Advanced Bionics® Cochlear Implants in Patients with Prelingual Hearing Loss
Received: 15-Jan-2014 / Accepted Date: 20-Feb-2014 / Published Date: 27-Feb-2014 DOI: 10.4172/2161-119X.1000159
Abstract
Introduction: Cochlear Implants (CI) have become standard in the treatment of prelingual, postlingual and perilingual deafness in children. Bilateral implants are considered standard for bilaterally affected children. Studies also find that the CI provides better access to speech for most children, and this access results in improved speech perception. In earlier times children who did not react to acoustic stimuli and were neither able to understand speech nor to acquire it spontaneously encountered severe discrimination, being dismissed as simple-minded or worse. Different studies broadly agree that one or two of every 1000 newborns have a hearing impairment that on current evidence warrants treatment or observation, i.e., permanent hearing loss with a lowering of the absolute threshold of hearing for speech perception by at least 35 dB. Approximately 50% of severe hearing impairments arising in the inner ear are thought to be hereditary in origin. When new Cochlear Implant (CI) sound processors are being introduced by the manufacturers, usually the newest generation implants benefit first from the new technology in order to release the full potential of the new hardware.
Objective: Evaluate the improvement of speech language and sound perception in patients with prelingual deafness that underwent cochlear implant using Advanced Bionics® device.
Method: Retrospective study of the medical records of the patients fitted with Advanced Bionics® cochlear implant in our institution between 2011 and 2012.
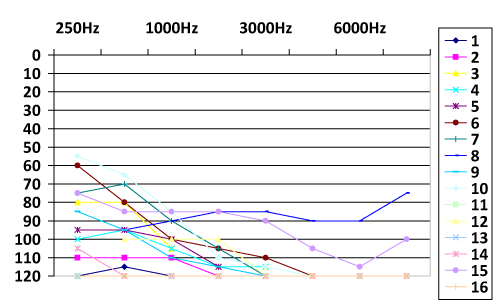
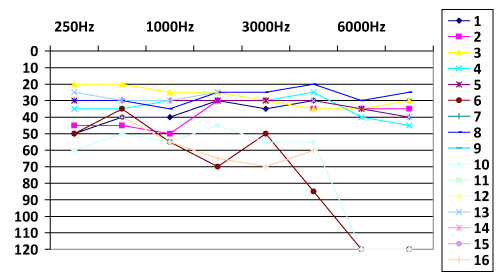
Results: Sixteen patients underwent to cochlear implantation using Advanced Bionics® devices. There were 43,75% prelingual and 43,75% postlingual patients with bilateral hearing loss. Mean age at implantation in the prelingual group was 3.6 years (ranged from 2 to 6 years). There was one case with medical history of deafness in family. All prelingual patients used hearing devices before the cochlear implant. The hearing levels improved after CI in all patients.
Conclusion: This study evaluated patients with pre-lingual deafness using the Advanced Bionics® cochlear implants demonstrated significant gains in neural stimulation and language development in children.
Introduction
Cochlear Implants (CI) have become the standard treatment of prelingual, postlingual and perilingual deafness and hearing loss in children and it is a revolutionary yet time-sensitive treatment for deaf children that must be performed within a critical window time for maximum benefit. Sensorineural hearing impairment alters speech perception in a complex nonlinear manner [1,2].
Reports on the speech of deaf children examined differences in the speech of deaf children as a function of hearing loss and/ or perceptual abilities; differences in the speech of deaf children as a function of hearing device via hearing aid or cochlear implants; longitudinal changes in the speech of deaf children; or deviation of speech acoustics of deaf children comparing to those of normal hearing children [1].
Cochlear implants have enabled a number of severely hearing impaired individuals to access auditory information and improve speech perception as well as speech production skills. Several studies demonstrate that multi-channel cochlear implants also promote the development of speech perception and speech production in prelingually deafened children.
Many factors such as sociocultural characteristics, teaching methodology, language skill, and phonological awareness contribute to the development of the ability to read in children with normal hearing [3]. Children with prelingual, profound hearing loss who use CIs tend to perform better on closed and open set word-identification tasks than their peers with profound hearing loss who use hearing aids [3]. It is vital that all newborn children undergo hearing screening to identify deaf children at birth [2]. Children with both postlingual and prelingual deafness and cochlear implants can acquire auditory–visual and visual–visual conditional discriminations using discrimination training regimens that were similar in character to those used with hearing populations, and subsequently exhibit both cross-modal (i.e. auditory-visual) and intramodal equivalence relations [4].
The skills involved in functional reading literacy include the following: a) reading lengthy, complex, abstract prose texts as well as synthesizing information and making complex inferences; b) integrating, synthesizing, and analyzing multiple pieces of information located in complex documents; and c) locating more abstract quantitative information and using it to solve multistep problems when the arithmetic operations are not easily inferred and the problems are more complex [5].
This manuscript aims to evaluate the improvement of sound perception in patients with prelingual deafness that underwent cochlear implant using Advanced Bionics® device.
Methods
Retrospective study of the medical records of the patients implanted with Advanced Bionics® cochlear implant in our institution between November, 2011 and November, 2012.
Device
For this study, we used the Advanced Bionics® devices (the HiFocus®1j electrode and the HiRes 90K® implant).
The HiFocus® 1j electrode consists of a fantail, electrode lead, and HiFocus 1j electrode array. The electrodes, composed of platinumiridium alloy, are housed in a silicone carrier and extend from the titanium case. The HiFocus® 1j intra cochlear electrode array is designed to be inserted approximately 25 mm into a normally patent cochlea. It consists of 16 planer contacts arranged along the medial (or inside) surface of the electrode array for stimulation of discrete segments of the cochlea. The electrode contacts are numbered 1 through 16 from apex to base. The neck refers to the jog at the proximal end of the array that transitions the array to the lead. The fantail is directly connected to the electronic implant. The lead, which extends from the fantail, refers to the silicone carrier in which the electrode wires are enclosed [6].
The HiRes 90K® implant has 16 independent output circuits with bi-directional communication link of telemetry, the information update rate is 90kHz, a stimulation rate up to 83,000 pulses per second, weights 12 grams and has an impact resistance value of 6 joules [6].
Subjects
There were 16 patients that underwent to a cochlear implant using the Advanced Bionics® devices. The selected patients were informed about the surgery risks and benefits and postoperative expectations and signed an informed consent form. All ethical guidelines established by the institution were respected. The study was approved by the institution’s Medical Ethics Committee (004/2013).
Inclusion criteria
The following criteria are based on the guidelines of the Brazilian Otolaryngology Association (ABORL-CCF) aiming to guide medical professionals and standardize criteria for cochlear implantation:
• Severe or deep sensorineural bilateral hearing loss;
• Patient without benefit after experience with the use of Hearing Aids (HA) for a minimum period of 3 months in severe hearing loss;
• Proper motivation of the family to use the cochlear implant and to develop intervention; and
• Presence of linguistic code established and properly rehabilitated by the oral method.
Exclusion criteria
• Appropriate gain after fitting a hearing aid;
• Improperly rehabilitation by the oral method;
• Absence of cochlea and cochlear nerve; and
• Unfavorable psychological assessment.
Audiological tests
Included subjects were tested before implantation with and without their HAs and four months after CI activation.
PTAs
Preoperative PTAs were performed in free field conditions, with and without HAs, as well as postoperative using CI. Measures were performed for 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz using the AC30-SD25 audiometer calibrated according to ISO 389/64.
Data analysis and statistic
The limited number of patients required the use of non-parametric statistics for all variables. Wilcoxon tests for paired samples were used to compare the SP results obtained pre and post CI surgery. The cut-off level for statistical significance was set at 0.05.
Results
Sixteen patients underwent to cochlear implantation using Advanced Bionics® devices (Table 1). There were seven (43.75%) prelingual, two (12.5%) perilingual and seven (43.75%) postlingual patients with bilateral hearing loss. Mean age at implantaion was 3.6 years (range 2 to 6 years) in the prelingual group. There was one case with medical history of deafness in family. All prelingual patients used hearing device before the cochlear implant. We compared the hearing levels before and after the cochlear implantation and observed the improvement of hearing levels after the procedure in all patients (Chart 1 and 2). In this study, 43.75% of the patients were diagnosed with hearing loss since birth (after Universal Newborn Hearing Screening was performed).
| Subject | Gender | Age | Lingual | OFL | Cause | PST pre-CI | Side of CI |
|---|---|---|---|---|---|---|---|
| 1 | F | 11 | Pre | Y | Rubeola | 0% | Left |
| 2 | F | 66 | Post | Y | - | 0% | Right |
| 3 | F | 31 | Peri | Y | Meningitis | 0% | Right |
| 4 | F | 22 | Post | Y | - | 0% | Right |
| 5 | F | 18 | Post | Y | - | 0% | Right |
| 6 | F | 57 | Peri | Y | - | 26% | Left |
| 7 | F | 50 | Post | Y | Trauma | 0% | Left |
| 8 | F | 60 | Post | Y | - | 14% | Right |
| 9 | F | 17 | - | Y | - | 0% | Right |
| 10 | F | 24 | Post | Y | Mondini dysplasia | 26% | Left |
| 11 | F | 21 | Post | Y | - | 0% | Right |
| 12 | F | 2 | Pre | N | - | Child | Right |
| 13 | F | 2 | Pre | N | - | Child | Left |
| 14 | F | 5 | Pre | Y | Congenital rubeola | Child | Right |
| 15 | M | 2 | Pre | N | - | Child | Right |
| 16 | F | 4 | Pre | Y | Premature | Child | Right |
Table 1: Subjects by gender, age, type of hearing loss (pre, peri or postlingual), orofacial language, cause of hearing loss, PST before CI and side of CI.
There were no technical difficulties during the cochlear implantation and only one patient referred local pain after the procedure. In this study, ten (62.5%) of the cases had an unknown etiology for the hearing loss after the investigation.
Only one male patient underwent to a surgical procedure.
Discussion
Cochlear implants have been approved for use in profoundly deaf children as young as 1 year of age. Longitudinal studies of outcomes in deaf children have established that a Cochlear Implant (CI) leads to gains in spoken language [7]. Since the approval by the Food and Drug Administration in 1984, the communicative benefits provided by Cochlear Implants (CIs) to postlingually deafened adults have been well documented [8].
Profound hearing loss affects people of all ages. For children, hearing is central to neurocognitive development, since sound deprivation early in life degrades the multiplicity of neural circuits that are responsible for information processing, especially those involved in the acquisition of speech and language [9]. Despite objections from the deaf community, thousands of prelingually deaf children have also received CIs, and many have shown excellent outcome on a wide range of measures of hearing, speech, and language. Using auditory inputs from their CIs, some prelingually deafened pediatric CI users have been able to acquire spoken language at a pace that is similar to normalhearing children [8].
The 1990s heralded major advances in speech-encoding strategies for cochlear implants, offering speech recognition without lip reading to the majority of recipients [9]. Several recent studies have suggested that the latest implant technology could indeed provide some openset speech perceptual abilities to these patients. These conclusions, however, are based on analyses of results obtained with only a very small number of patients, and the data often showed enormous variability among individuals, making the true assessment of their effectiveness an exceedingly difficult task [8].
It appears that the deafness-induced changes along the entire auditory pathway, including the degeneration of the auditory nerve, the alteration of synaptic structures in the midbrain, and the failure to establish appropriate intracortical projections in the auditory cortex, all contribute to the gradual deterioration of auditory performance with increasing duration of auditory deprivation [10]. Different studies broadly agree that one or two of every 1000 newborns have a hearing impairment that on current evidence warrants treatment or observation, i.e., permanent hearing loss with a lowering of the absolute threshold of hearing for speech perception by at least 35 dB [11].
As with other sensory impairments, there is hereditary and nonhereditary or congenital and pre-, peri-, or postnatal causes of hearing disorders. While the cause of conductive hearing loss can usually be identified relatively simply (e.g., by means of otoscopy in the case of tympanic effusion or accumulation of earwax), even thorough diagnostic investigation fails to uncover the reason for around half of the cases of inner ear hearing impairment in childhood. Approximately 50% of severe hearing impairments arising in the inner ear are thought to be hereditary in origin (Table 2).
| Hearing impairment in early childhood: signs and risk factors – Ptok, 2011. |
| •Concern on the part of parents/guardians regarding the hearing, speech development, or general development of their child |
| •Family history of permanent hearing impairment in childhood |
| •Stay of more than 5 days in the neonatal intensive care unit, possibly including the need for ventilation, extracorporeal membrane oxygenation, assisted breathing, administration of ototoxic drugs or loop diuretics, and hyperbilirubinemia requiring transfusion |
| •Intrauterine infections such as cytomegalovirus, herpes, rubella, syphilis, and toxoplasmosis |
| •Craniofacial anomalies, including malformation of the earlobe, auditory canal, or auricular appendages and anomalies of the auditory pit and petrosa |
| •External signs that may indicate a syndrome involving sensorineural hearing loss or permanent conductive hearing loss, e.g., a white forelock |
| •Syndromes involving immediate, progressive, or late-onset hearing loss, such as neurofibromatosis, osteopetrosis, and Usher syndrome; other complexes associated with hearing disorders are Waardenburg, Alport, Pendred, and Jervell-Lange-Nielsen syndromes |
| •Neurodegenerative diseases such as Hunter syndrome or sensorimotor neuro - pathies such as Friedreich ataxia and Charcot-Marie-Tooth syndrome |
| •Demonstration in culture of infections associated with sensory hearing loss, including bacterial or viral (especially herpes or varicella) meningitis |
| •Head injury, particularly fractures of the skull base or petrosa requiring inpatient treatment |
| •Chemotherapy |
| •Otitis media recurring frequently or persisting for more than 3 months |
Table 2: Hearing impairment in early childhood: signs and risk factors – Ptok, 2011.
The realization that children who had been born deaf could also derive substantial benefit, with some developing speech and language trajectories similar to those of their hearing peers, was transformational for childhood deafness, making mainstream schooling a viable option for many deaf children [9]. The benefits for speech and language development, as well as speech intelligibility brought by CI-enabled hearing are greatest if these are received as soon after diagnosis as possible. Continued improvements in preoperative diagnostics, electrode design, speech coding strategies and surgical techniques, have broadened the CI applications spectrum. Nowadays-with the exception of cochlear- and cochlear nerve aplasia-almost all malformations are manageable with CIs [12].
Conclusion
For this study we evaluated patients with prelingual, perilingual and postlingual deafness using the Advanced Bionics® cochlear implants and have demonstrated significant gains in hearing levels in both children and adults.
References
- Kant AR, Patadia R, Govale P, Rangasayee R, Kirtane M (2012) Acoustic analysis of speech of cochlear implantees and its implications. ClinExpOtorhinolaryngol 5 Suppl 1: S14-18.
- Russell JL, Pine HS, Young DL (2013) Pediatric cochlear implantation: expanding applications and outcomes. PediatrClin North Am 60: 841-863.
- Spencer LJ, Oleson JJ (2008) Early listening and speaking skills predict later reading proficiency in pediatric cochlear implant users. Ear Hear 29: 270-280.
- Almeida-Verdu AC, Huziwara EM, de Souza DG, De Rose JC, Bevilacqua MC, et al. (2008) Relational learning in children with deafness and cochlear implants. J Exp Anal Behav 89: 407-424.
- Spencer LJ, Tomblin JB (2009) Evaluating phonological processing skills in children with prelingual deafness who use cochlear implants. J Deaf Stud Deaf Educ 14: 1-21.
- Technical Specifications HiRes 90K® Implant, HiResolution® Bionic Ear System, Advanced Bionics, 2011.
- Horn DL, Pisoni DB, Sanders M, Miyamoto RT (2005) Behavioral assessment of prelingually deaf children before cochlear implantation. Laryngoscope 115: 1603-1611.
- Teoh SW, Pisoni DB, Miyamoto RT (2004) Cochlear implantation in adults with prelingual deafness. Part I. Clinical results. Laryngoscope 114: 1536-1540.
- O'Donoghue G (2013) Cochlear implants--science, serendipity, and success. N Engl J Med 369: 1190-1193.
- Teoh SW, Pisoni DB, Miyamoto RT (2004) Cochlear implantation in adults with prelingual deafness. Part II. Underlying constraints that affect audiological outcomes. Laryngoscope 114: 1714-1719.
- Ptok M (2011) Early detection of hearing impairment in newborns and infants. DtschArzteblInt 108: 426-431.
- Mlynski R, Plontke S (2013) [Cochlear implants in children and adolescents]. HNO 61: 388-398.
Citation: Pauna HF, Carvalho GM, Guimarães AC, Schuch LH, Muranaka EB, et al. (2014) Advanced Bionics® Cochlear Implants in Patients with Prelingual Hearing Loss. Otolaryngology 4:159. DOI: 10.4172/2161-119X.1000159
Copyright: © 2014 Pauna HF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16101
- [From(publication date): 5-2014 - Aug 17, 2025]
- Breakdown by view type
- HTML page views: 11397
- PDF downloads: 4704