Research Article Open Access
Novel Therapy for Nicotine Addiction in Alcohol Dependent Rats
Mona Boules*, Bethany Stennett, Naveen Muhktar, Zhimin Li, Shui Cai and Elliott RichelsonNeuropsychopharmacology Laboratory, Mayo Foundation for Medical Education and Research, USA
- *Corresponding Author:
- Mona Boules
Mayo Clinic, 4500 San Pablo Rd
Jacksonville, FL 32224, USA
Tel: (904)953-7136
Fax: (904)953-7117
E-mail: boules.mona@mayo.edu
Received date: August 05, 2013; Accepted date: September 18, 2013; Published date: September 28, 2013
Citation: Boules M, Stennett B, Muhktar N, Li Z, Cai S, et al. (2013) Novel Therapy for Nicotine Addiction in Alcohol Dependent Rats. J Addict Res Ther 4:161. doi:10.4172/2155-6105.1000161
Copyright: © 2013 Boules M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: NT69L, a non-selective neurotensin agonist, provides a potential novel therapy for nicotine addiction in alcoholics by interacting with the common neurotransmitter circuits supporting the rewarding process for both nicotine and alcohol. Considering the behavioral effects of NT69L in attenuating nicotine self-administration in rats and alcohol consumption in mice, this study was designed to assess the effects of NT69L on nicotine self-administration in alcoholdependent rats.
Methods: Wistar rats pre-exposed to alcohol vapor or air were allowed to self-infuse nicotine (0.03 mg/kg/infusion) or saline. When the rats reached a stable level of responding, the effect of pretreatment with NT69L (1 mg/kg i.p.) on the reinforcing effect of nicotine was determined. The effect of NT69L on withdrawal signs caused by the discontinuation of nicotine and alcohol were recorded. Additionally, the effect of NT69L on dopamine and glutamate in the nucleus accumbens of rats that were co-injected with nicotine (0.5 mg/kg s.c.) and alcohol (1 g/kg i.p.) was determined with the use of in vivo microdialysis with HPLC and capillary electrophoresis.
Results: Animals self-infused nicotine at a significantly (P<0.05) higher rate compared to saline in both air and alcohol vapor exposed groups. Acute treatment with a single injection of NT69L significantly (P<0.05) reduced nicotine self-infusion in both the alcohol vapor and the air exposed groups for 5 days post-injection. NT69L also reduced the withdrawal signs associated with the discontinuation of alcohol and nicotine administration. Additionally, NT69L attenuated the alcohol- and nicotine-induced increase in dopamine and glutamate in the nucleus accumbens.
Conclusion: NT agonists may represent a potential novel therapy to treat alcohol and nicotine addiction simultaneously by modulating dopamine and glutamate.
Keywords
Neurotensin; Nicotine; Alcohol; Addiction; Microdialysis; Dopamine; Glutamate; Rat
Introduction
Nicotine and alcohol addiction are frequently co-morbid problems [1]. The co-dependence of nicotine and alcohol addiction complicates treatment and is often associated with significant morbidity and mortality. Prevalence of smoking in alcoholics is as high as 90% compared to 30% for the general population [2] and alcohol consumption is associated with high nicotine use [3,4]. Co-morbid cigarette smoking and alcoholism has been associated with higher rates of depression in comparison with non-alcoholic smokers [5] and increased cravings for nicotine in comparison with non alcohol dependent smokers [6]. Additionally, smoker alcoholics are more likely to die from smoking related diseases rather than directly from an alcohol related medical condition [7]. Pharmacotherapeutic options for the treatment of alcohol and tobacco co-dependence are limited. Thus, there is a critical need for the development of novel drugs implementing new therapeutic targets.
Neurotensin (NT) is a neuropeptide that is closely associated with, and modulates dopamine (DA) [8], acetylcholine (ACh), glutamate, and γ-aminobutyric acid (GABA) neurotransmission, all of which are involved in addiction and reward pathways. Local administration of NT in the prefrontal cortex (PFC) increases extracellular levels of ACh and GABA [9]. Similar data were obtained with the extracranial injection of the NT agonist NT69L [10], a compound that was developed in our laboratory. NT also enhances GABAergic activity in rat hippocampus [11] and reduces glutamatergic neurotransmission in dorsolateral striatum [12]. However, NT must be administered centrally to have an effect because it is easily degraded by peptidases. Our laboratory has developed many NT agonists that can be administered extracranially and mimic the central effects of NT. The most studied of these agonists is NT69L, which binds with equal affinity to the two well-characterized NT receptors, subtype 1 (NTS1) and subtype 2 (NTS2). Our work with NT69L shows that it blocks nicotine-induced sensitization and nicotine self-administration [13-15].
In addition to its role in animal models for nicotine addiction, the NT system has been strongly implicated in the neurochemical and behavioral effects of alcohol use [16,17]. Chronic administration of NT69L reduces alcohol preference and consumption in mice through modulation of the NTS1 [18]. Biochemically, NT69L normalizes the nicotine-induced changes in DA [19], the neurotransmitter that promotes the motivational process for both nicotine and alcohol, and the alcohol-induced increase in DA and glutamate in the striatum [20]. Additionally, NT modulates other neurotransmitters implicated in alcohol use disorder (AUD). It causes neurochemical changes similar to acamprosate (the calcium salt of acetylhomotaurine), which is one of three FDA-approved drugs to treat AUD. Administration of acamprosate or NT69L increases extracellular concentration of DA in striatum [10,11] and both acamprosate and NT69L modulate glutamate [21,22].
Considering the behavioral effects of NT69L in attenuating nicotine self-administration in rats and alcohol consumption in mice and its modulatory effects on neurotransmitters implicated in nicotine and alcohol addiction, we hypothesized that NT69L might be a potential novel therapy for co-morbid nicotine and alcohol addictions. To test this hypothesis, we used alcohol-dependent rats that were simultaneously allowed to self-administer nicotine. The results support our hypothesis.
Materials and Methods
Animals
Wistar rats were used and weighed 200-220 g at the start of the experiments. Animals were housed in temperature controlled rooms with free access to food and water unless otherwise indicated. All animal procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee.
Behavioral studies
Operant behavior: i) Apparatus: Six operant conditioning chambers (Med Associates, St Albans, VT, USA) placed in soundattenuated outer chambers were used. The two sidewalls of the chambers were aluminum with the front and back walls made of clear Plexiglas. Two response levers were located on one side of the chamber on either side of a food trough. A 28-V DC white cue light was located above each of the response levers. An infusion pump (Med Associates) was used to deliver the nicotine, NT69L, or saline to the animal’s jugular vein via a tether/swivel line assembly (Instech Labs. PA, USA) that was mounted above each chamber. The swivel allowed unrestricted movement of the rats throughout the chamber.
ii) Operant training: Rats were trained to self-administer sucrose orally in 5 daily 1 h sessions to facilitate the learning process of the operant response. The rats were trained on fixed ratio 1 schedule of reinforcement (FR1) in which the rats received a maximum of 20 reinforcements. Reinforcement was paired with a 20 s cue light (during which the animal’s responding was reinforced upon pressing the active lever) followed by a 60 s period during which the cue light was turned off and responding was not reinforced. Responding to the inactive (right) lever had no scheduled consequences. Lever responses (active and inactive), and infusions were recorded by Med Associates computer software. During the entire phase of the experiment, food access was restricted to 20 g/rat/day given immediately after the operant session. When the rats reached a stable level of responding they were allowed free access to food prior to undergoing surgery to insert the jugular catheter as described below.
Alcohol dependence: Rats received intermittent (14 h on/10 h off) ethanol vapor exposure for 3 weeks to induce alcohol dependency as described in detail by others [23,24]. Briefly, the rats were placed in sealed Plexiglas standard rodent cages (Alcohol Research Inc, La Jolla San Diego, CA, USA). Ethanol vapor was produced by dripping 95% ethanol into a 2000 ml Erlenmeyer flask kept on a warming tray (50°C) and subsequently blowing air into the animal cage at a rate of (2.268 l/ min). Control animals were exposed to air.
Measurement of blood alcohol levels (BAL): Blood alcohol levels (BAL) were determined at the end of the 14 h ethanol vapor exposure period. Blood samples were centrifuged to separate the serum from blood. The serum (5 μl) was extracted and then injected into an Analox AM1 analyzer for measurement (Analox Instruments LTD, Lunenberg, MA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol+O2→acetaldehyde + H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration [24]. Control rats were treated equally except they were exposed to air.
Nicotine self-infusion: Nicotine self-infusion was assessed during 1 h daily sessions. Two separate groups of rats (n=12) (rats exposed to alcohol vapor and rats exposed to air) were allowed to self-infuse nicotine (0.03 mg/kg/infusion in 90 μl volume) contingent upon pressing the active lever (left). The active lever for nicotine infusion was the same lever used for sucrose reinforcement. When the animals reached a stable level of responding, the rats in each group were further sub-divided into 2 groups. One group was pretreated with NT69L (1 mg/kg i.p.) and the other with an equal volume of saline 30 min before the nicotine self-infusion session. The rats were tested daily for nicotine self-infusion for 5 d post-NT69L injection. The dose of nicotine used in this study has been reported by several investigators to be at or near the peak of the dose-response curve for nicotine self-administration on a fixed ratio schedule of reinforcement [25,26]. The dose of NT69L was that used in our laboratory to block the initiation and expression of nicotine sensitization in rats, a mechanism that is thought to underlie craving and risk of relapse to smoking [13,14,27].
Withdrawal studies: i) Nicotine withdrawal: Two groups of Wistar rats (n=12) were exposed to either alcohol vapor or air for 3 weeks. Animals were then injected with nicotine (0.35 mg/kg s.c.) twice daily for 15 d. On day 15, nicotine administration was discontinued and the animals were injected with either NT69L (1 mg/kg i.p.) or saline. Thirty minutes later somatic nicotine withdrawal signs were recorded for a 10 min observation period. Somatic withdrawal signs included body shakes, chews, cheek tremors, escape attempts, eye blinks, foot licks, gasps, genital licks, head shakes, ptosis, scratches, teeth chattering, and writhes as described by Skjei and Markou [28]. These data were recorded as the sum of individual occurrences of the abovementioned withdrawal signs in a 10 min period.
ii) Alcohol withdrawal: Two groups of Wistar rats (n=12) were exposed to either alcohol vapor (14 h on/10 h off) or air for three weeks. Acute alcohol withdrawal was induced by discontinuation of the alcohol exposure. Two hours following the anticipated time of exposure to alcohol vapor, the rats were tested for acute alcohol withdrawal behavior.
Alcohol withdrawal behavior included observation for tail rigidity [24], body tremors, and wet dog shakes [29]. Additionally, the rats were tested for hyperactivity, muscle rigidity (catatonia) [29], hyperalgesia (excessive sensitivity to pain) [30], and hyperthermia.
iii) Hyperlocomotion: Locomotor activity was recorded in a Plexiglas Opto-Varimex activity chamber (Columbus, OH, USA) equipped with infrared photocell emitters and detectors. The rats were acclimated in the room for 2 h and then placed in the activity chambers. Activity was recorded as distance travelled in cm every 10 min in a 3 h period.
iv) Hyperalgesia: Excessive sensitivity to pain (hyperalgesia) was assessed during alcohol withdrawal with the use of tail flick latency to radiant heat in an Analgesia Meter (Harvard Apparatus, Holliston, MA, USA). The latency for the rat to flick its tail was measured in seconds. A cutoff time of 15 s was implemented to prevent tissue damage.
v) Muscle rigidity (catatonia): muscle rigidity was evaluated with the use of the bar test. The rats’ front paws were placed on a suspended metal bar 10 mm in diameter and 11 cm from the base of a plastic standard rat cage. The latency for the rat to remove its paws from the bar was recorded. Each animal was tested 3 times and the average time in seconds was recorded.
vi) Hyperthermia: Body temperature was measured by means of a thermistor probe inserted approximately 2 cm into the rectum of the rat. Baseline readings were recorded prior to placing the rats in the alcohol chamber. Body temperature was recorded again after the rats were exposed to air or alcohol for three weeks and finally 30 min post NT69L injection. Data were recorded as change in body temperature from baseline in °C for each rat. The average of all rats in each group was illustrated.
Surgical procedures
Microdialysis: Surgeries were implemented to insert a guide cannula in the nucleus accumbens (NA) in Wistar rats as described previously by our group [19]. Briefly, each rat was anesthetized with gasiform isoflurane (1-2% isoflurane in a mixture of 20% oxygen and 80% nitrogen gas), and immobilized in a stereotaxic frame (KOPF Inc., Tujunga, CA, USA). The anesthesia was maintained during the entire experiment. Each guide cannula (CMA Microdialysis Inc., Acton, MA, USA) was stereotaxically implanted into the NA (A1.7, L 0.7, V6.0, relative to bregma and skull then secured to the skull by screws and dental cement. Following surgery, each rat was housed individually and allowed 3 days of recovery. On the testing day, the microdialysis probe was placed in the guide cannula and the animals were placed in the microdialysis bowl for 2 h to acclimate. After collecting three baseline fractions, the animals were pretreated with either saline or NT69L and 40 min later with nicotine (0.5 mg/kg s.c.), alcohol (1 g/kg i.p.) or the combination of the two (the combination doses of nicotine and alcohol were chosen to reflect moderate doses of both drugs that will result in adequate DA release without possible ceiling effect as reported previously [31]. Samples were collected every 20 min over a 3-h period. The dialysate samples were then applied to the HPLC to determine DA. 10 μl of the dialysate was derivatized and applied to the capillary electrophoresis to determine levels of glutamate and glycine. Control groups were injected with NT69L or saline and neurotransmitters were determined as described previously.
i) Capillary electrophoresis: Glutamate and glycine in the microdialysate were measured by capillary electrophoresis (CE) coupled with laser induced fluorescence (CE-LIF, Agilent 3D CE & Picometrics ZETALIF evolution detector) as previously described by our group [22].
ii) HPLC-ECD: DA was determined in the microdialysate with the use of an HPLC coupled with Coulochem II electrochemical detector system (ESA Inc., Chelmsford, MA, USA) with a 20-μl sample loop. DA was separated on an MD-150 analytical column (3×150 mm, 3 μm C18, ESA Inc., Chelmsford, MA, USA) with MDTM mobile phase (ESA Inc., Chelmsford, MA, USA) at 0.6 ml/min. Potential settings for detection were E1 at -175 mV, E2 at 250 mV, GC at 350 mV. Peaks were displayed, integrated, and stored with ESA 501 Chromatography data system (ESA Inc., Chelmsford, MA, USA).
Jugular catheterization: Animals underwent surgery to place an indwelling catheter for nicotine self-infusion as done previously in our laboratory and described in details by our group and others [15,32] with slight modifications. Briefly, the rats were anesthetized by intramuscular injection of a cocktail containing ketamine (30 mg/kg), xylazine (6 mg/ kg), and acepromazine (1 mg/kg). A silastic catheter was inserted into the jugular vein and exited and secured between the scapulae with the use of vascular access button and covered with a protective cap (Instech Labs., PA, USA). The vascular access button permits quick, aseptic connection and disconnection of the catheterized rat to the infusion tether, and the protective cap allows for group housing without the possibility of animals chewing the catheters. To manage pain, 100- 300 mg/kg of 30 ml Children’s Tylenol (160 mg/5 ml formulation) was added to drinking water 48 h before and 48 h after surgery.
Drugs
NT69L was synthesized by Mayo Clinic Chemistry Core facility as described previously [33]. NT69L, a 6-mer, is modified from NT at amino acid positions located in the C-terminal 8-13 sequence. Table 1 shows the structure of NT69L as compared to neurotensin.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | p-Glu | L-Leu | L-Tyr | L-Glu | L-Asn | L-Lys | L-Pro | L-Arg | L-Arg | L-Pro | L-Tyr | L-Ile | L-Leu |
| NT69L | - | - | - | - | - | - | - | N-methyl-Arg | L-Lys | L-Pro | L-neo-Trp | tert-Leu | L-Leu |
Table 1: Structures of NT and NT69L.
Nicotine hydrogen tartrate salt was purchased from Sigma Chemical Co., (St Louis, Mo., USA) and was dissolved in sterile physiological saline (0.9% sodium chloride). For nicotine self-infusion, the pH of the nicotine solution was adjusted to 7 (± 0.5) and filtered through a 0.2 μm filter. Ethanol (95%) was purchased from Fisher Thermo Scientific Inc., (Houston, TX, USA).
Statistical Analysis
One-way analysis of variance (ANOVA) was used to analyze the data. Individual group comparisons were carried out using the Tukey’s test with the use of SigmaStat 2.0 software (SPSS, Inc., Chicago, IL), with P<0.05 being considered significant.
Results
Behavioral studies
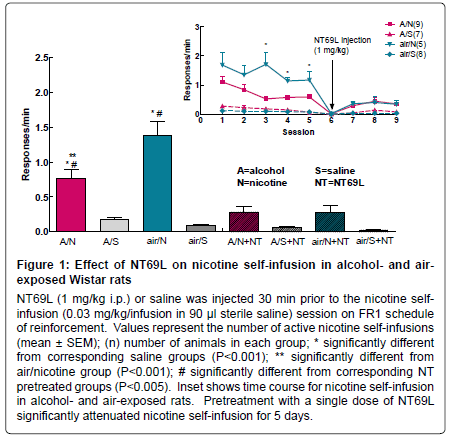
Operant Behavior: i) Effect of NT69L on nicotine self administration in rats exposed to alcohol vapor or air (Figure 1): One way ANOVA shows a significant effect of treatment on nicotine self-administration, F7,50=25.293, P<0.001. Nicotine self-administration was significantly higher than saline self-administration in both the alcohol vapor and air exposed groups (P<0.001). Exposure to alcohol significantly decreased nicotine self-administration (P<0.001). The blood alcohol level (BAL) in the alcohol exposed rats was significantly (P<0.001) higher 126.4 ± 1.8 mg/dl as compared to the air exposed rats (10.3 ± 0.8 mg/dl). BAL is considered undetectable (<20 mg/100 ml) in nondependent (control) animals [34].
Pretreatment with NT69L 30 min prior to a nicotine selfadministration session significantly reduced nicotine selfadministration in both the alcohol vapor and air exposed groups (P<0.005), Figure 1. Inset of Figure 1 shows the time course of nicotine self-administration after the responding had stabilized. Remarkably, the attenuating effect of a single NT69L injection on nicotine selfadministration lasted for 5 days post-injection. Previous studies in our laboratory showed that NT69L has no reinforcing effect and that there was no significant difference between NT69L and saline selfinfusion [15]. Since all the rats were trained to press the lever for sucrose pellets, the lack of response in the saline treated group indicates that the responding to nicotine was specific and that the responding to the sucrose pellets was extinguished and was not carried over to compromise responding to nicotine.
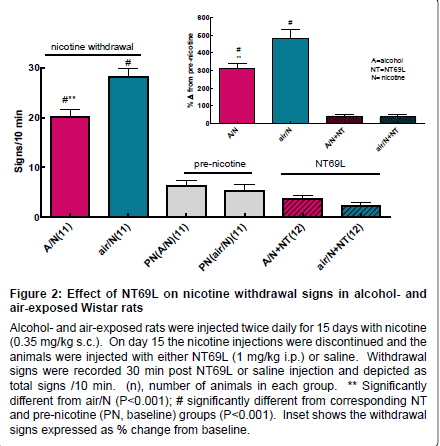
Withdrawal studies: i) Effect of NT69L on nicotine withdrawal signs in rats exposed to alcohol vapor or air: After two weeks of twice daily nicotine injections, the discontinuation of nicotine injections resulted in the development of acute nicotine somatic withdrawal signs. Rats showed body shakes, chews, cheek tremors, eye blinks, foot licks, genital licks, head shakes, teeth chattering, and writhes. All responses were summed in a 10-min observation period for each rat and the average of all the rats’ responses are shown in Figure 2. Discontinuation of nicotine treatment significantly increased withdrawal signs, F5,62=80.869, P<0.001. Both alcohol and air exposed groups showed significantly higher withdrawal responses as compared to pre-nicotine treatment (baseline), P<0.001. Alcohol vapor exposed rats showed significantly lower withdrawal signs than the air exposed group (P<0.001). Pretreatment with NT69L 30 min prior to observation attenuated the nicotine withdrawal signs (P<0.001) to levels that were not significantly different from pretreatment values in the air-exposed group (P=0.531) and the alcohol vapor-exposed group (P=0.640). Inset shows the withdrawal signs expressed as % change from baseline.
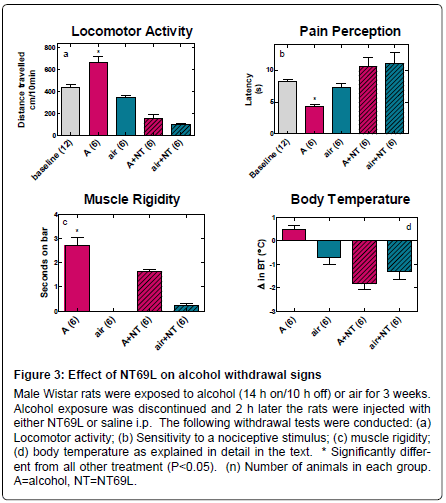
ii) Effect of NT69L on alcohol withdrawal signs: Acute alcohol withdrawal following 3 weeks of exposure to alcohol vapor resulted in development of alcohol withdrawal signs. The rats that were exposed to alcohol vapor showed “wet dog” shakes, body tremors, and Straub tail upon acute withdrawal (Table 2). Additionally, acute alcohol withdrawal resulted in: 1) Increased locomotor activity (F4,34=38.305, P<0.001), Figure 3a; 2) Hyperalgesia as measured by reduced latency to radiant heat in an Analgesia Meter as compared to air exposed rats (F4,35=7.738, P<0.001), Figure 3b; 3) Increased catatonia (muscle rigidity) (F 3,22=47.899, P<0.001), Figure 3c; and 4) Hyperthermia (F3,22=12.850, P<0.001), Figure 3d.
| Withdrawal sign | Alcohol | Air | Alcohol/ | Air/ |
|---|---|---|---|---|
| (10 min observation period) | NT | NT | ||
| Wet dog shake | 6.3 ± 0.8** | 0.0 | 0 | 0 |
| Tremors | 1.2 ± 0.5* | 0.0 | 0 | 0 |
| Straub tail | ++++ | - | - | - |
Significantly different from air exposed group and NT69L pretreatment (**P<0.001; *P=0.035).
Table 2: Effect of NT69L on observed acute alcohol withdrawal signs.
Microdialysis studies
Effect of NT69L on nucleus accumbens brain neurotransmitters of Wistar rats co-injected with nicotine and alcohol
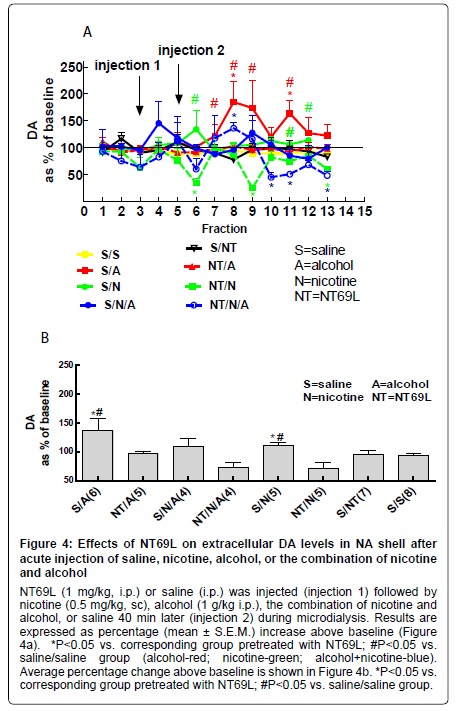
i) Effect of NT69L on nucleus accumbens DA levels: Both nicotine and alcohol significantly increased DA levels in the NA (P<0.05). Pretreatment with NT69L 30 min prior (injection 1) to nicotine or alcohol administration (injection 2) reduced DA levels to saline control (P<0.05). Interestingly, the combination of nicotine and alcohol did not increase DA levels (Figure 4a, Figure 4b).
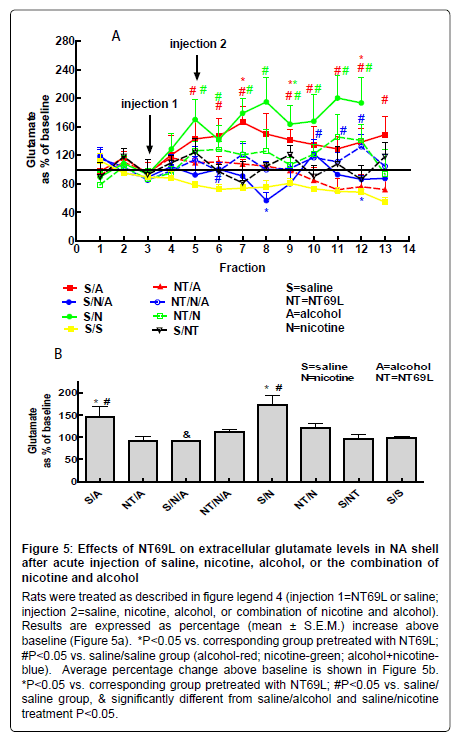
Effect of NT69L on nucleus accumbens glutamate levels: Both nicotine and alcohol significantly increased glutamate levels in the NA (P<0.05). Pretreatment with NT69L 30 min prior to nicotine or alcohol administration reduced glutamate levels (P<0.05). Interestingly the combination of nicotine and alcohol significantly reduced glutamate levels (Figure 5a and 5b).
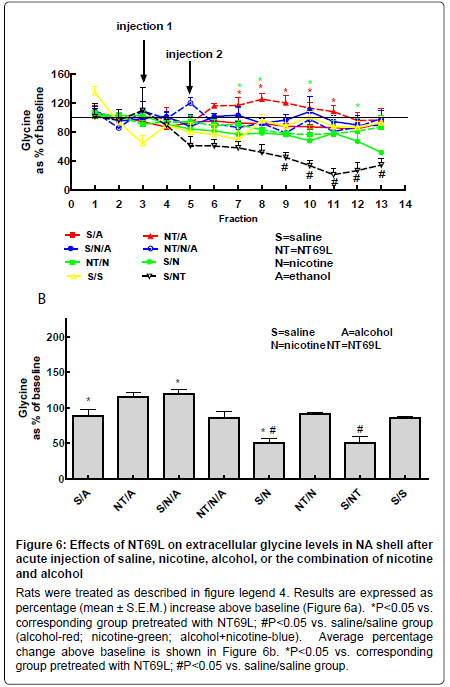
Effect of NT69L on nucleus accumbens glycine levels: Injection with nicotine, alcohol, or the combination of both did not cause a significant change from the saline-treated group. Pretreatment with NT69L significantly increased glycine levels in the NA in the alcohol and nicotine groups while reduced glycine levels in the animals injected with both alcohol and nicotine (P<0.05) (Figure 6a and 6b).
Figure 7 shows the microdialysis probe placement in the nucleus accumbens. Numbers indicate distance (mm) from bregma.
Discussion
The need for new therapy for nicotine addiction in alcohol dependent subjects
Humans that co-abuse alcohol and nicotine have a worse clinical outcome than individuals who use either substance [35]. Although nicotine and alcohol interact in their rewarding effects [36], few clinical studies have addressed the possibility of a co-treatment, and the data have produced mixed results for treating nicotine-dependence in alcoholdependent subjects [37-39]. Similar conflicting results were obtained in treating alcohol- dependent patients who smoke. For example, the opiate antagonist naltrexone, used to treat alcohol dependence [40], has been shown to have conflicting effects on smoking with some studies reporting reductions [41], while others report increases in the urge to smoke [42]. Thus, there is a critical need for the development of novel drugs with new therapeutic targets that will help with smoking cessation and alcohol dependence.
NT69L is a neurotensin receptor agonist that attenuates nicotine self-administration in rats [15] and alcohol consumption in mice [18]. NT69L works by interacting with the common neurotransmitter circuits supporting the rewarding process for both nicotine and alcohol. This action makes NT69L a good candidate for treating nicotine dependence in alcohol-dependent rats.
Effect of NT69L on nicotine self-administration in alcohol dependent rats
The most important finding in this study is that NT69L significantly attenuated nicotine self-administration in alcohol-dependent rats, as well as in air exposed rats. The results with the air-exposed rats are similar to previous results from our laboratory [15]. Interestingly, in contrast to alcohol-preferring (P) rats that readily self-administer more nicotine and express greater nicotineseeking behavior than do the alcohol non-preferring (NP) rats [43], the alcohol-exposed rats self-infused less nicotine as compared with air-exposed rats. The effect of alcohol exposure on nicotine selfadministration in our study is in agreement with a previous study where access to alcohol reduced nicotine self-administration in Wistar rats [36].
The difference between our data and those conducted using P rats might be due to the genetic liability of the P rats selected for high alcohol drinking. Genetic studies in humans have established that the liability to use either drug or both drugs is inherited [44]. Additionally, twin studies have shown that there may be common genetic mechanisms for alcohol and/or nicotine dependence [45-47]. Another consideration for the limited effect of alcohol dependence on nicotine self-administration might be due to the passive exposure of the rats to alcohol vapor. Passive exposure could remove the conditioning effect of drinking and smoking seen in humans. This conditioning is thought to be the reason why alcoholics tend to smoke more than non-alcoholic individuals. Nonetheless, NT69L reduced nicotine self-administration in both the alcohol- and air-exposed groups.
Effect of NT69L on nicotine withdrawal
Smoking cessation is known to produce an aversive withdrawal syndrome in humans [48,49]. Nicotine withdrawal is associated with somatic signs and symptoms such as bradycardia, gastrointestinal discomfort, and increased appetite as well as affective symptoms which include craving, depressed mood, dysphoria, anxiety, irritability, and difficulty concentrating [48,50,51]. The aversive aspects of nicotine withdrawal contribute to high relapse rates to tobacco smoking after cessation attempts [28]. In animals, the nicotine withdrawal syndrome is precipitated following either abrupt discontinuation of nicotine administration or the administration of nACh receptor antagonists, such as mecamylamine [52-54]. Nicotine abstinence in animals is manifested in somatic signs such as shakes, ptosis, jumps, piloerection, gasps, foot licks, chews, or scratches [55].
In addition to blocking the rewarding effects of nicotine, NT69L reversed withdrawal signs induced by the cessation of nicotine administration in both the alcohol and air exposed groups (Table 1). These results are in agreement with our previous studies showing that NT69L effectively blocks the initiation and expression of nicotineinduced sensitization [13,14]. Nicotine-induced sensitization is a mechanism that is thought to underlie craving and risk for relapse to smoking [27]. Interestingly, exposure to alcohol attenuated the nicotine withdrawal signs, similar to reports by Gulick and Gould, who found that ethanol reversed nicotine withdrawal symptoms [56]. Thus, alcohol might have a protective effect against nicotine withdrawal. This protective effect can be mediated via NMDA [57] or nACh receptors [58,59].
Effect of NT69L on alcohol withdrawal
Alcohol withdrawal refers to a group of signs and symptoms that result from terminating alcohol use after prolonged exposure [60]. These withdrawal signs and symptoms make attempts to abstain from alcohol difficult and increase the risk of relapse in recovering alcoholics [61,62]. The somatic alcohol withdrawal signs are thought to be due to neural hyperexcitability [63]. Alcohol withdrawal signs in rodents include tremors, rigidity, and convulsions [64-66], which are also observed in humans [67].
Unlike previous reports where neurotensin (10-100 μg, IC) had no effect on ethanol withdrawal signs [68], systemic administration of the neurotensin agonist, NT69L, significantly attenuated alcohol withdrawal signs (Table 1). The discrepancy between our data and those of Frye et al. may relate to differences in the dose of alcohol and to the route of administration of the NT receptor agonist.
Effect of NT69L on nicotine-and alcohol-induced biochemical changes in rats
The association between smoking and alcohol consumption involves biochemical interactions as well. The rewarding effects of both alcohol and nicotine are associated with the release of DA in the NA. This part of the brain is strongly implicated in the reinforcing effects of drugs, including alcohol and nicotine [69] and receives heavy dopaminergic input from the ventral tegmental area (VTA). Together the NA and VTA comprise an important component of the mesolimbic ‘reward pathway’ [31,70]. Acute nicotine elevates dialysate DA levels in the NA [19,71,72]. Similarly, alcohol enhances DA neurotransmission by increasing the firing rate of DA neurons [73] and somatodendritic DA release in the VTA [74,75], subsequently leading to an increase in extracellular levels of DA in the NA [71,75].
Despite having similar effects on DA release in the NA, nicotine and alcohol differ in their mechanism of action. The reinforcing effects of nicotine are thought to be highly specific to a subtype of nACh receptors. In contrast, the effects of alcohol are less specific. Alcohol seems to have selective effects on a variety of neurotransmitter systems including nACh receptors, DA, serotonin, GABA, and glutamate receptors [76]. Nonetheless, it is interesting to note that varenicline, a partial nACh receptor agonist, was recently shown to have some efficacy in treatment of alcohol abuse [77].
The clinical results with varenicline may relate to the animal data showing that desensitization of ACh receptors reduces alcohol [78], nicotine [79], and both nicotine and alcohol self-administration [80], since the rewarding effects of both alcohol and nicotine depends on ACh receptors [81]. It is thought that the nicotinic receptors in the brains of subjects during smoking are in a desensitized state. When the desensitized receptors start to recover (during no smoking periods), the smoker craves more nicotine. NT69L increases ACh release [10,82]. The increase in ACh could induce desensitization to the nicotinic receptors without the reinforcing properties associated with nicotine and alcohol use.
In agreement with previous studies from our laboratory and others [19,83,84], nicotine increased the release of DA. This increase is consistent with enhanced activity of the DAergic system, effects that were decreased by pretreatment with NT69L. Similarly, alcohol injection increased extracellular DA in the NA. This increase was also decreased by NT69L pretreatment. NT regulates DA through decreasing the DA binding affinity for the DA D2 receptor [85] and opposing DA D2 receptor agonist-induced auto-inhibition of DA cell firing [86]. Allosteric receptor-receptor interactions between NT and DA D2 receptors as well as second messenger-dependent receptor alteration, such as phosphorylation and dephosphorylation, have also been implicated in this regulation of DA by NT [87]. The possible desensitization of ACh receptors by NT69L in the VTA might indirectly attenuate alcohol and nicotine-induced increase in DA in the NA as the increase in DA caused by the nicotine and alcohol is a result of activation of ACh receptors.
In contrast, the co-administration of nicotine and alcohol did not result in an additive effect on accumbal DA levels as has been reported previously [70,88]. The difference in the results could be attributed to the difference in the doses of alcohol and nicotine [88] as well as the experimental design where the rats were exposed to either alcohol or nicotine before the co-administration of both alcohol and nicotine [31].
In addition to DA and ACh, the neurobiological substrates underlying drug dependence involve a complex interplay between other neurotransmitter systems such as glutamate [89]. Glutamate increases the activity and facilitates the release of DA from dopaminergic neurons [90]. Compounds that enhance inhibitory transmission and block excitatory glutamatergic neurotransmission play an important role in alcohol dependence. Similarly, activation of excitatory ACh receptors on glutamate terminals contributes to the acute reinforcing of nicotine; and NMDA antagonists block nicotine-induced DA release in the NA [91].
In our study, nicotine or alcohol administration increased glutamate levels in the NA. Co-administration of nicotine and alcohol did not increase glutamate levels in the NA, results similar to those of others [92]. Pretreatment with NT69L significantly reduced glutamate to levels that were not different from controls. Thus, in addition to its role as modulator of DA, NT plays a modulatory role on other neurotransmitter systems implicated in addiction to nicotine and alcohol, such as ACh, GABA, and glutamate. Anatomically, NT is co-localized with DAergic [93,94] and GABAergic [95] neurons in the VTA, NA, and PFC [96], areas that have been implicated in drug reward and reinforcement [31,70]. Biochemically, NT increases extracellular GABA in the NA [97]. This effect can decrease the glutamatergic excitatory effect on DA activity decreasing reward.
Glycine also plays an important role in regulating alcohol consumption. Glycine receptors in the NA and nicotinic Ach receptors in the VTA have been implicated in the positive reinforcing and DA elevating effects of ethanol [98]. Administration of the glycine transporter 1 (GlyT-1) inhibitor decreases alcohol intake in Wistar rats [99-101] and in alcohol preferring rats [102]. Blockade of GlyT-1 also interferes with compulsive alcohol consumption and relapse [103]. The effect of GlyT-1 inhibitor has been attributed to reducing the alcohol-induced DA response in the NA and restoring the altered balance between the glutamatergic and glycinergic signaling function [104]. Similarly, GlyT- 1 inhibitor blocks nicotine cue potentiated reinstatement and elevates NMDA signaling [105]. The effect of nicotine on glycine is region specific and dose- and Ca++-dependent. Intraperitoneally administered nicotine does not alter glycine levels [106]. Intracerebral administration of nicotine at a dose of 50 mM increases glycine in the hippocampus of anesthetized rats but s.c. administration does not [107]. Similarly perfusion of 1 mM of nicotine does not alter glycine levels but 5 mM increases glycine in the striatum and prefrontal cortex [108]. In the present study, pretreatment with NT69L significantly increased glycine levels in the alcohol and nicotine treated animals and lowered it in the alcohol/nicotine group indicating that in addition to its effect on DA and glutamate NT69L might affect alcohol and nicotine-dependence through modulating glycinergic neurotransmission.
In summary, the neurotensin agonist NT69L and possibly other neurotensin agonists may represent a new treatment for nicotine dependence in alcoholics through modulating neurotransmitters involved in nicotine and alcohol addiction. NT69L possess an additional important property that is crucial in smoking cessation. NT69L, and other NT agonists, reduce food intake and reduce weight gain [109,110]. Thus, NT69L will decrease the weight gain associated with smoking cessation. Weight gain acts as a barrier to quitting smoking particularly among women.
Acknowledgements
This work was supported by Mayo Foundation for Medical Education and Research and grant number 2KF01 04NIR-02 from the Florida Department of Health to Mona Boules.
References
- DiFranza JR, Guerrera MP (1990) Alcoholism and smoking. J Stud Alcohol 51: 130-135.
- Istvan J, Matarazzo JD (1984) Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull 95: 301-326.
- Zacny JP (1990) Behavioral aspects of alcohol-tobacco interactions. Recent Dev Alcohol 8: 205-219.
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, et al. (2000) Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol 35: 171-175.
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC (1997) Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treat 14: 521-527.
- Hertling I, Ramskogler K, Dvorak A, Klingler A, Saletu-Zyhlarz G, et al. (2005) Craving and other characteristics of the comorbidity of alcohol and nicotine dependence. Eur Psychiatry 20: 442-450.
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, et al. (1996) Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA 275: 1097-1103.
- Bissette G, Nemeroff CB (1988) Neurotensin and the mesocorticolimbic dopamine system. Ann N Y Acad Sci 537: 397-404.
- Petkova-Kirova P, Rakovska A, Della Corte L, Zaekova G, Radomirov R, et al. (2008) Neurotensin modulation of acetylcholine, GABA, and aspartate release from rat prefrontal cortex studied in vivo with microdialysis. Brain Res Bull 77: 129-135.
- Prus AJ, Huang M, Li Z, Dai J, Meltzer HY (2007) The neurotensin analog NT69L enhances medial prefrontal cortical dopamine and acetylcholine efflux: potentiation of risperidone-, but not haloperidol-, induced dopamine efflux. Brain Res 1184: 354-364.
- Li S, Geiger JD, Lei S (2008) Neurotensin enhances GABAergic activity in rat hippocampus CA1 region by modulating L-type calcium channels. J Neurophysiol 99: 2134-2143.
- Yin HH, Adermark L, Lovinger DM (2008) Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling. Neuropharmacology 54: 79-86.
- Fredrickson P, Boules M, Yerbury S, Richelson E (2003) Blockade of nicotine-induced locomotor sensitization by a novel neurotensin analog in rats. Eur J Pharmacol 458: 111-118.
- Fredrickson P, Boules M, Yerbury S, Richelson E (2003) Novel neurotensin analog blocks the initiation and expression of nicotine-induced locomotor sensitization. Brain Res 979: 245-248.
- Boules M, Oliveros A, Liang Y, Williams K, Shaw A, et al. (2011) A neurotensin analog, NT69L, attenuates intravenous nicotine self-administration in rats. Neuropeptides 45: 9-16.
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, et al. (1999) Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience 93: 227-236.
- Erwin VG, Campbell AD, Myers R, Womer DE (1995) Cross-tolerance between ethanol and neurotensin in mice selectively bred for ethanol sensitivity. Pharmacol Biochem Behav 51: 891-899.
- Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, et al. (2010) Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol Biochem Behav 95: 235-241.
- Liang Y, Boules M, Shaw AM, Williams K, Fredrickson P, et al. (2008) Effect of a novel neurotensin analog, NT69L, on nicotine-induced alterations in monoamine levels in rat brain. Brain Res 1231: 6-15.
- Li Z, Boules M, Richelson E (2011) NT69L blocks ethanol-induced increase of dopamine and glutamate levels in striatum of mouse. Neurosci Lett 487: 322-324.
- Dahchour A, De Witte P (2000) Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol 60: 343-362.
- Li Z, Boules M, Williams K, Peris J, Richelson E (2010) The novel neurotensin analog NT69L blocks phencyclidine (PCP)-induced increases in locomotor activity and PCP-induced increases in monoamine and amino acids levels in the medial prefrontal cortex. Brain Res 1311: 28-36.
- Gilpin NW, Richardson HN, Cole M, Koob GF (2008) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9: Unit 9.
- Gilpin NW, Richardson HN, Lumeng L, Koob GF (2008) Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res 32: 1688-1696.
- Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99: 473-478.
- Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107: 285-289.
- Fredrickson P, Boules M, Riley J, Baxter L, Richelson E Effect of extracranially administered neurotensin analog on wakefulness. Sleep 28: A4-A5.
- Skjei KL, Markou A (2003) Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 168: 280-292.
- Uzbay IT, Kayaalp SO (1995) A modified liquid diet of chronic ethanol administration: validation by ethanol withdrawal syndrome in rats. Pharmacol Res 31: 37-42.
- Gatch MB, Lal H (1999) Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res 23: 328-333.
- Tizabi Y, Bai L, Copeland RL Jr, Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol 42: 413-416.
- Corrigall WA (1999) Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1: 11-20.
- Fauq A H, Hong F, Cusack B, Tyler B M, Pang Y P, et al. (1998) Synthesis of (2S)-2-amino-3-(1H-4-indolyl) propanoic acid, a novel trryptophan analog for structural modification of bioactive peptides. Tetrahedron: Asymmetry 9: 4127-4134.
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, et al. (2009) Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res 33: 2113-2123.
- Lajtha A, Sershen H (2010) Nicotine: alcohol reward interactions. Neurochem Res 35: 1248-1258.
- Lê AD, Lo S, Harding S, Juzytsch W, Marinelli PW, et al. (2010) Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 208: 475-486.
- Hays JT, Schroeder DR, Offord KP, Croghan IT, Patten CA, et al. (1999) Response to nicotine dependence treatment in smokers with current and past alcohol problems. Ann Behav Med 21: 244-250.
- Kozlowski LT, Skinner W, Kent C, Pope MA (1989) Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addict Behav 14: 273-278.
- Stuyt EB (1997) Recovery rates after treatment for alcohol/drug dependence. Tobacco users vs. non-tobacco users. Am J Addict 6: 159-167.
- O'Brien CP, Volpicelli LA, Volpicelli JR (1996) Naltrexone in the treatment of alcoholism: a clinical review. Alcohol 13: 35-39.
- Wong GY, Wolter TD, Croghan GA, Croghan IT, Offord KP, et al. (1999) A randomized trial of naltrexone for smoking cessation. Addiction 94: 1227-1237.
- Sinha R, Krishnan-Sarin S, Farren C, O'Malley S (1999) Naturalistic follow-up of drinking behavior following participation in an alcohol administration study. J Subst Abuse Treat 17: 159-162.
- Lê AD, Li Z, Funk D, Shram M, Li TK, et al. (2006) Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26: 1872-1879.
- Dani JA, Harris RA (2005) Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 8: 1465-1470.
- Scherrer JF, Waterman BM, Heath AC, Bucholz KK, True WR, et al. (2004) Are substance use, abuse and dependence associated with study participation? Predictors of offspring nonparticipation in a twin-family study. J Stud Alcohol 65: 140-144.
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, et al. (1999) Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry 56: 655-661.
- Volk HE, Scherrer JF, Bucholz KK, Todorov A, Heath AC, et al. (2007) Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug Alcohol Depend 87: 225-232.
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW (1991) Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48: 52-59.
- Shiffman SM, Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 50: 35-39.
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, et al. (1990) Smoking, smoking cessation, and major depression. JAMA 264: 1546-1549.
- Parrott AC (1993) Cigarette smoking: effects upon self-rated stress and arousal over the day. Addict Behav 18: 389-395.
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, et al. (1994) The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology (Berl) 115: 180-184.
- Hildebrand BE, Nomikos GG, Bondjers C, Nisell M, Svensson TH (1997) Behavioral manifestations of the nicotine abstinence syndrome in the rat: peripheral versus central mechanisms. Psychopharmacology (Berl) 129: 348-356.
- Watkins SS, Stinus L, Koob GF, Markou A (2000) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther 292: 1053-1064.
- Kenny PJ, Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531-549.
- Gulick D, Gould TJ (2008) Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 196: 483-495.
- Prendergast MA, Harris BR, Mayer S, Littleton JM (2000) Chronic, but not acute, nicotine exposure attenuates ethanol withdrawal-induced hippocampal damage in vitro. Alcohol Clin Exp Res 24: 1583-1592.
- Prendergast MA, Harris BR, Mayer S, Holley RC, Hauser KF, et al. (2001) Chronic nicotine exposure reduces N-methyl-D-aspartate receptor-mediated damage in the hippocampus without altering calcium accumulation or extrusion: evidence of calbindin-D28K overexpression. Neuroscience 102: 75-85.
- Prendergast MA, Harris BR, Mayer S, Holley RC, Pauly JR, et al. (2001) Nicotine exposure reduces N-methyl-D-aspartate toxicity in the hippocampus: relation to distribution of the alpha7 nicotinic acetylcholine receptor subunit. Med Sci Monit 7: 1153-1160.
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, et al. (2011) Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res 35: 747-755.
- Anton RF (1999) What is craving? Models and implications for treatment. Alcohol Res Health 23: 165-173.
- Spanagel R (2003) Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol 17: 507-518.
- Chester JA, Price CS, Froehlich JC (2002) Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcohol Clin Exp Res 26: 19-27.
- Cooper BR, Viik K, Ferris RM, White HL (1979) Antagonism of the enhanced susceptibility to audiogenic seizures during alcohol withdrawal in the rat by gamma-aminobutyric acid (GABA) and "GABA-mimetic" agents. J Pharmacol Exp Ther 209: 396-403.
- Freund G (1975) Induction of physical dependence on alcohol in rodents. Adv Exp Med Biol 56: 311-325.
- Majchrowicz E (1975) Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43: 245-254.
- Walker B, Anderson M, Hauser L, Werchan I (2013) Ethanol for alcohol withdrawal: the end of an era. J Trauma Acute Care Surg 74: 926-931.
- Frye GD, Luttinger D, Nemeroff CB, Vogel RA, Prange AJ Jr, et al. (1981) Modification of the actions of ethanol by centrally active peptides. Peptides 2 Suppl 1: 99-106.
- Larsson A, Engel JA (2004) Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev 27: 713-720.
- Tizabi Y, Copeland RL Jr, Louis VA, Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res 26: 394-399.
- Imperato A, Mulas A, Di Chiara G (1986) Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol 132: 337-338.
- Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105: 849-856.
- Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508: 65-69.
- Campbell AD, Kohl RR, McBride WJ (1996) Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol 13: 569-574.
- Kohl RR, Katner JS, Chernet E, McBride WJ (1998) Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology (Berl) 139: 79-85.
- Little HJ (2000) Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Res Health 24: 215-224.
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, et al. (2013) A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence. J Addict Med 7: 277-286.
- Ericson M, Blomqvist O, Engel JA, Söderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358: 189-196.
- Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278-284.
- Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, et al. (2010) Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology (Berl) 211: 161-174.
- Söderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA (2000) Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res 113: 85-96.
- Rakovska A, Giovannini MG, Della Corte L, Kalfin R, Bianchi L, et al. (1998) Neurotensin modulation of acetylcholine and GABA release from the rat hippocampus: an in vivo microdialysis study. Neurochem Int 33: 335-340.
- Iyaniwura TT, Wright AE, Balfour DJ (2001) Evidence that mesoaccumbens dopamine and locomotor responses to nicotine in the rat are influenced by pretreatment dose and strain. Psychopharmacology (Berl) 158: 73-79.
- Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000) The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res 113: 73-83.
- Li XM, Ferraro L, Tanganelli S, O'Connor WT, Hasselrot U, et al. (1995) Neurotensin peptides antagonistically regulate postsynaptic dopamine D2 receptors in rat nucleus accumbens: a receptor binding and microdialysis study. J Neural Transm Gen Sect 102: 125-137.
- Shi WX, Bunney BS (1992) Roles of intracellular cAMP and protein kinase A in the actions of dopamine and neurotensin on midbrain dopamine neurons. J Neurosci 12: 2433-2438.
- Fuxe K, Von Euler G, Agnati LF, Merlo Pich E, O'Connor WT, et al. (1992) Intramembrane interactions between neurotensin receptors and dopamine D2 receptors as a major mechanism for the neuroleptic-like action of neurotensin. Ann N Y Acad Sci 668: 186-204.
- Ericson M, Löf E, Stomberg R, Söderpalm B (2009) The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther 329: 225-230.
- Guo JZ, Tredway TL, Chiappinelli VA (1998) Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J Neurosci 18: 1963-1969.
- Adell A, Artigas F (2004) The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev 28: 415-431.
- Sziráki I, Sershen H, Benuck M, Hashim A, Lajtha A (1998) Receptor systems participating in nicotine-specific effects. Neurochem Int 33: 445-457.
- Lallemand F, Ward RJ, Dravolina O, De Witte P (2006) Nicotine-induced changes of glutamate and arginine in naive and chronically alcoholized rats: an in vivo microdialysis study. Brain Res 1111: 48-60.
- Nicot A, Rostène W, Bérod A (1995) Differential expression of neurotensin receptor mRNA in the dopaminergic cell groups of the rat diencephalon and mesencephalon. J Neurosci Res 40: 667-674.
- Palacios JM, Kuhar MJ (1981) Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294: 587-589.
- Zahm DS (1987) Neurotensin-immunoreactive neurons in the ventral striatum of the adult rat: ventromedial caudate-putamen, nucleus accumbens and olfactory tubercle. Neurosci Lett 81: 41-47.
- Petrie KA, Schmidt D, Bubser M, Fadel J, Carraway RE, et al. (2005) Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci 25: 1629-1636.
- Tanganelli S, O'Connor WT, Ferraro L, Bianchi C, Beani L, et al. (1994) Facilitation of GABA release by neurotensin is associated with a reduction of dopamine release in rat nucleus accumbens. Neuroscience 60: 649-657.
- Chau P, Stomberg R, Fagerberg A, Söderpalm B, Ericson M (2010) Glycine receptors involved in acamprosate's modulation of accumbal dopamine levels: an in vivo microdialysis study. Alcohol Clin Exp Res 34: 32-38.
- Lidö HH, Marston H, Ericson M, Söderpalm B (2012) The glycine reuptake inhibitor Org24598 and acamprosate reduce ethanol intake in the rat; tolerance development to acamprosate but not to Org24598. Addict Biol 17: 897-907.
- Lidö HH, Stomberg R, Fagerberg A, Ericson M, Söderpalm B (2009) The glycine reuptake inhibitor org 25935 interacts with basal and ethanol-induced dopamine release in rat nucleus accumbens. Alcohol Clin Exp Res 33: 1151-1157.
- Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol 42: 11-18.
- Chau P, Höifödt-Lidö H, Löf E, Söderpalm B, Ericson M (2010) Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res 34: 39-45.
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R (2010) Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry 68: 704-711.
- Aliyev NA, Aliyev ZN (2005) Application of glycine in acute alcohol hallucinosis. Hum Psychopharmacol 20: 591-594.
- Uslaner JM, Drott JT, Sharik SS, Theberge CR, Sur C, et al. (2010) Inhibition of glycine transporter 1 attenuates nicotine- but not food-induced cue-potentiated reinstatement for a response previously paired with sucrose. Behav Brain Res 207: 37-43.
- Fedele E, Varnier G, Ansaldo M A, and Raiteri M (1998) Nicotine administration stimulates the in vivo N-methyl-D-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol 125: 1042-1048.
- Toth E (1996) Effect of nicotine on the level of extracellular amino acids in the hippocampus of rat. Neurochem Res 21: 903-907.
- Toth E, Vizi ES, Lajtha A (1993) Effect of nicotine on levels of extracellular amino acids in regions of the rat brain in vivo. Neuropharmacology 32: 827-832.
- Boules M, Cusack B, Zhao L, Fauq A, McCormick DJ, et al. (2000) A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res 865: 35-44.
- Feifel D, Goldenberg J, Melendez G, Shilling PD (2010) The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 58: 195-198.
- Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates. (3 edn), San Francisco, Academic Press, Inc.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15726
- [From(publication date):
September-2013 - Dec 01, 2025] - Breakdown by view type
- HTML page views : 10976
- PDF downloads : 4750