A New Approach to Treating Non-Small Cell Lung Cancer: Direct Thrombin Inhibitors Block the Disease's Spread and Spontaneous Metastasis
Received: 09-Nov-2023 / Manuscript No. IJRDPL-23-119792 / Editor assigned: 13-Nov-2023 / PreQC No. IJRDPL-23-119792 (PQ) / Reviewed: 27-Nov-2023 / QC No. IJRDPL-23-119792 / Revised: 02-May-2025 / Manuscript No. IJRDPL-23-119792 (R) / Published Date: 12-May-2025
Abstract
Two potentially lethal effects of advanced cancer are cancer cachexia and cancer-associated thrombosis. However, there is still much to learn about the expression of thrombin in primary tumor tissues of Non-Small Cell Lung Cancer (NSCLC) and how this may relate to the prognosis of NSCLC patients. A clinical pathological examination was conducted to ascertain the correlation between the advancement of the cancer and thrombin. We assessed the impact of r-hirudin and Direct Thrombin Inhibitor Peptide (DTIP) on the advancement of cancer. Immunohistochemistry, immunofluorescence, and Western blotting were employed to investigate the mechanism of r-hirudin and DTIP inhibition. It was established how well DTIP and chemotherapy worked together therapeutically. The presence of thrombin in NSCLC tissues was strongly associated with clinicopathogenic characteristics and the patients' prognosis. Tumor growth was reduced by thrombin insufficiency. R-Hardin, one of the new thrombin inhibitors and DTIP, respectively, prevented in vitro cell invasion and metastasis. In an orthotropic lung cancer model, they prevented tumour development and metastasis, impeded cell penetration, and extended survival following tumour cell injection through the tail vein. Additionally, they prevented spontaneous metastases and angiogenesis from tumours that were subcutaneously injected. In PAR-1-deficient NSCLC cells, thrombin's stimulation of invasion and metastasis was eliminated. Tissue growth was suppressed by r-hirudin and DTIP via the thrombin-PAR mediated NF-κB and RhoA signalling cascades by suppressing the expression of MMP9 and DTIP prevented chemotherapy-induced resistance in mice and enhanced chemotherapy-induced growth and inhibition of metastasis. Thrombin contributes significantly when combined with PAR-1, to cancerous NSCLC. Anti-tumor therapy may make use of the anticoagulants r-hirudin and DTIP, or a combination of and DTIP prevented cell invasion and in vitro metastasis. In an orthotopic lung cancer model, they slowed the growth and spread of the tumours, prevented cell invasion, and increased the length of survival following tumour cell injection via the tail vein. They also prevented spontaneous metastases from tumours that were subcutaneously injected and angiogenesis. In NSCLC cells lacking PAR-1, thrombin's stimulation of invasion and metastasis was eliminated. Through the thrombin-PAR-pathway, rhirudin and DTIP prevented the growth of tumours. DTIP, two anticoagulants, in conjunction.
Keywords: Combination therapy; Direct thrombin inhibitors; Metastasis; Non-small cell lung cancer; Thrombin
Introduction
Non-Small Cell Lung Cancer (NSCLC) makes up about 85% of all occurrences of lung cancer, and is the primary cause of cancer-related deaths in people worldwide [1]. With a median survival of fewer than 10–12 months, NSCLC has a significant risk of recurrence and metastasis even in cases when the disease is discovered at an operable stage and chemotherapy is used as treatment [2-4]. The majority of patients respond to platinum or gemcitabine-based chemotherapy initially, but they typically relapse and develop chemoresistant disease, with a 5 years survival rate of less than 0.5 percent [5]. Accordingly, focusing on medication resistance and tumour metastasis may be a potential approach to treating NSCLC [6]. It has long been assumed that tumours could exploit the circulatory system. Numerous noteworthy haemostatic irregularities, such as disseminated intravascular coagulation, hemorrhagic episodes, and migrating thrombo-phlebitis, have been reported in cancer patients [7]. According to Joseph et al., haemostatic problems do indeed frequently result in death for cancer patients. The metastatic potential of tumour cells has been clearly demonstrated by prior mouse studies involving the tumour cell-associated tissue factor, circulating prothrombin, and multiple downstream thrombin procoagulant targets (i.e., platelets, fibrinogen, and factor XII) [8-11].
Nonetheless, the involvement of hemostatic variables at the stage of initial tumour formation has been less certain. Thrombin is a complexly structured allosteric enzyme that interacts with different receptors on the surface of vascular and nonvascular cells to produce a range of biological effects. Additionally, it has been demonstrated that thrombin promotes the growth of tumours in both coagulation dependent and independent ways [12]. A significant body of research substantiates the notion that thrombin plays key roles in the development of tumours by mediating angiogenesis, inflammation, and the dissemination of cancer cells metastatically via its receptor PAR-1 [13,14]. A very specific set of serine proteases activates PAR-1, the model member of the PAR family. Strong inflammatory reactions are triggered when thrombin cleaves PAR-1, including the induction ofof cell surface adhesion molecules, hyperpermeability induction, and NF-κB pathway activation [15,16]. However, little is known about the expression of thrombin in the original tumour tissues of NSCLC and how it relates to the prognosis of NSCLC patients.
Chemotherapy is currently a recognised multimodal therapy for Non-Small Cell Lung Cancer (NSCLC), yet its advantages are restricted by a low response rate or developed tumour resistance. According to Queiroz et al., PAR-1 in tumours causes cancer cells to become resistant to chemotherapy. Since thrombin is the archetypal PAR-1 agonist, addressing it in conjunction with standard chemotherapy may be especially beneficial.
Our group created recombinant hirudin (r-hirudin) and direct Thrombin Inhibitor Peptide (DTIP), which are derivatives of wild-type hirudin variant 2 [17,18]. In order to suppress the activity of thrombin, DTIP and r-hirudin bind to exosite I and the apolar region of thrombin, while their N-terminal moiety prevents access to the thrombin active site. Phase I clinical studies for thrombin and r-hirudin have begun, while DTIP is a novel anti-thrombotic drug that may be utilised to prevent thrombosis without increasing the risk of bleeding after subcutaneous injection. Here, we looked at the thrombin protein levels in clinical NSCLC samples and examined the connection between the prognosis of NSCLC patients and the amount of thrombin expression and clinicopathological characteristics. We assessed the effects of DTIP and r-hirudin on the development, spread, and spontaneous metastasis of tumours both in vivo and in vitro. We further showed that the majority of the invasive signal is explained by the presence of PAR-1 and thrombin. The new research presented here suggests that rhirudin and DTIP, which are now used as anticoagulant medications, may have a wider clinical application in the treatment of tumours. Our hypothesis is that r-hirudin plus DTIP would be a novel cancer therapeutic breakthrough.
Materials and Methods
Patients and tissues
After study participants gave their informed agreement, clinical samples of lung cancer patients were obtained from Fudan University Shanghai Cancer Centre (Shanghai, China). Some research used primary non-small cell lung cancers. The tissues included samples from 132 patients (68 men, 64 women, and a median age of 58) who had Non-Small Cell Lung Cancer (NSCLC), of which 100 had Adenocarcinoma (ADC), 23 had Squamous Cell Carcinoma (SCC), and 9 had other forms of NSCLC at various stages. Every tumour sample was accompanied by lung tissues that were in good condition.
Animals
When it came to the care of the animals and the conduct of experimental experiments. Research involving animals or animal tissue is reported in accordance with the British journal of pharmacology's criteria and the ARRIVE guidelines [19]. We acquired male C57/BL6 mice (6–8 weeks, 22 ± 3 g, RRID: IMSR_JAX:0 00664) from Fudan University's animal centre. Each of the five or fewer mice per individually ventilated cage (width 24.5 cm, length 41.5 cm, and depth 18.5 cm) was kept in a controlled environment at 23°C ± 2°C with a 12-hour light/dark cycle and free access. to rat food and water of SPF grade. Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) was the supplier of the food. Before being used, the mice were housed in these circumstances for a week. Every animal study was planned with blinded analyses and randomization to create groups. However, the use of several assays and the unanticipated death of individual animals during the procedures are to blame for the uneven group size.
Real-time quantitative PCR
TRIzol (Invitrogen) was used to isolate total RNA from cells in accordance with the manufacturer's instructions. The following primers were used for q-PCR using the SYBR Green PCR master kit (Invitrogen). Thrombin: forward 50-TTGTCCTC CAGCGACTTCT-30 and reverse 50-GGGTACTGCGACCTCAACTA-30; GAPDH: forward 50-TGAGCTTGACAAAGTGGTCG-30 and reverse 50- GGCTCTCCAGAACATCATCC-30.
Assay for wound healing
Culture-Inserts (ibidi GmbH, Martinsried, Germany) were used to investigate wound healing. Following the separation of the cells from the plates using 0.05% (w/v) trypsin, A549 (RRID: CVCL_0023), 95D (RRID: CVCL_7110), and Lewis cells (RRID: CVCL_4358) were seeded onto culture-insert plates at a density of 2 × 104 cells per culture well. Culture inserts were taken out after the incubation period of 24 hours. Using a light microscope, pictures of the cells' migration into the scratch region were captured after a full day.
Migration of cells
Transwell inserts (Corning Life Sciences, Tewksbury, MA) were used in accordance with the manufacturer's instructions to assess cell migration. In short, 50 μl was coated on Transwell chambers (8 mm pore size). of Matrigel in dilution. 1.5 × 105 cells/ml−1 of serum-free medium were planted (0.1 ml) in the upper chambers. The bottom chambers were then filled with 0.5 mL of medium containing 10% FBS, PBS, 10 nmol/L−1 thrombin, and 25 nmol/L−150 nmol/L−1 DTIP, 10 nmol/L−1 thrombin+25 nmol/L−1 r-hirudinIn the top and lower chambers, r-hirudin, 10 nmol•L−1 thrombin, and 50 nmol•L−1 DTIP were added. Following a 24 hours’ culture, the cells were dyed with 0.1% crystal violet and fixed in methanol. We counted the cells on the bottom of the filters.
Assay for Factin staining as well as confocal microscope
Cells were cultured on 35 mm glass-bottom dishes, fixed for 15 minutes with 4% paraformaldehyde, and permeabilized for 10 minutes with 0.5% Triton X-100 in PBS. After that, the cells were blocked for 20 minutes using 1% BSA. After 30 minutes of YF-488 phalloidin (US Everbright Inc.) incubation, cells were stained for 5 minutes with DAPI. A confocal microscope was used to view the cells.
Determining the RhoA activation
With commercially available kits, RhoA activations (RhoA-GTP) were determined and tracked. In short, a cell lysis tool was used to lyse cells.
Buffer that the producer supplied. To quantify total RhoA by western blotting, half of the lysates were kept. The leftover sample was subjected to a 1 hour incubation period at 4°C with rotation containing 20 μg of GST fusion protein RBD (Rhotekin Rho-binding domain), which was attached to coloured glutathione-sepharose beads. The active GTPbound form of RhoA is the only form to which the RBD protein motif binds. Proteins were separated on 12% SDS-PAGE, transferred to a PVDF membrane, and then Western blotting was performed using an anti-RhoA monoclonal antibody in accordance with the manufacturer's instructions after the beads had been cleaned and resuspended in loading buffer.
Assay for tube formation
50 μl of liquid Matrigel was precoated onto each well of ninety-six well plates. Following a one-hour incubation period at 37°C, HUVESs (RRID: CVCL_2959, 1.5 by 104. cells/well) suspended in DMEM supplemented with PBS. The following were seeded and cultured for eight hours: 10 nmol•L−1 thrombin, 25 nmol•L−1 r-hirudin, 50 nmol•L −1 DTIP, 10 nmol•L−1 thrombin+25 nmol•L−1 r-hirudin, and 10 nmol•L−1 thrombin+50 nmol•L−1 DTIP.
Lentivirus infection and gRNA design
LentiCRISPRv2 was created by cloning human and mouse sequences with CRSIPR guides aimed at thrombin and PAR-1 at BsmBI restriction sites. These were the oligo sequences displayed. mouse thrombin: forward 50-GGTAGCGGCTCCGCCACAGC-30 and reverse 50-AGCTGTGG CGGAGCCGCTACC-30; human thrombin: forward 50-GAGGACCTTTGGCTCGGGAG-30 and reverse 50-CTCCCGAGCCAAAGGTCCTC-30; PAR-1: 50- GAAGGTC AAGAAGCCGGCGG-30 in forward direction and 50- CCGCCGGCTTCTTGACC TTC-30 in reverse; mouse Forward 50- GCGACGATCAGCAAGCGCCG-30 to PAR-1 and 50- CGGCGCTTGCTGATCGTCGC-30 in reverse. 293T cells (RRID: CVCL_0063) were transfected with LentiCRISPRv2 and packing constructs. 48 hours after the virus was transfected, supernatants were collected. Lewis Lung Cancer and A549 (LLC) cells were chosen in growth media containing puromycin after being infected with viral supernatants in the presence of polybrene. Karyotyping has been performed to evaluate and validate each of the cell lines that were utilised. The empty plasmid was transfected, and this was utilised as a negative command (NC). −/− A549THR, LLCA549PAR-1−/−, LLC, and Thr−/−Cell Par-1−/− lines were examined using sequencing and PCR.
Immunostained specimen scoring
Using a multihead microscope, two researchers who were blind to the clinical status of the patients examined immunostained materials. The level of protein staining was rated by consensus on a scale from 0 to 3 (0=negative staining; 1=faintly positive; 2=moderately positive; 3=strongly positive) in order to assess protein expression using Immunohistochemistry (IHC). The number of cells that were positive.
Ranked by agreement on a 0–4 scale (0=less than 1%; 1=1%)
10% to 20%; 10% to 50% in 2; 50% to 80% in 3; and > 80% in 4. Staining intensity and positive cells were used to calculate the Immunohistochemistry Scores (HIS). The score of thrombin or PAR-1 was defined as positive (thrombin+ >2) based on the cut-off value of the central positive staining area in the tumour segment and negative (thrombin ≤ 2) and high production of PAR-1 >6) low expression of PAR-1.
Injections into tail veins
The IACUC's guidelines and procedures were followed for conducting this animal study at Fudan University's Department of Laboratory Animal Science in Shanghai, China. Following collection, LLC cells were resuspended in medium without serum. 1 × 106 cells in 0.1 ml were injected into C57/BL6 mice via the tail vein. The mice were split up into several groups at random. PET scanning was performed on the animals 14 days after the cells were injected. After administering an overdose of pentobarbital sodium to kill the mice, all of the lungs were removed and examined further using fluorescence stereomicroscopy. The lungs were then preserved in Bouin's solution, and the number of metastatic nodules on the surface of the lungs was tallied.
Subcutaneous tumour inoculation in mice
The IACUC's guidelines and procedures were followed for conducting this animal study at Fudan University's Department of Laboratory Animal Science in Shanghai, China. Subcutaneous injections of 0.1 ml serum-free medium containing 1 × 106 tumour cells were made. The mice's right flank. The mice were randomised into distinct groups and their cancer progression was tracked daily. Three times a week, a tumour that was clearly visible was clipped, and its volume was calculated using the formula V=(LW2)/2, in which L is the longest diameter and W is the shortest. Pentobarbital sodium overdose was used to kill the mice, and the tumours were excised. While the remaining tumours were lysed for western blotting, some were fixed in 4% paraformaldehyde.
Mouse orthotopic lung cancer model
After receiving an intraperitoneal injection of 300 mg kg−1 of chloral hydrate, C57/BL6 mice were put in the correct lateral decubitus position. One × 106 cells in total 50 μl of Matrigel and 50 μl of RPMI-1640 media were injected into the left via the left rib cage to the lung parenchyma. Mice were observed every day after being split into several groups at random. An overdose of pentobarbital sodium was used to kill the mice, and the lung tissues were extracted.
Data and statistical analysis
For all experiments, plates were prepared so that all conditions were randomly present in each plate. Organoid counts and measures were performed by an observer unaware of the treatments.
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology. In all analysis, the number of independent experiments was at least five (exact numbers are provided in the figure legends). All data are expressed as mean ± SD. All the data including the out liers were included in data analysis and presentation. Unless otherwise stated, differences between the two groups were analysed by unpaired t test when variances are equal, and one-way ANOVA was used for multiple comparisons with Prism 6 (GraphPad Inc.). In multigroup studies with parametric variables, post hoc tests were conducted only if F values had achieved the significance level of P<.05, and there was no significant variance inhomogeneity. Values of P<.05 were considered statistically significant.
Results
Outcomes
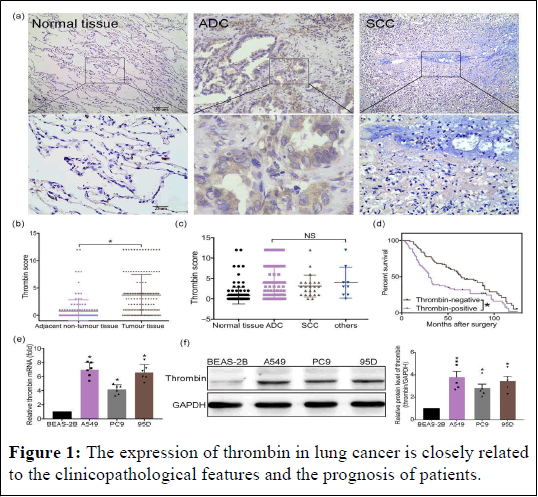
The clinicopathological characteristics and patient prognosis in non-small cell lung cancer are strongly correlated with thrombin expression. It has also been demonstrated that thrombin aids in the growth of tumours. Nevertheless, there hasn't been any information published regarding the expression of thrombin in NSCLC tissues or the connection between thrombin expression and clinicopathological characteristics as well as patient prognosis. In order to verify whether thrombin (prothrombin) is present in NSCLC, Analysis was done on 132 patients whose diagnosis of NSCLC was confirmed pathologically. When compared to the surrounding non-tumor lung tissues, we discovered that the expression of thrombin was significantly higher in tumours of all various types of NSCLC tissues. The various NSCLC subtypes did not significantly differ from one another. To assess the predictive significance of Univariate and multivariate analyses were carried out using the clinicopathological feature for NSCLC patients' hrombin. The substantial correlation between thrombin expression in NSCLC tumour tissue and the Tumour, Nodes, and Metastasis (TNM) stage of NSCLC. Compared to thrombin-negative patients, thrombin-positive patients had substantially worse 5 years overall survival rates. Furthermore, as shown by q-PCR and western blot, the mRNA and protein levels in three NSCLC cell lines were significantly higher than those in the normal lung cell line (BEAS-2B) (Figure 1).
Figure 1: The expression of thrombin in lung cancer is closely related to the clinicopathological features and the prognosis of patients.
Note: (a–c) Thrombin expression in NSCLC patients. (a) Paraffin sections obtained from patients with resectable NSCLC tissues were stained for thrombin (n=132 per group). (b) Score of thrombin expression in adjacent non-tumour lung tissue and in NSCLCs. (c) Thrombin expression in different types of NSCLC. (d) The overall survival rates of thrombin-positive patients and thrombin-negative patients. (e) The mRNA level of thrombin in BEAS-2B, A549, 95D, and PC9 cells was determined by q-PCR (n=6 per group). (f) Theexpression of thrombin in BEAS-2B, A549, 95D, and PC9 was determined by western blotting analysis (n=5 per group). Right: summary data of western blotting is shown. Data shown are means ± SD. *P<.05, significantly different as indicated; in (e, f), significantly different from BEAS-2B cells; NS, not significant.
Thrombin is crucial to the development of lung cancer
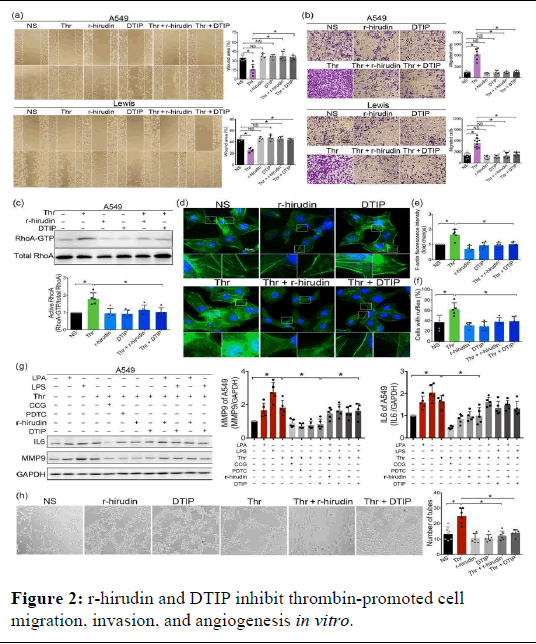
We cultivated A549THR−/− and LLCThr−/− cells to evaluate the function of thrombin in lung cancer cells inadequate cohort. A deficiency in thrombin could significantly extend the survival duration of mice in an orthotopic lung cancer model. Furthermore, animals with a high burden of lung tumours were produced by control LLC cells. Notably, a lower burden of lung tumours was caused by thrombin deficiency lung slice H and E staining further showed that the control group's tumour area was greater than that of the thrombin-deficient group's In mice, thrombin deficiency also prevented liver metastases. In conjunction with the in vitro investigations, we deduced that thrombin is crucial to the advancement of lung cancer (Figure 2).
Figure 2: r-hirudin and DTIP inhibit thrombin-promoted cell migration, invasion, and angiogenesis in vitro.
Note: (a) A549 (n=6 per group) and Lewis (n=5 per group) cells were pretreated with PBS, 10 nmol·L−1 thrombin, 25 nmol·L−1 r-hirudin, 50 nmol·L−1 DTIP, 10 nmol·L−1 thrombin+25 nmol·L−1 r-hirudin, and 10 nmol·L−1 thrombin+50 nmol·L−1 DTIP for 24 h. The migration of rhirudin and DTIP-treated A549 and Lewis cells were assessed using wound healing assay. Right: Quantitative analysis of wound area. (b) Transwell assay was performed to assess cell migration of A549 (n=6 per group) and Lewis (n=10 per group) cells. Right: Quantitative analysis of invasive cells. (c) Endogenous GTP-bound form of RhoA was enriched by a pull-down assay and detected by western blotting.
Total RhoA was detected using anti-RhoA antibody. Lower panel: Summary data of Western blotting (n=5). (d) Representative images of each group stained with phalloidin in A549 cells. (e) Quantification of the F-actin fluorescence intensity. (f) Percentages of ruffle-positive cells in different groups were calculated based on the immunofluorescence. (g) Expression of IL-6 and MMP9 in different groups. Right: Summary data of Western blotting of expression of MMP9 and IL-6 in A549 cells (n=5 per group). (h) Effect of r-hirudin and DTIP on HUVEC tube formation on Matrigel. Left: Representative photographs of five independent experiments were shown. Right: Quantification of the inhibitory activity of DTIP on tube formation (n=6 per group). Data shown are means ± SD. *P<.05, significantly different as indicated; NS: Not Significant.
lamellipodia in thrombin-stimulated NSCLC cells, which consequently lead to decreased cell motility and migration ability. In previous studies, we found thrombin deficiency could reduce the expression of MMP9 and IL-6. Furthermore, the expression of MMP9 and IL-6 also could be increased by exogenous thrombin, which could be inhibited by r-hirudin and DTIP. RhoA could be activated by thrombin. CCG, an inhibitor of RhoA, could inhibit thrombin-induced expression of MMP9 and IL-6. LPA, an activator of RhoA, could prevent the inhibition induced by r-hirudin and DTIP. Thrombin has been reported to activate NF-κB signalling in human pleural mesothelial cells. The effect of r-hirudin and DTIP on the NF-κB pathway in thrombin-stimulated NSCLC cells was analysed. The results indicated that thrombin could activate NF-κB signalling in NSCLC cells. Compared with the thrombin-treated group, r-hirudin and DTIP exhibited diminished IκBα and p65 phosphorylation, suggesting that r-hirudin and DTIP can inhibit thrombin-induced NF- κB activation. CCG could inhibit the thrombin-induced NF-κB activation, whereas RhoA and NF-κB activators (LPA and LPS) could prevent the inhibition induced by r-hirudin and DTIP. The results indicated that thrombin could activate NF-κB signalling via RhoA in NSCLC cells.
Studies have shown that NF-κB pathway activation upregulates the expression of cell adhesion molecules and inflammatory cytokines. We also found that RhoA and NF-κB inhibitor could inhibit thrombin induced expression of MMP9 and IL-6 and RhoA and NF-κB activator could prevent the inhibition induced by r-hirudin and DTIP, suggesting that thrombin can regulate the expression of MMP9 and IL-6 via RhoA and NF-κB pathway. Thrombin is known to promote the release of VEGF and induce angiogenesis. Hence, we examined the effects of rhirudin and DTIP on angiogenesis using a tube formation assay. After thrombin treatment, tubule formation was increased and r-hirudin and DTIP significantly inhibited thrombin-induced tube formation. These results demonstrated that r-hirudin and DTIP exhibited an antiangiogenic potential. r-hirudin and DTIP exert anti-invasive and antimetastatic abilities in a mouse lung cancer model.
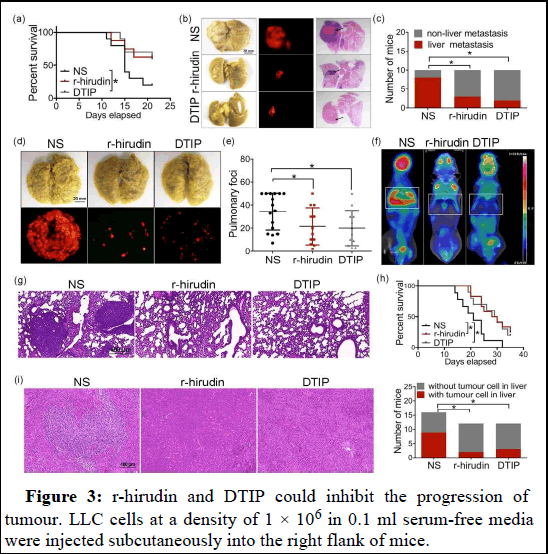
Our results so far suggested anti-metastatic and anti-angiogenic activity of r-hirudin and DTIP in vitro. We further confirmed the effects in vivo. In the orthotopic lung tumour model, DTIP improved the survival time of mice compared with the control group, and mice in r-hirudin or DTIPtreated groups had smaller tumour burden and had fewer mice with liver metastases. Murine models of experimental metastasis have been used frequently to investigate the effects of anti-haemostatic agents on cancer metastasis. Although such artificial models do not encompass the entire metastatic process, they remain useful for “proof-of-concept” experiments, focusing on the haematogenous phase of tumour dissemination [20]. Gross examination of the lungs harvested from r-hirudin or DTIP-treated mice revealed a median of 21 or 20 surface pulmonary metastases per animal. In contrast, the lungs harvested from normal saline-treated mice had confluent metastases that were too numerous to count and were clearly enlarged. Micro-PET scan and histological analyses revealed scattered small foci of tumour tissue within the lungs harvested from r-hirudin or DTIP-treated mice, whereas the lungs harvested from normal salinetreated mice were nearly completely effaced by tumour tissue. We found that the number of mice with tumour cells colonized in the liver was largely reduced in the r-hirudin and DTIP group compared with the normal saline group. It is important to note that 78% of control mice were dead at 24 days, with all dead at 32 days, whereas 25% of r-hirudintreated mice and 30% of DTIP-treated mice were dead at 24 days. We also examined spontaneous metastasis through subcutaneous inoculation of tumour cells in mice, which involves a more comprehensive process. Treatment of r-hirudin and DTIP for 1 week inhibited tumour growth slightly. Six of the nine tumours analysed from normal saline-treated mice showed signs of panniculus invasion, whereas only two of nine tumours from r-hirudin-treated mice and two of 10 tumours from DTIPtreated mice had any noticeable signs of panniculus invasion. We also administered 1.0 mg·kg−1 DTIP or 0.5 mg·kg−1 r-hirudin for 21 consecutive days after 1 week of the injection of LLC cells. r-hirudin and DTIP significantly inhibited tumour growth. The number of mice with lung and liver metastases was largely reduced in r-hirudin or DTIPtreated groups. Tumour angiogenesis was assessed using IHC analysis for CD31. The r-hirudin or DTIP-treated groups showed a significant reduction of CD31-positive microvessels compared with controls. We performed immunohistochemical analysis on tumour samples to determine the expression levels of MMP9 and IL-6. There was decreased expression of MMP9 and IL-6 after treatment with r-hirudin and DTIP, compared with normal saline-treated mice. We also examined phosphorylation levels of p65 and the key downstream signalling molecules in tumour tissue. We observed a marked inhibition of phospho-p65, phospho-ERK, phospho-STAT3 and phospho-Akt levels in the r-hirudin and DTIP-treated groups. Furthermore, we did not find increased bleeding after administration of DTIP, but slight subcutaneous haemorrhage was observed after r-hirudin administration for 3 weeks continuously (data are not shown). These results show that DTIP, a direct thrombin inhibitor, could be extended to anti-cancer therapy (Figure 3).
Figure 3: r-hirudin and DTIP could inhibit the progression of tumour. LLC cells at a density of 1 × 106 in 0.1 ml serum-free media were injected subcutaneously into the right flank of mice.
Note: (a–d) Mice that were injected with LLC cells were immediately treated with normal saline (NS), 0.5 mg.kg−1 r-hirudin or 1.0 mg.kg−1 DTIP for 1 week. (a) The curve of tumour growth after injection of LLC cells (NS n=9; r-hirudin n=9; DTIP n=10). (b) In vivo imaging of each group at 4 weeks after cell injection. (c) H and E-stained sections of tumour. (d) Number of saline, r-hirudin-and DTIP-treated mice, with panniculus invasion. (e–n) After 1 week of the injection of LLC cells, mice were treated with normal saline, 1.0 mg.kg−1 DTIP or 0.5 mg.kg−1 r-hirudin for 21 consecutive days. (e) The curve of tumour growth after injection of LLC cells (NS n=10; r-hirudin n=9; DTIP n=10). (f) Weight of resected tumours from normal saline, r-hirudin and DTIP-treated groups. (g) In vivo imaging of each group at 45 days after cell injection. (h) Representative photograph of metastatic nodules on lungs (upper). Gross appearance of representative lung harvested from different groups viewed under a fluorescence stereoscope (bottom). (i) Number of NS, rhirudin and DTIP-treated mice with lung metastasis. (j) Representative photograph of metastatic nodules on livers (upper). Gross appearance of representative single liver lobes harvested from different groups viewed under a fluorescence stereoscope (bottom). (k) Number of NS, r-hirudin, and DTIP-treated mice with liver metastasis. (l) Effect of r-hirudin and DTIP against primary tumor angiogenesis. Left: A typical photograph of immunohistochemical staining for CD31. Right: The histogram represents the number of microvessels. (m) Immunohistochemical analysis was performed on tumour samples to determine the expression of MMP9 and IL-6 on tumours from NS, r-hirudin and DTIP-treated mice. (n) Inhibition of phosphorylation of p65, ERK, STAT3, and Akt in tumours by r-hirudin and DTIP. Right: Summary data of Western blotting. Data shown are means ± SD. *P<.05, significantly different from saline (NS).
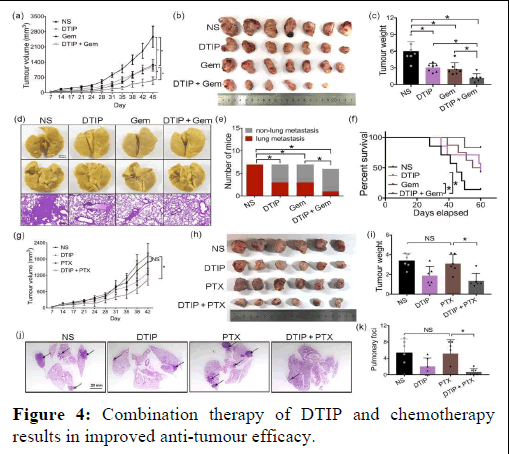
PAR-1 is a major determinant in thrombin-promoted metastases of lung cancer: Thrombin is the main activator of PAR-1. Overexpression of PAR-1 has been detected in various types of cancers, including ovarian, breast cancer (Figure 4).
Figure 4: Combination therapy of DTIP and chemotherapy results in improved anti-tumour efficacy.
Note: (a–f) 1 × 106 LLC cells were subcutaneously injected in the right dorsal region of mice. One week after LLC inoculation, the mice were randomly divided into four groups: Normal Saline (NS), DTIP (1 mg·kg−1 per day, s.c.), gemcitabine (Gem, 120 mg·kg−1 once a week; i.p.) and a combination of DTIP with gemcitabine. The mice were treated with normal saline or DTIP for 21 consecutive days and gemcitabine for 4 consecutive weeks (NS, DTIP, Gem n=7; DTIP +Gem n=6). (a) The curve of tumour growth after injection of LLC cells. (b) Mice were humanely killed and the tumours were resected at 45 days after cell injection. (c) Weight of resected tumours was determined in different groups. (d) Representative photograph of metastatic nodules on lungs (upper). H and E-stained sections of representative single lung lobes harvested (bottom). (e) Number of mice with liver metastasis. (f) Survival of mice in different treatment groups. (g–k) 1 × 106 LLC cells were subcutaneously injected in the right dorsal region of mice. One week after LLC inoculation, the mice were randomly divided into four groups: Normal saline, DTIP (1 mg·kg−1 per day, s.c.), paclitaxel (20 mg·kg−1 every two days; i.p.) and combination DTIP with paclitaxel treatment groups, and then the mice were administered normal saline or DTIP for 21 consecutive days and paclitaxel (PTX) for 4 consecutive weeks (n=6 per group). (g) The curve of tumour growth after injection of LLC cells. (h) Mice were humanely killed and the tumours were resected at 42 days after cell injection. (i) Weight of resected tumours was determined in different groups. (j) H and E-stained sections of representative single lung lobes harvested. (k) The number of pulmonary foci in lungs. All the results were expressed as mean ± SD. *P<.05, significantly different as indicated; NS, not significant.
After deleting PAR-1, the activation of RhoA was inhibited, and the ability of thrombin to activate RhoA was also inhibited. Similar responses were also observed via immunofluorescence staining of Factin, as PAR-1 deletion decreased the formation of membrane ruffles. These data suggest that thrombin enhanced cell motility and migration can be completely inhibited by PAR-1 deletion, in vitro. PAR-1 deficiency exhibited diminished IκBα phosphorylation and p65 phosphorylation. Importantly, thrombin-driven NF-κB activation was inhibited by pretreatment with the specific PAR-1 inhibitor ML161.
LPA or LPS could rescue th activation of NF-κB, but thrombin could not. r-hirudin and DTIP could not inhibit NF-κB activation induced by LPA or LPS, suggesting that r-hirudin and DTIP inhibit thrombininduced RhoA and NF-κB activation via PAR-1 signalling. We also found PAR-1 deficiency led to decreased expression of MMP9 and IL-6. Besides thrombin, LPA or LPS could increase MMP9 and IL-6 expression in PAR-1-deficient cells. r-hirudin and DTIP could not inhibit MMP9 and IL-6 expression induced by LPA or LPS, suggesting that r-hirudin and DTIP inhibit thrombin-induced MMP9 and IL-6 expression through RhoA and NF-κB activation via PAR-1 signalling. To further analyse the effects of PAR-1 on lung cancer growth and metastasis, we established lung cancer model in mice using LLC cells infected by gRNA-PAR-1 lentivirus (Par-1−/− group) or LV-NC (vehicle group). In orthotopic lung tumour model, PAR-1 deficiency markedly increased the survival rate and inhibited tumour growth in lung. In subcutaneous tumours, the PAR-1-deficient group was significantly smaller than control group in addition, based on the results of CD31 staining, we confirmed that CD31-positive microvessels decreased in the PAR-1-deficient tumours.
In the metastatic colonization model, our results confirmed that PAR-1-deficient lung cancer cells lead to less lung metastatic nodes than control cells, as well as significantly decreased signal intensity in the lungs, as seen on micro-PET scans. In addition, PAR-1 deficiency could markedly increase the survival rate. Together with the in vitro experiments, we concluded that PAR-1 plays an important role in the thrombin induced progression of lung.
DTIP potentiates chemotherapy-induced growth and metastasis inhibition and inhibits chemotherapeutic drug tolerance of NSCLC in mice
Chemotherapy is been commonly prescribed in the treatment of patients with NSCLC; however, its benefits are limited due to a low response rate or acquired tumour resistance. Queiroz et al. have shown that the presence of PAR-1 in tumours induces resistance to chemotherapy in the cancer cells. Meanwhile, chemotherapeutic agents such as gemcitabine, cisplatin and paclitaxel are associated with a significant increase in the risk of arterial thromboembolic events. We hypothesized that DTIP could potentiate chemotherapy-induced inhibition of tumour progression.
When combining DTIP and gemcitabine, the tumour volume and tumour weight in the combination treatment group were significantly smaller than that in the groups administered DTIP alone or gemcitabine alone. The number of mice with lung metastases was clearly reduced in the combination treatment group. We also measured the survival of mice in different groups. It is important to note that 85% of control mice (untreated and bearing tumours) were dead by 60 days, 57% of DTIP-treated mice and 50% of gemcitabine-treated mice were dead at 60 days and only 16% in the combination-treated group were dead at 60 days. Paclitaxel did not significantly inhibit the growth of LLC in vivo. However, when combined with DTIP, the growth and metastasis of LLC were significantly inhibited. Combination of DTIP and cisplatin had a smaller tumour volume, but there was no significant difference in metastasis and survival of mice, compared with the group given cisplatin alone. In addition, we evaluated the chemotherapy effects in thrombin-deficient NSCLC mouse models. We found that gemcitabine or paclitaxel-treated mice in the thrombin-deficient group had smaller tumours and longer survival time compared with control group treated with gemcitabine or paclitaxel. These results indicated that therapy with a combination of DTIP and chemotherapy might achieve a better therapeutic effect.
Discussion
Metastasis has been recognized as the main cause of fatal outcomes in lung cancer patients. It is of interest that low-grade intravascular coagulation has been observed in most patients with solid tumours. Malignant tumours often exhibit hypercoagulability, which is correlated with high levels of activated thrombin. Studies have shown that exogenous thrombin is capable of enhancing tumour adhesion to platelets, endothelial cells, and fibronectin in vitro and revealed that exogenous thrombin promotes tumour growth. In this study, we demonstrated that the level of thrombin expression in tumour tissues was significantly correlated with the metastatic potential of NSCLC, post-operative tumour recurrence and poor prognosis in NSCLC patients. This finding is supported by the fact that a high thrombin expression was significantly associated with the aggressive histopathological characteristics of NSCLC, such as high TNM stage. Thrombin deficiency impaired tumour progression. This indicates that thrombin may serve as an independent predictor for tumour recurrence and prognosis of NSCLC patients, which might be an explanation as to why malignant tumours often exhibit hypercoagulability.
To further explore the role of thrombin in lung cancer, we used exogenous thrombin to treat NSCLC cell lines in vitro. We found that 10 nmol·L−1 thrombin increased NSCLC cell invasion, angiogenesis and metastasis in vitro. This led to the suggestion that cancer cells.
These findings demonstrated that DTIP and r-hirudin could prevent thrombin-induced angiogenesis, metastasis, and cell invasion in vitro. In mice with NSCLC, r-hirudin and DTIP prevented the disease's spread and spontaneous metastasis, extending the mice's survival period in lung cancer models. Anticoagulants, however, cause bleeding issues that may shorten the survival of non-responders, hiding any potential advantages that may exist for a small percentage of patients. Additionally, we found a small amount of subcutaneous haemorrhage following the treatment of r-hirudin for three weeks straight, but no enhanced bleeding following the administration of DTIP in our investigations. These findings demonstrated that DTIP may be included in anti-cancer treatment and that thrombin is a therapeutic target for NSCLC.
The thrombin receptor PAR-1 is cleaved by According to McLaughlin et al. thrombin reveals a novel N-terminus that attaches to the receptor to cause trans-membrane signalling. Malignant invasive cancer cell lines have been shown to overexpress PAR-1. According to our current results, there was no discernible variation in PAR-1 expression levels between human NSCLC tissues depending on subtype or clinical stage. In an effort to learn more about the function of thrombin in PAR-1-mediated NSCLC metastasis, we treated PAR-1-deficient NSCLC cells in vitro with exogenous thrombin and discovered that both in vitro and in vivo, the invasion and metastasis of PAR-1-deficient cell lines were suppressed. Exogenous thrombin, however, had little effect on their capacity for invasion and metastasis. These findings demonstrate that the thrombin and PAR-1 levels Patients should be categorised using expression.
In this investigation, we demonstrated that thrombin boosted RhoA activation via PAR-1 activation, resulting in cytoskeleton rearrangements, actin stress fibre production, and NF-κB activation. When cytosolic NF-κB is activated, MMP9 and IL-6, an inflammatory marker, are expressed. In order to prevent RhoA and NF-κB activation and reduce the expression of MMP9 and IL-6 in NSCLC cells, rhirudin and DTIP can specifically bind to thrombin and block the binding of thrombin to PAR1. This would be extremely helpful in suppressing metastasis. One of the main adverse effects of chemotherapy for Non-Small Cell Lung Cancer (NSCLC) is vascular problems, such as venous or arterial thromboembolic events. The addition of LMWH to conventional gemcitabine/cisplatin chemotherapy dramatically increased patient survival in a nonrandomized research. Patients pancreatic cancer that is either metastatically or locally progressed. Patients treated with LMWH had an almost 60% overall response rate, while those treated simply with chemotherapy had a mere 12%. Our research indicates that DTIP plus chemotherapy improves anti-tumor activity, and that DTIP may enhance gemcitabine-induced suppression of lung cancer and prevent mice from developing paclitaxel resistance. This strategy deserves greater testing in larger-scale research and with patients with NSCLC.
Conclusion
This study has produced compelling evidence that thrombin is essential for the progression of Non-Small Cell Lung Cancer (NSCLC) as well as an explanation for the observation of intravascular coagulation and thrombosis in the majority of patients with solid tumours. We draw the conclusion that thrombin has a significant function in increasing MMP9 and IL-6 production, which in turn triggers RhoA and NF-κB signalling cascades that drive PAR-1 mediated NSCLC invasion and metastasis. Additionally, DTIP, a highly selective and powerful thrombin inhibitor suppresses the tumor's natural tendency to spread throughout the body and invade important organs in non-small cell lung cancer. We recommend that DTIP treatment be initiated as soon as possible following diagnosis (before to the development of a large tumour) and should be administered in addition to chemotherapy.
References
- Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, et al. (2018) Global, regional, and national Disability-Adjusted Life-Years (DALYs) for 359 diseases and injuries and Healthy Life Expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1859–1922.
- Mlak R, Krawczyk P, Ciesielka M, Koziol P, Homa I, et al. (2016) The relationship between RRM1 gene polymorphisms and effectiveness of gemcitabine-based first-line chemotherapy in advanced NSCLC patient. Clin Transl Oncol 18: 915–924.
[Crossref] [Google Scholar] [PubMed]
- Alvarez MP, Westeel V, Cortes‐Jofre M, Cosp XB (2013) Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev CD001990.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Cai XJ, Chen LY, Cheng B, Shi L, et al. (2018) Factors potentially associated with gemcitabine-based chemotherapy-induced thrombocytopenia in Chinese patients with nonsmall cell lung cancer. J Cancer Res Ther 14: S656–S660.
[Crossref] [Google Scholar] [PubMed]
- Morabito A, Carillio G, Daniele G, Piccirillo MC, Montanino A, et al. (2014) Treatment of small cell lung cancer. Crit Rev Oncol Hematol 91: 257–270.
[Crossref] [Google Scholar] [PubMed]
- Vyse S, Huang PH (2019) Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 4: 1–10.
[Crossref] [Google Scholar] [PubMed]
- Soff G (2019) Thrombosis and hemostasis in cancer. Scope of the prob lem and overview. Cancer Treat Res 179: 1–9.
[Crossref] [Google Scholar] [PubMed]
- Yokota N, Zarpellon A, Chakrabarty S, Bogdanov VY, Gruber A, et al. (2014) Contributions of thrombin targets to tissue factor dependent metastasis in hyperthrombotic mice. J Thromb Haemost 12: 71–81.
[Crossref] [Google Scholar] [PubMed]
- Joseph S, Palumbo KWK, Drew AF, Grimes TS, Kiser JH, et al. (2000) Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96: 3302–3309.
[Google Scholar] [PubMed]
- Plantureux L, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C (2018) Effects of platelets on cancer progression. Thromb Res 164: S40-S47.
[Crossref] [Google Scholar] [PubMed]
- Yokoyama Y, Mori S, Matsuura N (2008) Effect of platelet derived growth factor on development and progression of breast cancer. Tumor Biol 29: 77–77.
- Xue YH, Zhang XF, Dong QZ, Sun JA, Dai C, et al. (2010) Thrombin is a therapeutic target for metastatic osteopontin-positive hepatocellular carcinoma. Hepatology 52: 2012–2022.
[Crossref] [Google Scholar] [PubMed]
- Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, et al. (2014) Anticoagulation inhibits tumor cell-medi ated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood 123: 101–112.
[Crossref] [Google Scholar] [PubMed]
- Reddel CJ, Allen JD, Ehteda A, Taylor R, Chen VM, et al. (2017) Increased thrombin generation in a mouse model of cancer cachexia is partially interleukin-6 dependent. J Thromb Haemost 15: 477–486.
[Crossref] [Google Scholar] [PubMed]
- Feistritzer C, Riewald M (2005) Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 cross-activation. Blood 105: 3178–3184.
[Crossref] [Google Scholar] [PubMed]
- Jeffers A, Owens S, Koenig K, Quaid B, Pendurthi UR, et al. (2015) Thrombin down-regulates tissue factor pathway inhibitor expression in a PI3K/nuclear factor-κB-dependent manner in human pleural mesothelial cells. Am J Respir Cell Mol Biol 52: 674–682.
[Crossref] [Google Scholar] [PubMed]
- Mo W, Zhang YL, Chen HS, Wang LS, Song HY (2009) A novel hirudin derivative characterized with anti-platelet aggregations and thrombin inhibition. J Thromb Thrombolysis 28: 230–237.
[Crossref] [Google Scholar] [PubMed]
- Zhao B, Zhang Y, Huang Y, Yu J, Li Y, et al. (2017) A novel hirudin derivative inhibiting thrombin without bleeding for subcutaneous injection. Thromb Haemost 117: 44–56.
[Crossref] [Google Scholar] [PubMed]
- Lilley E, Stanford SC, Kendall DE, Alexander SPH, Cirino G, et al. (2020) ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. Br J Pharmacol 177: 3611–3616.
[Crossref] [Google Scholar] [PubMed]
- Sjoberg E, Meyrath M, Milde L, Herrera M, Lovrot J, et al. (2019) A novel ACKR2-dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res 25: 3702–3717.
[Crossref] [Google Scholar] [PubMed]
Citation: Sonwani HP, Sinha A (2025) A New Approach to Treating Non-small Cell Lung Cancer: Direct Thrombin Inhibitors Block the disease's Spread and Spontaneous Metastasis. Int J Res Dev Pharm L Sci 11: 273
Copyright: © 2025 Sonwani HP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 367
- [From(publication date): 0-0 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 276
- PDF downloads: 91