41-Year Systematic Literature Review of Early Infancy Osteomyelitis: What Have We Learned with the Entry of MRI into Diagnostic Protocol?
Received: 04-Jan-2019 / Accepted Date: 31-Jan-2019 / Published Date: 08-Feb-2019 DOI: 10.4172/2572-4983.1000177
Abstract
Background: A 41-year systematic literature review of early infancy osteomyelitis was performed. Observations were made regarding variables related to the diagnosis and treatment of the disease. We asked: 1) since magnetic resonance imaging (MRI) technology was introduced into diagnostic protocol in 1998, has there been an improvement in diagnosis and outcomes? 2) What additional aspects of the disease and diagnostic protocol may be affecting outcomes?
Methods: We performed a literature search and divided 36 cases into 2 groups; 1) all young infancy osteomyelitis cases before first reported MRI usage in 1998, 2) all young infancy osteomyelitis cases after first reported MRI usage in 1998. 34 cases from the literature and 2 of our cases were reviewed.
Results: Analysis of key variables related to diagnosis and treatment did not indicate statistically significant differences between the 2 groups. The following symptoms were common among both groups: pseudoparalysis in 28 (77.8%) and swelling in 22 (61.1%) patients.
Conclusion: Despite the benefits of MRI there have been few outcome changes with respect to the diagnosis and treatment of early infancy osteomyelitis. Early diagnosis is crucial; therefore, appropriate modifications to current diagnostic protocol may be warranted. Improvement may be possible if clinicians adopt a high index of suspicion for osteomyelitis and a low threshold of obtaining MRI.
Keywords: Early infancy osteomyelitis; MRI; Diagnostic protocol
Introduction
Osteomyelitis can present unique diagnostic and management challenges in neonates and young infants. Neonates are defined as less than 28 days-of-life (DOL). Infants up to 2 months of age were included. We describe the use of magnetic resonance imaging (MRI) to aid in the timely diagnosis of two of our own patients. Labs and vitals for these cases are presented (Table 1).
| Case 1 | Case 2* | |
|---|---|---|
| Laboratory Test Results | ||
| C-reactive protein (CRP) (reference range, 0.0-9.0) (mg/L) | Not available | 4.1 |
| Erythrocyte sedimentation rate (ESR) (reference range, 0-20) (mm/hr) | Not available | Not available |
| White blood-cell count (WBC) (reference range, 4.50-11.00) (x103 per nL) | 17.02 | 12.51 |
| Vital Signs | ||
| Blood pressure (mm Hg) | 90/60 | 77/33 |
| Heart rate (beats/min) | 116 | 132-172 |
| Respiratory rate (breaths/min) | 26 | 48-62 |
| Temperature (°C) | 37 (rectal) | 36.2-36.9 (axillary) |
| Oxygen saturation, on room air | 98% | 97-100% |
*Case 2 was admitted since birth in NICU - labs and vitals above are from DOL 54 when lower extremity edema was first noted
Table 1: Laboratory results for our young infancy osteomyelitis patients.
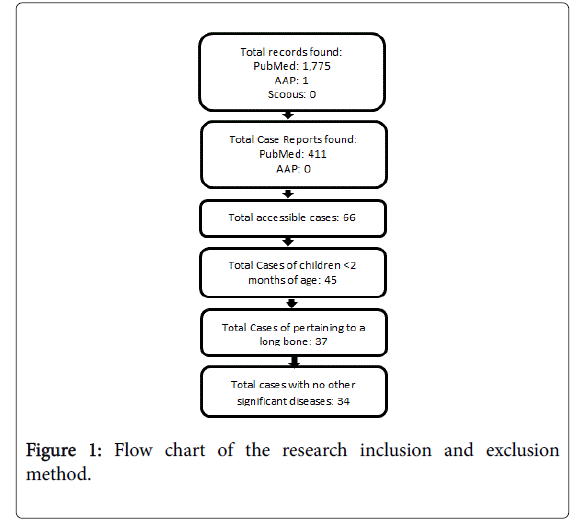
We also review case reports published over the past 41 years (Figure 1) to observe trends in symptoms, diagnostic methods, etiologic pathogens, and radiography to identify osteomyelitis (Tables 2 and 3). We asked: 1) since magnetic resonance imaging (MRI) technology was introduced into diagnostic protocol in 1998, has there been an improvement in diagnosis and outcomes? 2) What additional aspects of the disease and diagnostic protocol may be affecting outcomes?
| Case | Admittance age/Gender | Days symptomatic before admission | Pseudoparalysis/limited mobility | Swelling | Radiographic Findings | Antibiotic treatment start time | Initial diagnosis | Final diagnosis | Sequelae |
|---|---|---|---|---|---|---|---|---|---|
| Henderson, et al. (Case 1) [1] | 31 d/M | 14 days | Left knee | Left knee | Left femur: lytic proximal femoral metaphysis and periosteal reaction distal left femur | Post-diagnosis of osteomyelitis | Suspected infection | Beta-hemolytic streptococcus Group B osteomyeltis | 3 month follow-up, none reported |
| McCook, et al. (Case 1) [2] | 28 d/F | 2 days | Left arm | Left shoulder | N/A | Post-diagnosis of osteomyelitis | Osteomyelitis | Group B streptococcus osteomyelitis | 6 week follow-up, none reported |
| McCook, et al. (Case 2) [2] | 42 d/F | 4 days | Right arm | Right shoulder | N/A | Pre-diagnosis of osteomyelitis | Suspected sepsis | Group B streptococcus osteomyelitis | 3 month follow-up, none reported |
| McCook, et al. (Case 3) [2] | 49 d/F | 27 days | Left arm | Left shoulder | N/A | Post-diagnosis of osteomyelitis | Suspected infection | Group B hemolytic streptococcus osteomyelitis | Not reported |
| Svirsky-Fein, et al. (Case 1) [3] | 19 d/M | Already in NICU | Right arm | Right shoulder, later bilateral knees | Right shoulder: lateral dislocation with lytic lesions of the proximal humeral metaphysis | Pre-diagnosis of osteomyelitis | Sepsis | Candida tropicalis osteomyelitis | 1 month follow-up, none reported |
| Svirsky-Fein, et al. (Case 2) [3] | 14 d/M | Already in NICU | None | Right knee | Bilateral shoulder: left-widening of shoulder, lateral dislocation and lysis of humeral head also with lysis of right humeral head. Right knee: rarefaction at joint and metaphysis | Post-diagnosis of osteomyelitis | Sepsis | Candida tropicalis osteomyelitis | 5 month follow-up, none reported |
| Broughton, et al. (Case 1) [4] | 6 d/F | 6 days | Right leg | Right knee | Right femur: lytic lesion distal metaphysis and demineralization adjacent epiphysis | Pre-diagnosis of osteomyelitis | Suspected infection | Type III Group B streptococcus osteomyelitis | 17 months of age follow-up, none reported |
| Broughton, et al. (Case 2) [4] | 10 d/M | 8 days | Right arm and left leg | None | Left femur: metaphyseal lytic defects proximal femur with laterally displaced femoral head. Right shoulder: proximal humerus and left clavicle lytic lesions | Post-diagnosis of osteomyelitis | Suspected infection | Group B streptococcus osteomyelitis | 9 month follow-up, none reported |
| Broughton, et al. (Case 3) [4] | 28 d/F | 14 days | Bilateral arms | None | Bilateral shoulders: lytic defects proximal humeral metaphyses crossing into the epiphysis | Pre-diagnosis of osteomyelitis | Suspected infection | Osteomyelitis, suspected Type III Group B streptococcus. | Not reported |
| Isaacs, et al. (Case 1) [5] | 10 d/M | Unknown | Left arm | Developed in left shoulder, right foot, left groin, and left thigh | Left shoulder: lytic lesion humeral metaphysis, later showed chronic osteomyelitis left humeral metaphysis and periosteal reaction left femur | Pre-diagnosis of osteomyelitis | Erb's palsy | Staphlococcus aureus and coliforms osteomyelitis | Follow-up, none reported |
| Isaacs, et al. (Case 2) [5] | 14 d/F | Unknown | Left arm | Developed in left arm | Left humerus: lytic lesion humerus with periosteal reaction | Post-diagnosis of osteomyelitis | Erb's palsy | Group B streptococcus osteomyelitis | 2 month follow-up, none reported |
| Isaacs, et al. (Case 3) [5] | 17 d/M | Already in NICU | None | Left thigh, right shoulder and wrist, and left middle finger | N/A | Time N/A | Suspected infection | Staphlococcus aureus osteomyelitis | 9 month follow-up, head and neck of left femur were totally destroyed, left thigh was shorted by 1 cm, and limited dorsiflexion of right wrist and flexion contracture of left proximal interphalangeal joint |
| Orebaugh, et al. (Case 1) [6] | 28 d/M | 14 days | bilateral legs | None | Lower extremities: bilateral distal femoral periosteal reaction and osteolysis. | Post-diagnosis of osteomyelitis | Osteomyelitis and secondary pyarthrosis | Staphylococcus aureus osteomyelitis | 2 week follow-up, none reported |
| Obando, et al. (Case 1) [7] | 3 d/F | Already in NICU | Left leg and ankle | None | N/A | Pre-diagnosis of osteomyelitis | Acute neuropathy | Group B streptococcus osteomyelitis | 5 month follow-up, none reported |
| Oleinik, et al. (Case 1) [8] | 20 d/M | Already in NICU | None | Left elbow | Left humerus: lucency distal humerus, erosion of olceranon, periosteal reaction | Pre-diagnosis of osteomyelitis | Suspected infection | Candida lusitaniae and amphotericin ostoemyelitis | 6 month follow-up, none reported |
Table 2: All young infancy osteomyelitis cases before first reported MRI usage in 1998.
| Case | Admittance age/Gender | Days symptomatic before admission | Pseudoparalysis/limited mobility | Swelling | Radiographic or MRI Findings | Antibiotic treatment start time | Initial diagnosis | Final diagnosis | Sequelae |
|---|---|---|---|---|---|---|---|---|---|
| Sadlier and Connolly (Case 1) [9] | 3 d/M | Already in NICU | Left arm | Later in left shoulder | Left humerus xray: lytic lesion humerus | Post-diagnosis of osteomyelitis | Traumatic brachial-plexus injury | Group B streptococcus osteomyelitis | 3 month follow-up, none reported |
| Sadlier and Connolly (Case 2) [9] | 15 d/M | 2 days | Left arm | None | MRI normal | Post-diagnosis of osteomyelitis | Traumatic brachial-plexus injury | Group B streptococcus osteomyelitis | Follow-up, neurologically normal |
| Sadlier and Connolly (Case 3) [9] | 21 d/F | 18 days | Left arm | Later in left shoulder | Left shoulder xray: demineralization humeral head | Post-diagnosis of osteomyelitis | Traumatic brachial-plexus injury | Group B streptococcus osteomyelitis | 5 month follow-up, none reported |
| Wathne, et al. (Case 1) [10] | 5 d/M | Unknown | None | Left toe, left ankle, and chin | MRI normal | Pre-diagnosis of osteomyelitis | Sepsis | Beta-hemolytic streptococcus group A osteomyelitis | 4 month follow-up, none reported |
| Wathne, et al. (Case 2) [10] | 12 d/M | Unknown | Right leg | None | Right leg MRI: marrow edema distal femur | Post-diagnosis of osteomyelitis | Suspected infection | Osteomyelitis, culture negative | 1 month follow-up, none reported |
| Wathne, et al. (Case 3) [10] | 21 d/F | Unknown | Right leg | None | Right hip MRI: effusion hip with some light signal changes in caput femoris | Post-diagnosis of osteomyelitis | Arthritis/osteomyelitis | Osteomyelitis, culture negative | 1 month follow-up, none reported |
| Solebo, et al. (Case 1) [11] | 14 d/M | 7 days | Right arm | None | Right shoulder xray: lytic area humeral head and radioisotope ‘hot spot’ | Post-diagnosis of osteomyelitis | Erb's palsy | Osteomyelitis, causitive pathogen not reported | 4 month follow-up, none reported |
| Estienne, et al. (Case 1) [12] | 13 d/M | 8 days | Right and eventually left arm | None | Right shoulder xray: edema humeral head | Post-diagnosis of osteomyelitis | Suspected infection | Osteomyelitis, culture negative | 6 month follow-up, X-rays revealed mild avascular necrosis |
| Liao, et al. (Case 1) [13] | 31 d/F | 24 days | Right shoulder and arm | Right shoulder | Right humerus xray: large osteolytic lesion proximal humeral metaphysis, MRI consistant | Post-diagnosis of osteomyelitis | Erb's palsy | Klebsiella pneumoniae osteomyelitis and septic synovitis | 2 month follow-up, almost full recovery |
| Korakaki, et al. (Case 1) [14] | 15 d/F | Unknown | Left knee | Left leg | Left leg MRI: consistent with acute osteomyelitis left tibia septic arthritis knee joint | Pre-diagnosis of osteomyelitis | Suspected infection | MRSA osteomyelitis and septic arthritis | 1 year follow-up, none reported |
| Korakaki, et al. (Case 2) [14] | 28 d/M | 5 days | None | Left lateral chest wall and left knee | Rib xray: lytic lesions 10th rib and distal metaphysis left femur with new bone formation. Ultrasound revealed edema of the soft tissue | Post-diagnosis of osteomyelitis | Acute osteomyelitis and septit arthritis | MRSA osteomyelitis and septic arthritis | 2 year follow-up, limp, 2 cm discrepancy left lower limb, flexion deformity of the left knee joint and ankylosis |
| Waseem, et al. (Case 1) [15] | 21 d/F | Unknown | Right arm | None | Right shoulder MRI: shoulder joint effusion | Post-diagnosis of osteomyelitis | Erb's palsy | Osteomyelitis, culture negative | At follow-up, none reported |
| Qadir, et al. (Case 1) [16] | 35 d/M | 14 days | Right arm | Right shoulder joint | Right shoulder xray: periosteal reaction proximal humerus and irregularity proximal humeral metaphysis | Post-diagnosis of osteomyelitis | Suspected infection | Klebsiella pneumoniae osteomyelitis | 7 month follow-up,clinically normal; right humeral head smaller than left |
| Winkler, et al. (Case 1) [17] | 18 d/F | Unknown | Right arm | None | MRI: edema marrow clavicle with periosteal abscess and fluid extension into the sternoclavicular joint | Pre-diagnosis of osteomyelitis | Suspected infection | MSSA osteomyelitis | 2 year follow-up, none reported |
| Allagui, et al. (Case 1) [18] | 28 d/M | 10 days | Left shoulder | Near clavicle | Left shoulder ultrasound: left supraclavicular collection corresponding to a subperiosteal abscess | Post-diagnosis of osteomyelitis | Suspected infection | Haemophilus influenzae osteomyelitis | None reported |
| Berkowitz, et al. (Case 1) [19] | 28 d/F | 7 days | Left leg | None | Left leg MRI: edema marrow distal tibial osteomyelitis and effusion ankle joint | Post-diagnosis of osteomyelitis | Suspected infection | Group B streptococcus osteomyelitis | Not reported |
| Dessie and Constantine (Case 1) [20] | 28 d/F | 1 day | None | Right thigh | Right leg MRI: 1-cm intraosseous fluid collection in the right distal femoral metaphysis, extending into epiphysis and knee joint | Post-diagnosis of osteomyelitis | Suspected infection | Group B streptococcus osteomyelitis | None reported at discharge |
| Ben-Meir, et al. (Case 1) [21] | 35 d/F | 7 days | Bilateral arms | None | MRI: extensive edema involving C5, adjacent intervertebral discs, epidural space from C6 to the clivus, paravertebral space at C1–C4, neck and pharyngeal soft tissue | Pre-diagnosis of osteomyelitis | Suspected infection | MSSA osteomyelitis | 4 month follow-up, mild flattening of C5 vertebra with complete resolution of inflammation |
| Zhan, et al. (Case 1) [22] | 28 d/M | Unknown | None | Right leg, worse over time | Right leg MRI: significant soft tissue edema and destruction of the right distal tibia and periosteal reaction of the right distal tibia and fibula | Pre-diagnosis of osteomyelitis | Suspected sepsis | Salmonella typhi osteomyelitis | 3 month follow-up, none reported |
| Present (Case 1) | 28 d/M | 7 days | Left arm | None | Left arm MRI: fluid in elbow joint, edema of distal humerus, proximal radius, olecranon, lysis of the distal humerus | Pre-diagnosis of osteomyelitis | Arm sprain | MRSA osteomyelitis and septic elbow | 3 year follow-up, elbow lacked 15 degree flexion and continued defect from initial lytic lesion of the humerus |
| Present (Case 2) | 54 d/M | Already in NICU | None | Left leg | Left leg MRI: abscess of the left tibia and fibula, fluid crossing into the epiphysis | Pre-diagnosis of osteomyelitis | Osteomyelitis | Osteomyelitis, suspected Group B streptococcus | 5 year follow-up, none reported |
Table 3: All young infancy osteomyelitis cases after first reported MRI usage in 1998.
Materials and Methods
Literature search was performed using PubMed, AAP, and Scopus. Of the total records found, 1,775 were from PubMed, 1 from AAP, 0 from Scopus. Of the total case reports found 411 were from PubMed and 0 from AAP. 66 total cases were accessible. 45 of these cases included children <2 months of age. Of the 45 cases, 37 pertained to a long bone. 34 of these cases contained no other significant disease (Figure 1). In the end, 34 cases from the literature and our two cases were reviewed. These cases were divided into two groups; 1) all young infancy osteomyelitis cases before first reported MRI usage in 1998 (Table 2).
2) All young infancy osteomyelitis cases after first reported MRI usage in 1998 (Table 3). Groups consisted of 15 and 21 patients, respectively.
Case 1
4-week-old male with 5-day neonatal intensive care unit (NICU) stay for respiratory distress and polydactyly with supernumerary finger stub removal at birth presented with progressive upper extremity pseudoparalysis. After 7 symptomatic days, he was admitted to an outside hospital emergency department (ED). The x-ray and labs were normal, and he was sent home with an “arm sprain”, despite pseudoparalysis.
After 11 symptomatic days, the elbow and arm became edematous and the infant became irritable. Radiographs repeated by the pediatrician showed bony destruction within the left distal humeral metaphysis demonstrating osteomyelitis. Initial vitals and labs are in Table 1.
MRI with gadolinium was consistent with septic elbow and osteomyelitis of the distal humerus, proximal radius, and olecranon. The patient was taken to the operating room (OR) where he underwent elbow irrigation and debridement (I&D) with packing. The humeral supracondylar area contained yellow and lobulated pus.
He was treated with cefotaxime and vancomycin, postoperative vitals are shown in Table 1. I&D was repeated 3 days later with wound closure. Methicillin-resistant Staphylococcus aureus (MRSA) sensitive to clindamycin was identified. Blood cultures were negative. He was discharged on POD 4 and treated with 6-weeks intravenous clindamycin. At 3-year follow-up, the elbow lacked 15 degrees flexion. X-rays showed bone defect filling in.
Case 2
Male at 27 3/7 week twin gestation was admitted to the NICU for late-onset Group B streptococcus sepsis at DOL 17. He was treated with a 21-day course of ampicillin and intubated for poor oxygenation. Leg swelling was observed on DOL 54. Labs and vitals are presented (Table 1). Initial radiograph was normal. Follow-up radiograph on DOL 58 showed metaphyseal lucency with periosteal reaction suggestive of proximal tibial osteomyelitis. He was started on cefotaxime and vancomycin.
Orthopedic surgery was consulted. MRI demonstrated abscess of the tibia and fibula, fluid crossing into the epiphysis. An abscess containing clear fluid with fibrinous material was debrided around the proximal tibia and distal femur. Drill holes were made into the tibia epiphysis and metaphysis. OR cultures were negative. He was given a 6-week course of ampicillin for presumed Group B streptococcus and discharged on DOL 95. At 5-year follow-up, plain radiographs show the growth plate functioning with no central irregularity.
Results
Between the two groups, most common symptoms were pseudoparalysis, in 28 (77.8%), and swelling, in 22 (61.1%) patients. 14 (38.9%) patients experienced both symptoms; all experienced one. 11 (30.6%) were febrile. Patients were between 3 and 54 DOL (average 22 DOL).
In Group 1 (cases before first reported MRI usage in 1998; Table 2), 8 (53.3%) patients are male and 7 (46.7%) are female. In group 2 (cases after first reported MRI usage in 1998; Table 3), 12 (57.1%) patients are male, and 9 (42.9%) are female.
A comparison and analysis of key diagnostic variables between the 2 groups are presented (Table 4). The results indicated no statistical significance.
| Group 1 (All young infancy osteomyelitis cases before first reported MRI usage in 1998) (n=15) | Group 2 (All young infancy osteomyelitis cases after first reported MRI usage in 1998) (n=21) | p-value | |
|---|---|---|---|
| Average time between initial symptoms and admission | 11 days (8 patients) | 9.2 days (12 patients) | 0.6407 Mann Whitney |
| Required surgery | 6 patients (40%) | 10 patients (47.6 %) | 0.741 |
| Initially misdiagnosed | 6 patients (40%) | 9 patients (42.9%) | >0.999 |
| Misdiagnosed with Erb’s palsy or neuropathy | 3 patients (20%) | 3 patients (14.3%) | 0.677 |
| Began antibiotic treatment before diagnosis | 7 patients (50%) | 7 patients (33%) | 0.499 |
| Causative pathogen is streptococcus B | 8 patients (53%) | 5 patients (23.8%) | 0.090 |
| Causative pathogen is Staphylococcus aureus | 3 patients (20%) | 5 patients (23.8%) | >0.999 |
| Negative cultures | 1 patient (6.7%) | 5 patients (23.8%) | 0.367 |
| Reported sequelae | 1 patient (6.7%) | 4 patients (19%) | 0.376 |
| Average time from admission to correct diagnosis | Not available | 10.3 days (10 patients) |
Table 4: Key diagnostic variables.
Discussion
We asked: 1) since magnetic resonance imaging (MRI) technology was introduced into diagnostic protocol in 1998, has there been an improvement in diagnosis and outcomes? 2) what additional aspects of the disease and diagnostic protocol may be affecting outcomes?
Over the past 41 years, despite the introduction of MRI into diagnostic protocol in 1998, diagnosis and treatment of early infancy osteomyelitis have not significantly changed. There is a low incidence of early-infancy osteomyelitis. Signs and symptoms are difficult to discern [23-25]. Prompt diagnosis and treatment are crucial [26-28].
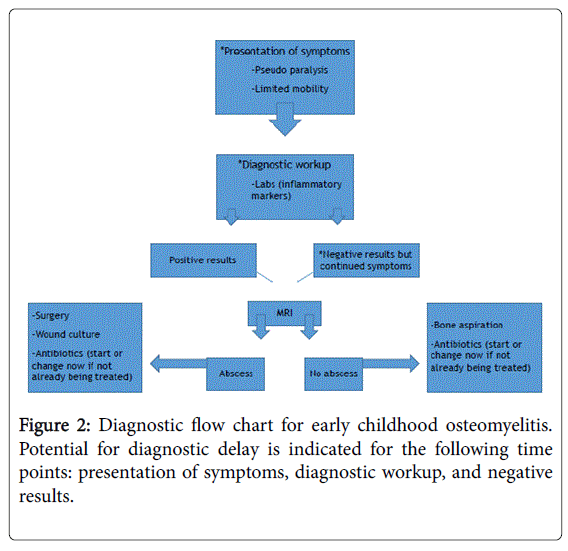
Earlier diagnosis mitigates serious sequelae, such as cartilage destruction, bone and growth plate deformity, joint dislocation, limited joint motion, and neurologic abnormalities [29-31]. A flow chart (Figure 2) highlights common time points for diagnostic delay.
Duration of symptoms before admission remains about the same, which may be unavoidable since parents are responsible for presenting during this subtle phase. Of the reported cases, patients were admitted after an average of 10 symptomatic days and diagnosed after 10 additional days (Tables 2 and 3). Fever occurs in one third or fewer cases [23,29]. Pseudoparalysis or extremity swelling are present in 60-95% of infections and should alert the clinician to possible osteoarticular infection [23-25]. Pseudoparalysis present at birth is indicative of a neurological problem or Erb’s palsy; however, pseudoparalysis which develops after birth indicates infection. There was a decrease in patients misdiagnosed with neuropathy after the use of MRIs, although about the same number of patients was misdiagnosed in general.
Radiologic abnormalities may be absent, subtle, or only positive in chronic infections when lytic lesions, sequestrum, or involucrum are present [23]. MRI is useful in defining bone edema, soft tissue involvement, delineating bone and soft tissue abscesses, and showing co-existing joint pathology [32,33]. MRI is the most sensitive radiological investigation for diagnosing osteomyelitis and can detect osteomyelitis 1 to 3 days post-infection [24,32].
Radiographs cannot pick up signs of osteomyelitis until after 2 weeks when the infection is chronic [32,34,35]. To prevent delay, MRI should be ordered immediately once patients develop pseudoparalysis, regardless of “normal” radiographs. MRI should not delay diagnosis, but rather hasten it and improve certainty of the underlying disorder.
Neonates are often treated empirically with broad spectrum antibiotics. Ideally, pathogen identification should be sought to optimize treatment with antibiotics. Continued advancements in PCRbased testing will enable pathogen identification to occur with higher degrees of specificity, sensitivity, and rapidness [36]. More aggressive pathogen analysis and treatment is warranted and will be especially helpful for treating neonates.
Despite the evident value of using MRI technology, the natural history of early infancy osteomyelitis may be impossible to avoid. However, an in-depth understanding of the disease and common time points of diagnostic delay may give insight as to why outcomes are not improving as one might expect. Optimizing diagnostic protocol may be warranted if it will lead to clinicians lowering the threshold for attaining MRI and utilizing the technology earlier in their patient assessments. Emphasis on early diagnosis appears to be crucial for improving outcomes.
Statement of Informed Consent
Both patients’ parents were informed that the case data would be submitted for publication, to which they agreed.
Disclosures
The authors have no disclosures or conflicts of interest.
References
- Henderson KC, Roberts RS, Dorsey SB (1977) Group b beta-hemolytic streptococcal osteomyelitis in a neonate. Pediatrics 59: 1053-1054.
- McCook TA, Felman AH, Ayoub E (1978) Streptococcal skeletal infections: observations in four infants. AJR Am J Roentgenol 130: 465-467.
- Svirsky-Fein S, Langer L, Milbauer B, Khermosh O, Rubinstein E (1979) Neonatal osteomyelitis caused by Candida tropicalis: report of two cases and review of the literature. J Bone Joint Surg Am 61: 455-459.
- Broughton RA, Edwards MS, Haffar A, Baker CJ (1982) Unusual manifestations of neonatal group B streptococcal osteomyelitis. Pediatr Infect Dis 1: 410-412.
- Isaacs D, Bower BD, Moxon ER (1986) Neonatal osteomyelitis presenting as nerve palsy. Br Med J (Clin Res Ed) 292: 1071.
- Orebraugh S, Singer JI (1988) Limb disuse in a newborn. Pediatr Emerg Care 4: 256-258.
- Obando I, Martin E, Alvarez-Aldean J, Chileme A, Baca M, et al. (1991) Group B Streptococcus pelvic osteomyelitis presenting as foot drop in a newborn infant. Pediatr Infect Dis J 10: 703-705.
- Oleinik EM, Della-Latta P, Rinaldi MG, Saiman L (1993) Candida lusitaniae osteomyelitis in a premature infant. Am J Perinatol 10: 313-315.
- Sadlier LG, Connolly MB (1998) Acquired brachial-plexus neuropathy in the neonate: a rare presentation of late-onset group-B streptococcal osteomyelitis. Dev Med Child Neurol 40: 496-499.
- Wathne KO, Babovic A, Nordshus T (2001) Acute osteomyelitis in young children--a diagnostic challenge. Tidsskr Nor Laegeforen 121: 1693-1696.
- Solebo JO, Keane MR, Obaro RO, Browne LM (2004) Osteomyelitis of head of humerus presenting as Erbs palsy in a neonate. Eur J Pediatr 163: 262.
- Estienne M, Scaioli V, Zibordi F, Angelini L (2005) Enigmatic osteomyelitis and bilateral upper limb palsy in a neonate. Pediatr Neurol 32: 56-59.
- Liao SL, Lai SH, Lin TY, Chou YH, Hsu JF (2005) Premature rupture of the membranes: a cause for neonatal osteomyelitis? Am J Perinatol 22: 63-66.
- Korakaki E, Aligizakis A, Manoura A, Hatzidaki E, Saitakis E, et al. (2007) Methicillin-resistant Staphylococcus aureus osteomyelitis and septic arthritis in neonates: diagnosis and management. Jpn J Infect Dis 60: 129-131.
- Waseem M, Devas G, Laureta E (2009) A neonate with asymmetric arm movements. Pediatr Emerg Care 25: 98-99.
- Qadir M, Ali SR, Lakhani M, Hashmi P, Amirali A (2010) Klebsiella osteomyelitis of the right humerus involving the right shoulder joint in an infant. J Pak Med Assoc 60: 769-71.
- Winkler S, Dai L, Hauck F, Dinger J, Pessler F (2012) Primary osteomyelitis of the clavicle in the newborn period. Pediatr Infect Dis J 31: 211.
- Allagui M, Bellaaj Z, Zrig M, Abid A, Koubaa M (2014) Acute osteomyelitis of the clavicle in the newborn infant: a case report. Arch Pediatr 21: 211-213.
- Berkowitz T, Young D (2015) An infant not moving her leg. Am J Emerg Med 34: 756.e1-2.
- Dessie A, Constantine E (2016) Neonate with a swollen thigh. Ann Emerg Med 68: 87-88.
- Ben-Meir E, Rubinshtein M, Pessach I, Barkai G, Keller N, et al. (2017) Neonatal cervical osteomyelitis with bilateral upper limb paresis. Pediatr Infect Dis J 36: 1013-1015.
- Zhan C, Du J, Chen L (2018) Salmonella osteomyelitis in a previously healthy neonate: a case report and review of the literature. Ital J Pediatr 44: 28.
- Knudsen CJ, Hoffman EB (1990). Neonatal osteomyelitis. J Bone Joint Surg Br 72: 846-851.
- Davis S, Thompson S (2017) Paediatric orthopaedic infections. Surgery (Oxford) 35: 62-67.
- Narang A, Mukhopadhyay K, Kumar P, Bhakoo ON (1998) Bone and joint infection in neonates. Indian J Pediatr 65: 461-464.
- Pittard WB, Thullen JD, Fanaroff AA (1976) Neonatal septic arthritis. J Pediatr 88: 621-624.
- Frederiksen B, Christiansen P, Knudsen FU (1993) Acute osteomyelitis and septic arthritis in the neonate, risk factors and outcome. Eur J Pediatr 152: 577-580.
- Li Y, Zhou Q, Liu Y, Chen W, Li J, et al. (2016) Delayed treatment of septic arthritis in the neonate: a review of 52 cases. Medicine 95: e5682.
- Mascarenhas A, Almeida C, Constantino C, Soudo AP, Calado E, et al. (2011) Septic arthritis presenting as brachial plexus neuropathy. BMJ Case Rep 2011: bcr1220103562.
- Wong M (1996) Osteomyelitis and septic arthritis. Seminars in Fetal & Neonatal 1: 161-168. WB Saunders.
- Ilharreborde B (2015) Sequelae of pediatric osteoarticular infection. Orthop Traumatol Surg Res 101: S129-S137.
- Agarwal A, Aggarwal AN (2016) Bone and joint infections in children: acute hematogenous osteomyelitis. Indian J Pediatr 83: 817-824.
- Courtney PM, Flynn JM, Jaramillo D, Horn BD, Calabro K, et al. (2010) Clinical indications for repeat MRI in children with acute hematogenous osteomyelitis. J Pediatr Orthop 30: 883-887.
- https://www.mayoclinic.org/diseases-conditions/osteomyelitis/diagnosis-treatment/drc-20375917
- Hatzenbuehler J, Pulling TJ (2011) Diagnosis and management of osteomyelitis. Am Fam Physician 84: 1027-1033.
- Belgrader P, Benett W, Hadley D, Long G, Mariella R Jr, et al. (1998) Rapid pathogen detection using a microchip PCR array instrument. Clin Chem 44: 2191-2194.
Citation: Minkowitz B, Lillie E, Baorto E, Murphy R, Ristic JR, et al. (2019) 41-Year Systematic Literature Review of Early Infancy Osteomyelitis: What Have We Learned with the Entry of MRI into Diagnostic Protocol?. Neonat Pediatr Med 5: 178. DOI: 10.4172/2572-4983.1000177
Copyright: © 2019 Minkowitz B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3893
- [From(publication date): 0-2019 - Nov 06, 2025]
- Breakdown by view type
- HTML page views: 3009
- PDF downloads: 884