the agricultural soil helped avoid the inhibition on soil respiration rate caused by heat and further resulted in more than 100% stimulation, indicating that the resistance of soil respiration rate to heat in the agricultural soil can be greatly enhanced by the amendment of this sediment. Resistance to heat in urease and FDA hydrolysis was also improved with the sediment amendment. However, significant decrease in resistance to heat was found in dehydrogenase, arylsulfatase, and catalase. |
| Different from the agricultural soil, the amendment of sediment B into the forest soil improved the resistance to heat in only FDA hydrolysis. For soil respiration rate and catalase, the resistance to heat was markedly lessened. No pronounced improvement or deterioration in the resistance to heat was demonstrated with other 5 activities in the forest soil with the sediment amendment. |
| By comparing with blanks, the average resistance to heat of the agricultural soil with 0, 2 and 10% sediment amendment was calculated to be 43.4 ±2.3, 44.8 ±3.2, and 70.4 ±1.4%, respectively. That for the forest soil with 0, 2 and 10% sediment amendment was 51.9 ±1.5, 57.9 ±1.6, and 56.2 ±1.6%, respectively. Statistic analysis indicated that the resistance to heat of agricultural soil was not significantly (a=95%) affected when the sediment amendment ratio was increased from 0 to 2%, but it was significantly (a=95%) weakened when the ratio was further increased to 10%. On the contrary, the resistance to heat of the forest soil was found to be significantly (a=95%) reduced when the sediment amendment ratio was increased from 0 to 2%. But the further addition of sediment to 10% exerted no significant change (a=95%) on the resistance to heat. This phenomenon revealed that the resistance to heat of the agricultural soil could tolerate the sediment amendment at low percentage (≤ 2%), but the tolerance capacity was very limited. Although appearing more sensitive in the heat resistance to sediment amendment at low percentage than the agricultural soil, the forest soil displayed more resistant to heat with a high amendment percentage of sediment. As stated above, the forest soil contained less total heavy metals than the agricultural soil (shown in Table 2). When both of them were exposed to sediment B with high contents of heavy metals, especially Zn, the forest soil was more likely less stressed. The tolerance/ detoxification mechanisms established in soil to deal with heavy metals from the sediment, the first stress, were most probably different from those demanded to deal with heat, the additional disturbance. Hence, it could be expected that the sediment-amended forest soil (less stressed soil) became more resistant to heat than the amended agricultural soil (more stressed soil) [26]. |
| The impact of sediment amendment on soil microbial functional resilience from the heat disturbance |
| In the resilience experiment, non-amended unheated soil samples were incubated with those heated samples at the same time and were taken as blanks to calculate resilience values of heated samples. Values of microbial functional activities of non-amended unheated soils after resilience incubation were listed in Table 4 as well. |
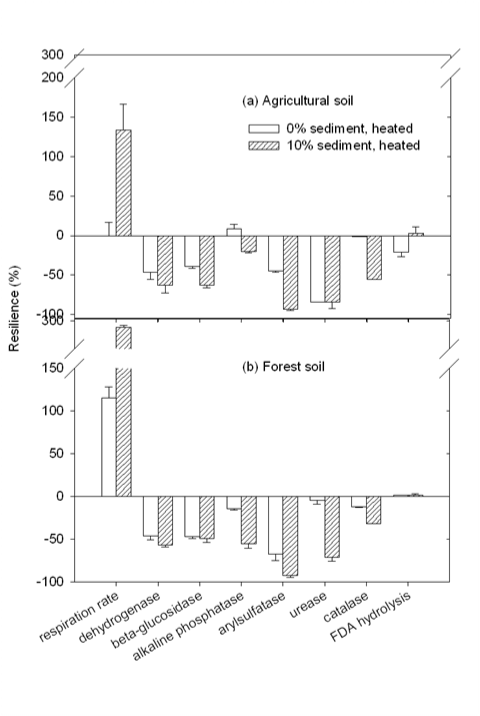
| The resilience of heated soils with and without 10% sediment B is shown in Figure 4. As demonstrated in figure 4a, soil respiration rate in the heated non-amended agricultural soil was well recovered after 30 days incubation, implying that the aerobic activity was resumed. A similar resilience was observed in alkaline phosphatase and catalase. However, more than 20% activity was not resumed for all other investigated microbial functions. Especially for urease, more than 84% activity was not resumed, remaining almost the same as that before 30- day incubation. Compared with the non-amended agricultural soil, the amendment of 10% sediment B demonstrated to have no significant (a=95%) effect on the resilience from heat in dehydrogenase and urease activity. Additionally, resilience enhancement was found in FDA analysis and deterioration was observed in respiration rate, a-glucosidase, alkaline phosphatase, arylsulfatase, and catalase activities. The average resilience for the heated agricultural soil with 0 and 10% sediment amendment was 30.6±2.7 and 64.4±4.6%, respectively, indicating that the resilience of the agricultural soil from heat disturbance was significantly (a=95%) reduced by the amendment of sediment B. |
| Similar phenomena were observed in the forest soil (shown in Figure 4b). Additional to catalase and alkaline phosphatase, urease and FDA hydrolysis activities in the non-amended forest soil were well recovered from heat disturbance. Soil respiration rate was recovered from the heat-caused inhibition and even greatly stimulated during incubation. For all other three microbial functions, more than 44% activity was not recovered. Different from the agricultural soil, the amendment of sediment B in the forest soil showed no impact on the resilience of b-glucosidase and FDA hydrolysis. Resilience deterioration caused by sediment amendment was observed in all other 6 functional activities. The average resilience from heat disturbance for the forest soil with sediment amendment at 0 and 10% was 38.7 ±2.1 and 80.5 ±1.3%, respectively. |
| Based on obtained values of average resilience from heat, the nonamended agricultural soil appeared to be significantly (a=95%) more resilient than the non-amended forest soil. The amendment of sediment B into the agricultural and the forest soil at 10% significantly (a=95%) reduced soil resilience from heat disturbance with an increase of Res at 33.8 ± 5.3 and 41.8 ± 2.5%, respectively. No significant (a=95%) difference was found between the resilience deteriorations caused by sediment amendment in two soils. |
| Conclusion |
| Based on the obtained values of average resistance, sediment A and B appeared to have no significant difference between their impacts on the microbial functional activities in the agricultural soil. The forest soil was found to be more resistant to sediment amendment than the agricultural soil. Though the resistance to heat of the forest soil appeared more sensitive to sediment amendment at low percentage than that of the agricultural soil, the former exhibited a bigger capacity to tolerate the sediment amendment than the later. Additionally, both soils were weakened in resilience from the heat disturbance with sediment amendment at 10%. No significant difference was found between the deteriorations in resilience caused by sediment amendment in two soils. |
| Overall, microbial functional stability in two local soils was markedly impacted by the amendment of sediments from St. Jones River. As indicated by functional activities, the nutrient cycling of N, P, and S in two local soils was greatly depressed and easily-available organic-C was promptly consumed with the sediment amendment. To minimize the impact on soil microbial functional stability, remediation treatment to remove heavy metals and/or alternative storage might need to be considered for sediment from St. Jones River when it is dredged in the future. |
| Acknowledgements |
| The authors would like to thank NSF Delaware EPSCoR RII-2 Program (National Science Foundation grant # EPS-0814251) for financial support through Delaware State University Seed Grant Program. The authors also thank the Visiting Scholar Program in Hefei University of Technology for the award to Shoujun Yuan for his visiting in Delaware State University. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15548
- [From(publication date):
specialissue-2012 - Oct 20, 2025] - Breakdown by view type
- HTML page views : 10916
- PDF downloads : 4632
