A Consecutive Series of all Subtypes of the Acute Coronary Syndrome Patient Admitted to an Academic Coronary Care Unit
Received: 08-Sep-2017 / Accepted Date: 13-Sep-2017 / Published Date: 18-Sep-2017
Abstract
Objective: Little is known of the in-hospital management and outcomes of consecutive acute coronary syndrome (ACS) patients admitted to an academic coronary care unit (CCU). We therefore surveyed the characteristics, inhospital management and complications in an unselected ACS cohort.
Methods and results: In this retrospective observational cohort study we analyzed 567 consecutive ACS patients, divided in subgroups of ST-segment elevation myocardial infarction (STEMI) (n=369), non-STEMI (NSTEMI) (n=129) and unstable angina (UA) (n=69), admitted to our CCU. An invasive strategy was chosen in 93.8% of ACS patients (STEMI 98.1%, NSTEMI 85.3%, UA 87.0%, p<0.001). NSTEMI patients with a GRACE risk score>140 compared to ≤ 140 were less frequently treated with percutaneous coronary intervention (PCI) (50.0% vs. 70.2%, p=0.024) and more frequently with coronary artery bypass grafting (CABG) (20.5% vs. 3.6%, p=0.002). Overall in-hospital mortality was 3.2% (1.8% at discharge from the CCU). Thirty-day and one-year mortality were 4.9% and 8.5% respectively. In-hospital (re)infarctions occurred in 1.8%, stroke in 1.6% and major bleeding in 3.4% of patients. Major adverse cardiac and cerebrovascular events (MACCE) occurred in 4.9% and net adverse clinical events (NACE) in 6.9%. Age, female gender, previous stroke and chronic kidney disease (CKD) were associated with higher one-year mortality.
Conclusion: Our consecutive and unselected ACS cohort comprised 65.1% STEMI, 22.8% NSTEMI and 12.2% UA patients. Independent of ACS subtype and GRACE risk score about 90% of patients were treated by an invasive strategy. Age, female gender, previous stroke and CKD were associated with higher one-year mortality.
Keywords: Acute coronary syndrome; Acute myocardial infarction; Coronary care unit; GRACE risk score; Cardiac risk factors and prevention; In-hospital management; Complications
5860Abbreviations
ACS: Acute Coronary Syndrome; AMC: Academic Medical Center; BMI: Body Mass Index; ABG: Coronary Artery Bypass Grafting; CAG: Coronary Angiography; Cath lab: Catheterization Laboratory; CCU: Coronary Care Unit; CIN: Contrast Induced Nephropathy; CKD: Chronic Kidney Disease; CPU: Chest Pain Unit; CVD: Cardiovascular Diseases; DAPT: Dual Antiplatelet Therapy; ECG: Electrocardiogram; ED: Emergency Department; Egfr: Estimated Glomerular Filtration Rate; GRACE: Global Registry of Acute Coronary Events; hsTropT: High Sensitive Troponin T; IQR: Interquartile Range; LAD: Left Anterior Descending; MACCE: Major Adverse Cardiac And Cerebrovascular Events; NACE: Net Adverse Clinical Events; NSTE-ACS: Non-ST-Segment Elevation Acute Coronary Syndrome; NSTEMI: Non-ST-Segment Elevation Myocardial Infarction; PCI: Percutaneous Coronary Intervention; SD: Standard Deviation; STEMI: ST-segment Elevation Myocardial Infarction; TIA: Transient Ischemic Attack; UA: Unstable Angina.
Introduction
The acute coronary syndrome (ACS) is a cardiovascular spectrum of ischemic diseases with potentially severe consequences, including death. Although clinical presentations of patients with ACS are different, they share a common pathophysiological expression of acute myocardial ischemia with different epicardial and endocardial etiologies. Based on findings on the electrocardiogram (ECG) and cardiac biomarkers, ACS has been categorized into ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina (UA) [1].
As little is known of the in-hospital management and outcomes of consecutive patients with all three subtypes within the ACS spectrum admitted to one academic coronary care unit (CCU), we surveyed the characteristics, in-hospital management and complications in an unselected ACS cohort. Our observational study provides a consecutive series of patients with STEMI, NSTEMI and UA admitted and treated simultaneously at one academic CCU. Furthermore, we investigated the route of ACS patients to our CCU as well as their discharge destination after hospitalization, which gives a unique insight into management practices at an academic CCU.
Methods
Study population
In this retrospective observational cohort study we included all consecutive ACS patients admitted to our academic CCU between July 2012 and March 2013. Patients were classified into STEMI, NSTEMI and UA, according to the discharge diagnosis stated in the clinical record of the patient. If this diagnosis was noted as ‘ACS’ or ‘non-STAtherosclerosis segment elevation acute coronary syndrome’ (NSTE-ACS), we classified the patient based on the available ECGs and troponins. Patients with an elevated high sensitive troponin T (hsTropT) from other conditions were excluded.
Baseline characteristics were obtained from the clinical records. A detailed list of the baseline characteristics definitions can be found in Online Appendix A.
Setting
This study was performed at an academic CCU (Academic Medical Center (AMC), University of Amsterdam, The Netherlands). It is a large tertiary center with both percutaneous coronary intervention (PCI) and on-site cardiac surgery facilities. At the time of the registration, the annual coronary artery bypass grafting (CABG) (without valve) volume was ~500. The annual PCI volume was ~2000, of which ~600 primary PCI.
We are embedded in a pre-hospital ECG triage network. If the diagnosis STEMI is likely based on the ECG and physical complaints, the patient is pre-treated in ambulance with intravenous heparin 5000 IU and dual antiplatelet therapy (DAPT) and brought to the catheterization laboratory (cath lab) directly to minimize system delay. After primary PCI, uncomplicated STEMI patients are transferred to referring hospitals after four hours observation on our CCU. Unstable STEMI patients have longer observation on our CCU.
Patients with suspected NSTE-ACS are first seen on the chest pain unit (CPU; Dutch: ‘Eerste Hart Hulp’) by a dedicated cardiologist and evaluated. An hsTropT assay is used to differentiate between NSTEMI and UA [1]. A representative hsTropT value below the upper limit of normal (50% is considered to be elevated, and makes the diagnosis NSTEMI likely. We use a value >0.05 μg/l as an absolute cut-off.
Patients who are diagnosed with NSTE-ACS are submitted to the CCU and treated with DAPT and anticoagulation according to the guidelines [1]. Patients’ risk for mortality and recurrent myocardial infarction is calculated using the GRACE (Global Registry of Acute Coronary Events) risk score [2], and the bleeding risk using the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) bleeding risk score [3].
The choice to perform and the timing of an invasive approachurgent coronary angiography (CAG) (1].
Endpoints
Three primary endpoints were pre-specified: the treatment strategy during the current hospital admission with ACS, 30-day mortality and one-year mortality. The treatment strategy was classified into medical treatment only or an invasive strategy. An invasive strategy was further specified into CAG only, PCI, CABG and revascularization (PCI and/or CABG). Treatment strategies were compared between high-risk (GRACE risk score on admission for in-hospital death>140) and lowto- intermediate-risk (GRACE score ≤ 140) NSTE-ACS patients.Mortality data were collected at 30 days and at one year follow-up from the Municipal Personal Records Database.
Secondary endpoints included the following
The route of ACS patients to our CCU and their discharge destination after CCU admission were registered, as well as the length of stay on the CCU. In-hospital events were collected from the AMC medical records and included mortality, (re)infarction [4], stroke [5-7], transient ischemic attack (TIA) [7] and bleeding. These outcomes were registered if occurring during hospitalization after presentation with ACS on our CCU, either in our institution or in the referring hospital awaiting for PCI or CABG (i.e. if a complication occurred in another hospital after a definite hospital transfer to a referring hospital this was no longer considered an in-hospital outcome).
For composite endpoints the following definitions were used. Major bleeding (not related to CABG) was defined according to the criteria of the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) [8] and ACUITY (Acute Catheterization and Urgent Intervention Triage strategy) [9] trials, as the in-hospital occurrence of intracranial bleeding, access site hemorrhage requiring surgical or interventional treatment, hematoma at the puncture site of ≥ 5 cm in diameter, an Hb drop of ≥ 1.9 mmol/l (3 g/dl) with an overt source of bleeding, an Hb drop of ≥ 2.5 mmol/l (4 g/dl) without an overt source of bleeding, reoperation for bleeding, or the use of any blood product transfusion, with the exclusion of CABG-related bleedings. Like the HORIZONSAMI investigators we also reported the major bleeding rate including CABG-related bleedings. Major adverse cardiac and cerebrovascular events (MACCE) comprised in-hospital all-cause mortality, (re)infarction and stroke (without TIA). Net adverse clinical events (NACE) were defined as the composite of in-hospital MACCE and major bleeding not related to CABG. After stratification by one-year mortality, we identified factors which were associated with higher oneyear mortality and higher one-year survival. Gender was related to the factors associated with higher one-year mortality and survival in a post-hoc analysis.
Data-analysis
Continuous variables were compared by means of the analysis of variance (ANOVA) and the independent samples t-test (normal distribution) or the Kruskal Wallis and the Mann Whitney U test (skewed distribution). Categorical outcomes were compared by means of the chi-square test. One-year mortality was estimated with the Kaplan-Meier method. All tests were considered statistically significant if p ≤ 0.05 (α=5%). Statistical analysis was performed with SPSS version 20.0 (IBM).
Results
Baseline characteristics
A total of 567 patients were admitted to our academic CCU with a diagnosis of ACS between July 2012 and March 2013, with 369 (65.1%) STEMI, 129 (22.8%) NSTEMI and 69 (12.2%) UA patients. The mean age was 63.8 years, with 69.1% of the patients being male (Table 1). STEMI patients were younger and less frequently had suffered a prior cardiovascular event compared to NSTEMI and UA patients. Hypertension showed the highest prevalence in the NSTEMI group and diabetes mellitus in the UA group.
| ACS study cohort | STEMI | NSTEMI | UA | P value | |
|---|---|---|---|---|---|
| n=567 | n=369 | n=129 | n=69 | ||
| Patient characteristics | |||||
| Age (years) – mean ± SD | 63.8 ± 12.6 | 62.5 ± 12.6 | 65.8 ± 13.1 | 67.2 ± 11.0 | 0.002 |
| Male gender – n (%) | 392 (69.1) | 261 (70.7) | 81 (62.8) | 50 (72.5) | 0.199 |
| Cardiovascular medical history | |||||
| Cardiac patientA – n (%) | 250 (44.1) | 116 (31.4) | 79 (61.2) | 55 (79.7) | <0.001 |
| Myocardial infarction – n (%) | 120 (21.2) | 53 (14.4) | 38 (29.5) | 29 (42.0) | <0.001 |
| PCI – n (%) | 128 (22.6) | 53 (14.4) | 45 (34.9) | 30 (43.5) | <0.001 |
| CABG – n (%) | 30 (5.3) | 11 (3.0) | 14 (10.9) | 5 (7.2) | 0.002 |
| Atrial fibrillation – (%) | 30 (5.3) | 12 (3.3) | 10 (7.8) | 8 (11.6) | 0.006 |
| Peripheral vascular disease – n (%) | 60 (10.6) | 30 (8.1) | 21 (16.3) | 9 (13.0) | 0.027 |
| Stroke/TIA – n (%) | 56 (9.9) | 27 (7.3) | 18 (14.0) | 11 (15.9) | 0.081 |
| Cardiovascular risk factors | |||||
| Past and current smokersB – n (%) | 312 (64.1) | 206 (65.8) | 73 (64.0) | 33 (55.0) | 0.278 |
| Diabetes mellitus – n (%) | 120 (21.2) | 63 (17.1) | 35 (27.1) | 22 (31.9) | 0.004 |
| Hypertension – n (%) | 280 (49.4) | 163 (44.2) | 79 (61.2) | 38 (55.1) | 0.002 |
| Family history for CVD – n (%) | 207 (36.5) | 133 (36.0) | 43 (33.3) | 31 (44.9) | 0.259 |
| Hypercholesterolemia – n (%) | 193 (34.0) | 115 (31.2) | 49 (38.0) | 29 (42.0) | 0.122 |
| BMIC – mean ± SD | 28.4 (5.2) | 27.6 (5.3) | 29.2 (5.2) | 29.0 (4.7) | 0.201 |
| Chronic kidney diseaseD – n (%) | 117 (20.7) | 69 (18.8) | 35 (27.1) | 13 (18.8) | 0.122 |
| eGFR <60 ml/min/1.73m2 – n (%) | 134 (23.6) | 83 (22.5) | 37 (28.7) | 14 (20.3) | 0.284 |
Table 1: Baseline characteristics; ACS: Acute Coronary Syndrome; BMI: Body Mass Index; CABG: Coronary Artery Bypass Grafting; CVD: Cardiovascular Diseases; eGFR: estimated Glomerular Filtration Rate; NSTEMI: Non-ST-segment Elevation Myocardial Infarction; PCI: Percutaneous Coronary Intervention; SD: Standard Deviation; STEMI: ST-segment Elevation Myocardial Infarction; TIA: Transient Ischemic Attack; UA: Unstable Angina; A) Cardiac patient: a patient with one or more events in the cardiac medical history; B) Missing for n=80 (ACS), n=56 (STEMI), n=15 (NSTEMI) or n=9 (UA); C) Missing for n=421 (ACS), n=304 (STEMI), n=75 (NSTEMI) or n=42 (UA); D) Chronic kidney disease: chronic kidney disease mentioned in the medical history and/or an eGFR<60 ml/min/1.73m2 on admission and during the whole current admission, calculated with the modification of diet in renal disease equation.
Routing of patients
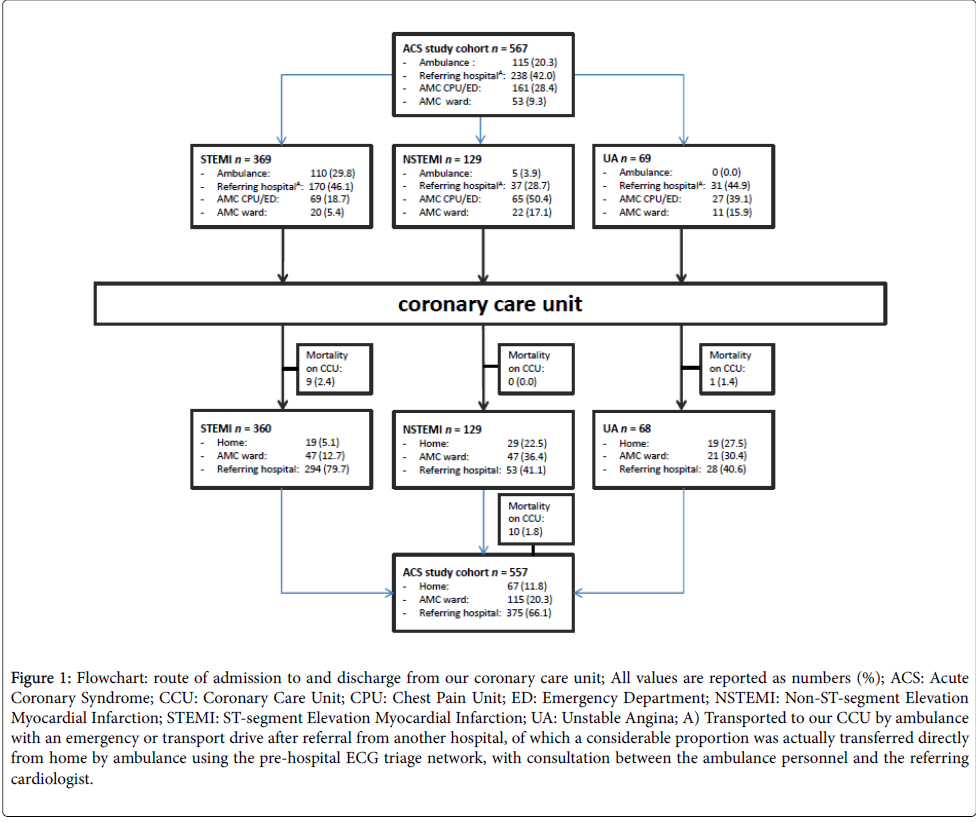
Our CCU is used as a step-up function (i.e. entering or in need for more intensive hospital management) for ACS patients in our region (Figure 1). One fifth (20.3%) of ACS patients came directly from home by ambulance. Most ACS patients (42.0%) were transported to our CCU by ambulance with an emergency or transport drive after referral from another hospital, of which a considerable proportion (STEMI patients) was actually transferred directly from home by ambulance using the pre-hospital ECG triage network, with consultation between the ambulance personnel and the referring cardiologist. The CPU or emergency department (shock room) was the location of presentation for 28.4% of ACS patients and another ward in our hospital for 9.3% of ACS patients.
Figure 1: Flowchart: route of admission to and discharge from our coronary care unit; All values are reported as numbers (%); ACS: Acute Coronary Syndrome; CCU: Coronary Care Unit; CPU: Chest Pain Unit; ED: Emergency Department; NSTEMI: Non-ST-segment Elevation Myocardial Infarction; STEMI: ST-segment Elevation Myocardial Infarction; UA: Unstable Angina; A) Transported to our CCU by ambulance with an emergency or transport drive after referral from another hospital, of which a considerable proportion was actually transferred directly from home by ambulance using the pre-hospital ECG triage network, with consultation between the ambulance personnel and the referring cardiologist.
Hemodynamic unstable NSTE-ACS patients were transferred to our CCU before revascularization, independent of their location of presentation. Part of ACS patients presenting at the CPU or shock room by ambulance were waiting for transportation to the cath lab or were admitted because of severe hemodynamic instability.
Elective PCI complicated by ACS accounted for 3.2% of admitted patients. Only 11 patients (1.9%) came as a step-down from the intensive care unit to our CCU.
The median duration of stay on our CCU was 14.5 hours (interquartile range (IQR) 5.8-31.3 hours), with significant shorter admission for STEMI patients (median 10.0 h, IQR 5.1-24.3 h, p<0.001), mostly awaiting for transfer to another hospital for further rehabilitation (79.7% in STEMI vs. 41.1% in NSTEMI and 40.6% in UA, p<0.001; Table 2 and Figure 1).
| ACS study cohort | STEMI | NSTEMI | UA | P value | |
|---|---|---|---|---|---|
| n=567 | n=369 | n=129 | n=69 | ||
| Duration of stay on CCU (hours) –median (IQR) | 14.5 (5.8-31.3) | 10.0 (5.1-24.3) | 27.4 (13.6-51.5) | 21.5 (6.4-37.9) | <0.001 |
| Treatment strategy | |||||
| Medical treatment only (1) – n (%) | 35 (6.2) | 7 (1.9) | 19 (14.7) | 9 (13.0) | <0.001 |
| Invasive strategy (2/3/4) – n (%) | 532 (93.8) | 362 (98.1) | 110 (85.3) | 60 (87.0) | <0.001 |
| CAG only (2) – n (%) | 45 (7.9) | 19 (5.1) | 16 (12.4) | 10 (14.5) | 0.003 |
| PCI (3) – n (%) | 456 (80.4) | 336 (91.1) | 82 (63.6) | 38 (55.1) | <0.001 |
| CABG (4) – n (%) | 38 (6.7) | 14 (3.8) | 12 (9.3) | 12 (17.4) | <0.001 |
| Revascularization (3/4) – n (%) | 487 (85.9) | 343 (93.0) | 94 (72.9) | 50 (72.5) | <0.001 |
Table 2: In-hospital treatment. ACS: Acute Coronary Syndrome; CABG: Coronary Artery Bypass Grafting; CAG: Coronary Angiography; CCU: Coronary Care Unit; IQR: Interquartile Range; NSTEMI: Non-ST-segment Elevation Myocardial Infarction; PCI: Percutaneous Coronary Intervention; STEMI: ST-segment Elevation Myocardial Infarction; UA: Unstable Angina.
Treatment of patients
An invasive strategy (CAG and/or PCI and/or CABG) was chosen during the current admission in 93.8% of ACS patients, with 98.1% in STEMI compared to 85.3% in NSTEMI and 87.0% in UA patients (p<0.001; Table 2). NSTEMI (72.9%) and UA patients (72.5%) were less frequently revascularized (PCI and/or CABG) compared to STEMI patients (93.0%, p<0.001). A higher proportion of NSTEMI (9.3%) and UA (17.4%) patients were revascularized by CABG compared to STEMI patients (3.8%, p<0.001). GRACE risk score on admission for in-hospital death did not statistically significantly differ between NSTE-ACS (NSTEMI and UA) patients managed with and without an invasive strategy.
High-risk (GRACE score>140) NSTEMI patients (34.4%) compared to low-to-intermediate-risk (GRACE score ≤ 140) NSTEMI patients (65.6%) were less frequently treated with PCI (50.0% vs. 70.2%, p=0.024) and more frequently with CABG (20.5% vs. 3.6%, p=0.002). High-risk patients tended to have more often a prior CABG (18.2% vs. 7.1%, p=0.057). In UA patients with a GRACE score>140 compared to ≤ 140 management strategies were similar.
Clinical outcomes
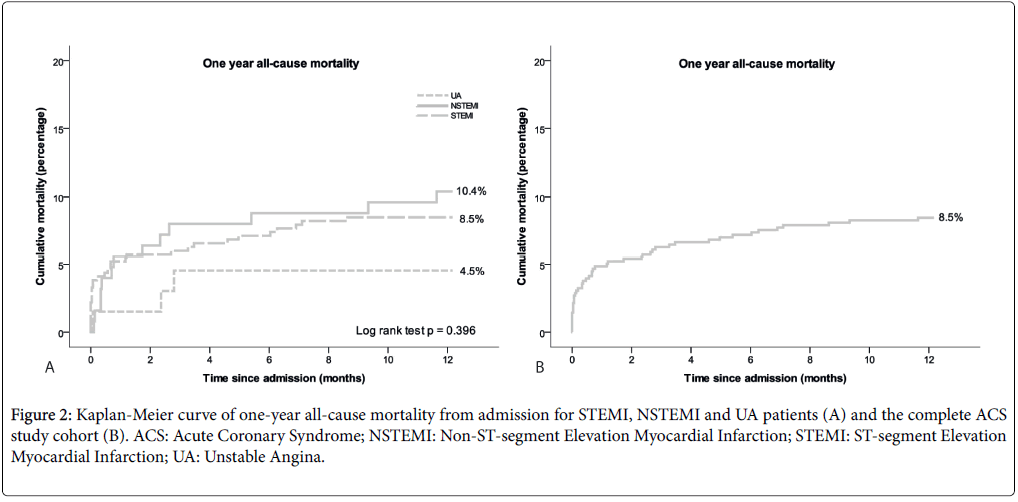
Mortality at discharge from the CCU was 1.8% and at hospital discharge it was 3.2% (Table 3 and Figure 1). Causes of in-hospital death were cardiac failure in 11 patients (STEMI 9, NSTEMI 1, UA 1), cardiac failure combined with a hemorrhagic shock in 1 patient (STEMI) and cardiac failure combined with sepsis in 2 patients (NSTEMI). Hemorrhagic stroke was the cause in 2 patients (STEMI) and termination of treatment in the context of failure of neurologic recovery in 2 other patients (STEMI 1, NSTEMI 1). At 30 days, allcause mortality was 4.9%, increasing to 8.5% at one year (Figure 2). Thirty-day mortality in STEMI patients was 5.2%, in NSTEMI patients 5.6% and in UA patients 1.5% (p=0.398).
| ACS study cohort | STEMI | NSTEMI | UA | P value | |
|---|---|---|---|---|---|
| n=567 | n=369 | n=129 | n=69 | ||
| In-hospital outcomes | |||||
| On-CCU all-cause mortality – n (%) | 10 (1.8) | 9 (2.4) | 0 (0.0) | 1 (1.4) | 0.189 |
| In-AMC all-cause mortality – n (%) | 18 (3.2) | 13 (3.5) | 4 (3.1) | 1 (1.4) | 0.665 |
| (Re)infarction-n (%) | 10 (1.8) | 4 (1.1) | 5 (3.9) | 1 (1.4) | 0.114 |
| Spontaneous (type 1) – n (%) | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0.764 |
| Secondary to ischemic imbalance (type 2) – n (%) | 1 (0.2) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0.183 |
| PCI-related (type 4a) – n (%) | 4 (0.7) | 2 (0.5) | 2 (1.6) | 0 (0.0) | 0.378 |
| In-stent thrombosis (type 4b) – n (%) | 2 (0.4) | 1 (0.3) | 0 (0.0) | 1 (1.4) | 0.236 |
| CABG-related (type 5) – n(%) | 2 (0.4) | 0 (0.0) | 2 (1.6) | 0 (0.0) | 0.033 |
| Stroke/TIA – n (%) | 9 (1.6) | 5 (1.4) | 2 (1.6) | 2 (2.9) | 0.641 |
| Bleeding – n (%) | 49 (8.6) | 30 (8.1) | 15 (11.6) | 4 (5.8) | 0.319 |
| Cause: | |||||
| Access site – n (%) | 20 (3.5) | 13 (3.5) | 5 (3.9) | 2 (2.9) | 0.939 |
| Femoral hematoma ≥5 cm – n (%) | 7 (1.2) | 4 (1.1) | 3 (2.3) | 0 (0.0) | 0.334 |
| Femoral <5cm or brachial/radial – n (%) | 13 (2.3) | 9 (2.4) | 2 (1.6) | 2 (2.9) | 0.792 |
| CABG-related – n (%) | 11 (1.9) | 4 (1.1) | 6 (4.7) | 1 (1.4) | 0.039 |
| Gastro-intestinal – n (%) | 6 (1.1) | 5 (1.4) | 1 (0.8) | 0 (0.0) | 0.564 |
| Non-gastro-intestinal – n (%) | 12 (2.1) | 8 (2.2) | 3 (2.3) | 1 (1.4) | 0.914 |
| Severity: | |||||
| With ≥1.9 mmol/l Hb drop – n (%) | 21 (3.7) | 12 (3.3) | 7 (5.4) | 2 (2.9) | 0.494 |
| With blood transfusion – n (%) | 14 (2.5) | 9 (2.4) | 4 (3.1) | 1 (1.4) | 0.774 |
| Major bleeding, non-CABG-related – n (%) |
19 (3.4) | 13 (3.5) | 4 (3.1) | 2 (2.9) | 0.950 |
| Major bleeding, including CABG – related-n (%) |
30 (5.3) | 17 (4.6) | 10 (7.8) | 3 (4.3) | 0.363 |
| MACCE – n (%) | 28 (4.9) | 17 (4.6) | 8 (6.2) | 3 (4.3) | 0.750 |
| NACE – n (%) | 39 (6.9) | 27 (7.3) | 8 (6.2) | 4 (5.8) | 0.848 |
| 30-day outcome | |||||
| All-cause mortalityA –n (%) | 27 (4.9) | 19 (5.2) | 7 (5.6) | 1 (1.5) | 0.398 |
| One-year outcome | |||||
| All-cause mortalityA – n (%) | 47 (8.5) | 31 (8.5) | 13 (10.4) | 3 (4.5) | 0.384 |
Table 3: Clinical outcomes. ACS: Acute Coronary Syndrome. CABG: Coronary Artery Bypass Grafting. CCU: Coronary Care Unit. MACCE: Major Adverse Cardiac and Cerebrovascular Events. NACE: Net Adverse Clinical Events. NSTEMI: Non-ST-segment Elevation Myocardial Infarction. PCI: Percutaneous Coronary Intervention. STEMI: ST-segment Elevation Myocardial Infarction. TIA: Transient Ischemic Attack. UA: Unstable Angina; A Missing for n=11 (ACS), n=4 (STEMI), n=4 (NSTEMI) or n=3 (UA).
Figure 2: Kaplan-Meier curve of one-year all-cause mortality from admission for STEMI, NSTEMI and UA patients (A) and the complete ACS study cohort (B). ACS: Acute Coronary Syndrome; NSTEMI: Non-ST-segment Elevation Myocardial Infarction; STEMI: ST-segment Elevation Myocardial Infarction; UA: Unstable Angina.
The in-hospital (re)infarction rate was 1.8% and (re)infarctions were classified according to the third universal definition of myocardial infarction [4], as shown in Table 3. (Re)infarctions occurred in 4 STEMI (1.1%), 5 NSTEMI (3.9%) and 1 UA patient (1.4%) during their hospital admission (p=0.114).
Bleeding complications occurred in 8.6% of patients, with access site bleedings being responsible for most of them (3.5%). Major bleedings not related to CABG occurred in 3.4% of patients. CABG-related bleedings (1.9%) were significantly more common in NSTEMI (4.7%) compared to STEMI (1.1%) and UA (1.4%) patients (p=0.039).
In-hospital MACCE occurred in 4.9% and NACE in 6.9% of ACS patients, without statistically significant differences between the subgroups.
Stratification by one-year mortality
ACS patients deceased at one year follow-up (group A) were significantly older (74.2 with standard deviation (SD) 9.7 years vs. 62.8 with SD 12.4 years, p<0.001) and more often female (44.7% vs. 29.9%, p=0.036) compared to those alive (group B). Chronic kidney disease (CKD) was more common in group A (54.3% vs. 17.3%, p<0.001) as was a prior stroke (25.5% vs. 8.3%, p=0.001). An invasive strategy (group A 85.1% vs. group B 94.7%, p=0.009) and revascularization (group A 72.3% vs. group B 87.2%, p=0.005) were significantly correlated with higher one-year survival.
In the complete ACS study cohort, female compared to male patients were significantly older and had more often CKD. Furthermore, women and men had similar percentages of CAG, but women less frequently underwent revascularization.
Discussion
The current unselected ACS population admitted to our academic CCU mainly consisted of STEMI patients (65.1%), followed by NSTEMI (22.8%) and UA patients (12.2%). Not all ACS patients are admitted to the CCU. Low-risk UA patients are sent home from the CPU, with a maximum observation time of 24 h, or are admitted to a ward. On the other end, STEMI patients deceased at the cath lab or those coming intubated and/or in a shock state after which they go immediately to the intensive care unit and decease, are never admitted to our CCU, creating a discriminating STEMI population as well.
The main finding of this observational study is that independent of ACS subtype and GRACE risk score, about 90% of patients were treated with an invasive strategy (CAG and/or PCI and/or CABG). Revascularization (PCI and/or CABG) was less frequently performed in NSTEMI and UA patients compared to STEMI patients. An invasive strategy and revascularization as treatment strategy for the current ACS correlated with higher one-year survival in the complete ACS study cohort.
In our study, short- and long-term mortality was low in UA patients. STEMI patients showed a higher in-hospital mortality and NSTEMI patients a higher one-year mortality, but 30-day mortality rates of about 5% were similar in these two subtypes. These mortality trends are comparable with the literature [10-13], although in our cohort these differences did not reach statistical significance, probably due to the small sized cohort.
We demonstrated the route of ACS patients to our CCU as well as their discharge destination after hospitalization, which gives a unique insight into management practices at an academic CCU.
GRACE risk score
Rates of PCI and CABG were substantially higher in our NSTEMI and UA patients compared to other study populations, probably as a consequence of the high-risk baseline profiles in our academic NSTEACS cohort [14-16]. These high-risk profiles were reflected in a higher median GRACE score, but also more disadvantageous risk factors which are not embedded in the GRACE score, in particular a history of PCI and stroke/TIA, compared to other NSTE-ACS CCU [14] and non-CCU populations [15,16].
The GRACE risk score did not differentiate between NSTE-ACS patients treated with and without an invasive strategy. This might be related to a relatively high cardiovascular risk profile not completely incorporated in the GRACE score in our academic ACS population, with high overall rates of PCI and CABG.
NSTEMI patients with a GRACE score>140 compared to ≤ 140 in our population were less frequently treated with PCI and more frequently with CABG. In a large cohort of the GRACE study PCI was also more frequently performed in low- than high-risk ACS patients [17]. Beigel et al. [18] concluded that a GRACE score>140 was a strong predictor of high-risk coronary anatomy (left main disease >50%, proximal left anterior descending (LAD) lesion>70% or two- to threevessel disease involving LAD) and therefore the CABG rate is likely higher in our NSTEMI patients who have a GRACE score>140. Alternatively, as NSTEMI patients with a higher GRACE score tended to have more frequently a prior CABG, an occluded graft may have been a reason to perform a CABG instead of a PCI. Another explanation might be that NSTEMI patients with a GRACE score>140 may have more often other cardiac morbidity requiring surgery, such as cardiac valve disease.
Gender differences
Female compared to male gender was associated with a higher oneyear mortality rate. Women were older and had more often CKD, two factors that were also associated with one-year mortality. Although treated with an invasive strategy as often as men, women ended up with undergoing revascularization less frequently, in accordance with prior research [19,20]. Hvelplund et al. demonstrated that this could just partially be ascribed to a lower percentage of significant stenosis on the diagnostic angiograms in female patients [20]. Hence, our higher one-year mortality rates in women might be caused by more comorbidities and lower rates of revascularization, especially in the NSTEMI and UA group [21-23]. A historical cohort from our center, published by Sjauw et al. found similar adverse baseline risk profiles in women, but without a higher mortality rate in women up to three years for STEMI patients [23].
Chronic kidney disease
Our ACS patients with CKD had an increased risk for death within one year follow-up. CKD is a recognized determinant of worse outcome and an independent predictor of short- and long-term mortality in ACS patients [24,25]. A recent meta-analysis revealed that in all ACS subtypes an early revascularization strategy compared to an initial medical approach is associated with increased survival, across all CKD stages [24]. STEMI patients treated by primary PCI have an increased risk of developing contrast induced nephropathy (CIN) compared to patients undergoing elective PCI with pre-hydration. Reasons are hemodynamic instability, impaired systemic perfusion as a consequence of left ventricular dysfunction, large volume of contrast medium, renal hypotension and lack of pre-hydration or other prophylactic measures [26]. In STEMI patients, decisions on reperfusion have to be made before assessment of renal function is available. Consequently, patients with unknown or undocumented CKD are not pre-hydrated, even if they have impaired renal function on admission. Having unknown CKD places these STEMI patients at an even higher risk for CIN when undergoing primary PCI. Acute (on chronic) kidney injury due to CIN may be worsened by systemic hypoperfusion as a consequence of myocardial infarction, inducing further kidney damage. We might speculate whether pre-hydration in every STEMI patient in whom renal function is unknown would decrease the incidence of CIN/acute kidney injury after primary PCI, with an improvement in clinical outcomes. Alternatively, a quick test for renal function in-ambulance and subsequent in-ambulance prehydration if necessary might improve outcomes after primary PCI.
Study limitations
Our retrospective study is likely to suffer from the limitations that arise from a retrospective study design.
Furthermore, in-hospital outcomes were only registered in our medical center. However, we have 100% completed mortality data up to one year follow-up. Most complications in STEMI patients-the subtype with shorter admission duration on our CCU compared to NSTEMI and UA patients-occur within the first four hours of admission; hence, these patients were only transferred if they were stable after this period. Previous studies demonstrated that transferring patients four hours after elective PCI proved to be safe and feasible [27,28]. Estévez-Loureiro et al. showed that returning low-risk STEMI patients early to referring hospitals after primary PCI was safe, without complications during transport or significant differences in cardiovascular events and mortality during 30-day follow-up [29]. In our ACS cohort, 30-day and one-year mortality did not statistically significantly differ between ACS patients who were admitted shorter compared to longer than the median duration of stay on our CCU, which was also true for the STEMI cohort.
Finally, the low patient number in this study sometimes restrained us from demonstrating statistical significance.
Conclusion
In the current observational cohort study we have provided a consecutive series of patients diagnosed with all ACS subtypes admitted and treated simultaneously at our academic CCU, with 65.1% STEMI, 22.8% NSTEMI and 12.2% UA patients. Independent of ACS subtype and GRACE risk score, about 90% of patients were treated by an invasive strategy (CAG and/or PCI and/or CABG). NSTEMI patients with a GRACE score>140 compared to ≤ 140 were less frequently revascularized with PCI and more frequently with CABG. Thirty-day mortality was about 5% in STEMI and NSTEMI patients and not significantly lowers in UA patients (1.5%). One-year mortality was 8.5% in STEMI, 10.4% in NSTEMI and 4.5% in UA patients (not significant). Age, female gender, previous stroke and CKD were associated with higher one-year mortality.
References
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, et al. (2011) ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32: 2999-3054.
- Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, et al. (2006) Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 333: 1091-1094.
- Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, et al. (2009) Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 119: 1873-1882.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, et al. (2012) Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) Third universal definition of myocardial infarction. Eur Heart J 33: 2551-2567.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, et al. (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045-1057.
- Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, et al. (2002) Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288: 2411-2420.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137: 263-272.
- Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al. (2011) Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet 377: 2193-2204.
- Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, et al. (2007) Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 49: 1362-1368.
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, et al. (2010) Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362: 2155–2165.
- Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, et al. (2006) The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J 27: 2285–2293.
- Goodman SG, Huang W, Yan AT, Budaj A, Kennelly BM, et al. (2009) The expanded Global Registry of Acute Coronary Events: baseline characteristics, management practices, and hospital outcomes of patients with acute coronary syndromes. Am Heart J 158: 193–201.
- Terkelsen CJ, Lassen JF, Nørgaard BL, Gerdes JC, Jensen T, et al. (2005) Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 26: 18-26.
- González-Pacheco H, Márquez MF, Arias-Mendoza A, Ãlvarez-Sangabriel A, Eid-Lidt G, et al. (2015) Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J Cardiol 66: 148-154.
- Brieger D, Fox KA, Fitzgerald G, Eagle KA, Budaj A, et al. (2009) Predicting freedom from clinical events in non-ST-elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 95: 888-894.
- Hutchinson-Jaffe AB, Goodman SG, Yan RT, Wald R, Elbarouni B, et al. (2010) Canadian Acute Coronary Syndromes (ACS) Registry I and II Investigators and Canadian Global Registry of Acute Coronary Events (GRACE/GRACE 2) Investigators: Comparison of baseline characteristics, management and outcome of patients with non-ST-segment elevation acute coronary syndrome in versus not in clinical trials. Am J Cardiol 106: 1389-1396.
- Fox KA, Anderson FA Jr, Dabbous OH, Steg PG, López-Sendón J, et al. (2007) Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 93: 177-182.
- Beigel R, Matetzky S, Gavrielov-Yusim N, Fefer P, Gottlieb S, et al. (2014) ACSIS and ACSIS-PCI 2010 Investigators - Predictors of high-risk angiographic findings in patients with non-ST-segment elevation acute coronary syndrome. Catheter Cardiovasc Interv 83: 677-683.
- Poon S, Goodman SG, Yan RT, Bugiardini R, Bierman AS, et al. (2012) Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 163: 66-73.
- Hvelplund A, Galatius S, Madsen M, Rasmussen JN, Rasmussen S, et al. (2010) Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J 31: 684-690.
- Hoenig MR, Doust JA, Aroney CN, Scott IA (2006) Early invasive versus conservative strategies for unstable angina & non-ST-elevation myocardial infarction in the stent era. Cochrane Database Syst Rev 3: CD004815.
- Alfredsson J, Clayton T, Damman P, Fox KA, Fredriksson M, et al. (2014) Impact of an invasive strategy on 5 years outcome in men and women with non-ST-segment elevation acute coronary syndromes. Am Heart J 168: 522-529.
- Sjauw KD, Stegenga NK, Engström AE, van der Schaaf RJ, Vis MM, et al. (2010) The influence of gender on short- and long-term outcome after primary PCI and delivered medical care for ST-segment elevation myocardial infarction. EuroIntervention 5: 780-787.
- Huang HD, Alam M, Hamzeh I, Virani S, Deswal A, et al. (2013) Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int J Cardiol 168: 3741-3746.
- Goldenberg I, Subirana I, Boyko V, Vila J, Elosua R, et al. (2010) Relation between renal function and outcomes in patients with non-ST-segment elevation acute coronary syndrome: real-world data from the European Public Health Outcome Research and Indicators Collection Project. Arch Intern Med 170: 888–895.
- Busch SV, Jensen SE, Rosenberg J, Gögenur I (2013) Prevention of contrast-induced nephropathy in STEMI patients undergoing primary percutaneous coronary intervention: a systematic review. J Interv Cardiol 26: 97-105.
- Heyde GS, Koch KT, de Winter RJ, Dijkgraaf MG, Klees MI, et al. (2007) Randomized trial comparing same-day discharge with overnight hospital stay after percutaneous coronary intervention: results of the Elective PCI in Outpatient Study (EPOS). Circulation 115: 2299-2306.
- Koch KT, Piek JJ, de Winter RJ, David GK, Mulder K, et al. (1997) Short-term (4 hours) observation after elective coronary angioplasty. Am J Cardiol 80: 1591-1594.
- Estévez-Loureiro R, Calviño-Santos R, Vázquez JM, Barge-Caballero E, Salgado-Fernández J, et al. (2009) Safety and feasibility of returning patients early to their originating centers after transfer for primary percutaneous coronary intervention. Rev Esp Cardiol 62: 1356-1364.
Citation: Hoorweg AJ, Grundeken MJD, Van de Hoef TP, Henriques JPS, Peters RJG, et al. (2017) A Consecutive Series of all Subtypes of the Acute Coronary Syndrome Patient Admitted to an Academic Coronary Care Unit. Atheroscler Open Access 2:115.
Copyright: © 2017 Hoorweg AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 3888
- [From(publication date): 0-2017 - Jul 06, 2025]
- Breakdown by view type
- HTML page views: 2957
- PDF downloads: 931