A New Method for the Screening of Ureolytic Bacteria Inducing Calcium Carbonate Precipitation
Received: 16-Jul-2016 / Accepted Date: 12-Oct-2016 / Published Date: 19-Oct-2016 DOI: 10.4172/2155-952X.1000248
Abstract
At present several studies are evaluating biological calcite precipitation as it has laid the concept of bioconcrete as a self-healing material which may be a green and more sustainable component for future architecture. This paper reports a 96 wells plate based rapid and easy method for the screening of urea hydrolyzing/calcite-precipitating bacteria. Arsenazo III was used as a colorimetric indicator in 96 wells plate assay. This method requires small amount of reagents and therefore was easy to manage. In this rapid, economic and reliable method that we have developed, Paenibacillus, a facultative anaerobe and Lysinibacillus, were screened as the most efficient bacteria in calcium carbonate precipitation among isolated urea hydrolyzing microbes or bacterial samples from the soil collected from Uttarakhand, India.
Keywords: Bacterial calcification; Calcium carbonate; Screening method; Urease
249661Introduction
“Microbial mineralization” of various elements in the form of carbonates, oxides, phosphate, sulfides, silicates, etc. is one of such processes which holds promising future prospects and is a major factor in the global cycling of various metals [1]. Some may participate directly in an extra or intracellular transformation of metals. Biomineralization of calcium in the form of carbonates finds wide range of scientific and technical applications. It offers an opportunity for developing a technique for remediationof heavy metals and radio nuclides contaminated water as well as lays down the concept of “Bioconcrete” [2]. These calcifying microorganisms more precisely bacteria are recently receiving more attention as they may be utilized for the healing of minor cracks in historical monuments, old buildings or can be used to develop self-healing bio-concrete [3,4]. As several factors like strain type, media composition, temperature, pH, surrounding chemical environment and many other physicochemical co-factors influence the bio-precipitation of calcium carbonate it is very important to optimize these parameters for effective bio-precipitation of calcium carbonate [5-7]. Till date, mostly calcite precipitating bacteria are screened based on traditional calcium urea plate/broth method which requires a large amount of reagent and time. Therefore, we attempted to devise a method for the [8] calcium binding dye which is well-known calcium chelator used for the detection of calcium in various biological samples [8,9].
Materials and Methods
All the chemicals used were of high purity (HIMEDIA). All the cultures were cultivated and maintained on nutrient agar. For calcium carbonate precipitation experiments traditional calcium-urea medium was used. The medium contained 3 g/L nutrient broth, 20 g/L urea, 2.12 g/L sodium bicarbonate, 10 g/L ammonium chloride, 3.7 g/L calcium chloride dehydrate. Before autoclaving the pH of the medium was adjusted to 6.0 with 6 N HCl, which was consequently increased to pH 7.2. Bacterial protein was estimated using Micro BCA protein assay kit from Thermo Scientific. Arsenazo III assay was performed using Calcium 150 kit from Accurex Biomedical Pvt. Ltd. The alkaline soil sample was collected from the area rich in deposits of calcite, located in Dehradun, Uttarakhand, India. The physicochemical analysis of the sample such as pH, oxidizable organic carbon, available phosphate, potassium, ammonical and nitrate nitrogen was performed using a soil testing kit from Hi-media. Bacteria were isolated from the samples using serial dilution plating method.1 g of the sample was suspended in 10 ml of autoclaved normal saline, vortexed and serial diluted ranging 10-2-10-6, again vortexed and left for 30 min. 100 μl of the saline was then pipetted and spread plated on a nutrient agar plate. The strains were identified on the basis of morphology, bio-chemical tests and, 16S rRNA gene sequencing. To screen the calcite-precipitating bacteria, we developed a 96 wells plate assay. For that traditional calcium-urea medium was used. For the growth of the culture, a 24 wells plate was used. 1ml of the culture media was suspended in the wells in duplicate for each strain. The cells of overnight grown cultures in nutrient broth at 37°C were harvested and dispensed in calcium urea medium at a fixed O.D 600nm of 0.6 10 μl of the inoculum was added to the respective wells. A negative control containing only calcium urea medium was left uninoculated. The plate was incubated at 37°C for 24 h. After 24 h the culture was transferred to a 1.5 ml micro centrifuge tube and centrifuged at 8000 rpm. Cells were collected for the total protein estimation. A 50 times dilution was prepared from the obtained supernatant by adding 490 μl of water to 10 μl of the supernatant for each strain including negative control 4 μl of this dilution was suspended in 96 wells plates in triplicate and then 100 μl of arsenazo solution was added to each well. For blank 100 μl arsenazo solution was added to 4 μl of deionized water. The plate was left for 2 min to complete the reaction and then the absorption at 650 nm was measured with Elisa reader. To determine the range of detection different mM concentrations of calcium were prepared in calcium-urea medium and diluted 50 times. 4 μl of the dilution was dispensed in the 96 wells plates in triplicates and arsenazo III was added to them. After 2 min absorption was measured with Elisa reader at 650 nm.
| S.No.Â? | Soil propertiesÂ? | Results |
|---|---|---|
| 1 | pH | 9.5 |
| 2 | Oxidizable organic carbon | Medium Low |
| 3 | Available phosphate | Not detectable |
| 4 | Potassium | Above 393Kg/Hec |
| 5 | Ammonical Nitrogen AboutÂ? | 15 Kg/Hec |
| 6 | Nitrate nitrogenÂ? | Not detectable |
Table 1: Results of soil properties.
Results and Discussion
The soil analysis results confirmed the alkaline nature of the soil, showing a pH value of 9.5 and medium to low content of oxidizable organic carbon Table 1. The potassium content of the soil was found to be very high, i.e., more than 393 kg/hec. Most of the available nitrogen was present as ammonical nitrogen as nitrate nitrogen was not detectable in the soil.
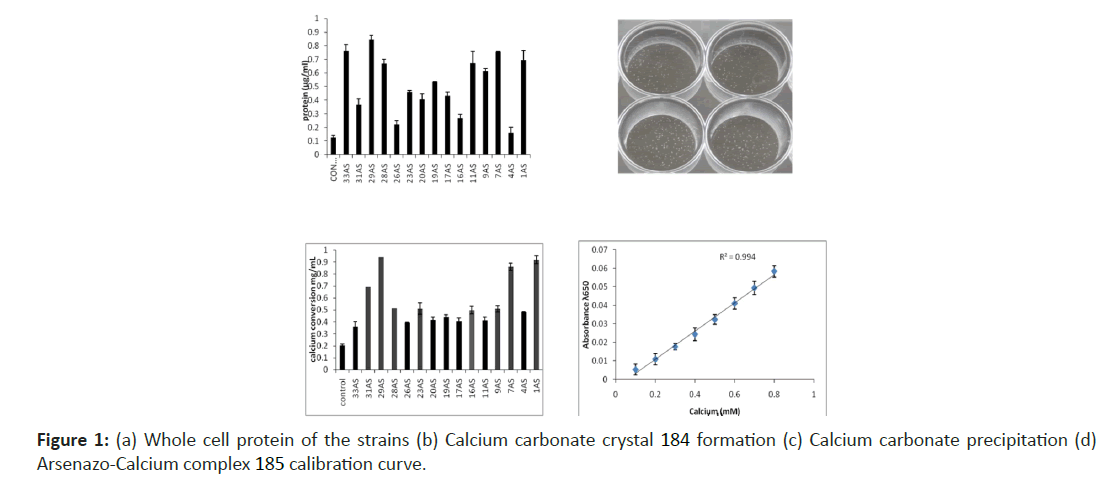
Arsenazo III, a highly sensitive calcium chelator has been used as the colorimetric indicator for the monitoring of the calcium carbonate precipitation by the strains. The intensity of the colour indicates the availability of free calcium ions in the solution. Based on the above principle arsenazo III was used as a colorimetric indicator for the screening purpose. Strains showing less or decreased absorbance than the negative control, were considered as positive for calcium carbonate precipitation. Based on this method 14 bacterial samples were found positive for calcium carbonate precipitation. Figure 1a shows the whole-cell protein of the strains grown in a calcium-urea medium.
Calcium carbonate precipitation was evident in 24 wells plates in case of bacteria efficiently inducing precipitation Figure 1b. Calcium carbonate particles adhered at the bottom of the plate. The absorption pattern by the arsenazo-calcium complex for all the urease positive strains is represented in Figure 1c. Arsenazo III-Ca complex intensity showed a maximum decrease for strains 1AS and 29AS comparative to control followed by 7AS. Although the absorption does not represent the actual concentration of calcium ions in the medium as it may be due the binding of calcium with other components in the media, but a relative estimation can be done with respect to control. 16S rRNA gene sequencing analysis results showed that the strain 29AS belongs to genera Lysinibacillus (accession no. KF053267) which is a gram positive, spore forming bacteria and strain 1AS is a facultative anaerobe of genera Paenibacillus (accession no. KF303139). An arsenazo-calcium binding calibration curve was drawn to check the linearity in calcium urea medium varying the concentration of calcium in the medium. Here also the concentrations of 0 to 40mM calcium were prepared and diluted to 50 times as in the case of microtiter assay. The range was found linear from 0.1 to 0.8 mM of calcium Figure 1d.
Conclusion
A new method for the screening of calcium carbonate precipitating bacteria. This paper reports a 96 wells plate method based on colorimetry which is rapid, economic reliable and is easy to perform. The new method will be very useful in the studies on Bioconcrete and in research on conservation of monuments and buildings in self-healing and sealing of concrete by the calcifying microorganisms.
Acknowledgement
We are grateful to Director of IMTECH for providing the necessary facilities for this work.
References
- Fortin D, Ferris FG and Beveridge TJ (1997) Surface-mediated mineral development by bacteria. Reviews in Mineralogy and Geochemistry 35: 161-180.
- De Muynck W, De Belie N and Verstraete W (2010) Microbial carbonate precipitation in construction materials: A review. Ecological Engineering 36: 118-136.
- Ghosh P, Mandal S, Chattopadhyay BD, Pal S (2005) Use of microorganism to improve the strength of cement mortar. Cement and Concrete Research 35: 1980-1983.
- Vempada SR, Reddy SSP, Rao MVS and Sasikala C (2011) Strength enhancement of cement mortar using microorganisms - An experimental study. Int J Earth SciEngg 4: 933-936.
- Hammes F, Boon N, De Villiers J, Verstraete W,Siciliano SD (2003) Strain-specific ureolytic microbial calcium carbonate precipitation. Applied and Environmental Microbiology 69: 4901-4909.
- Ferris FG, Phoenix V, Fujita Y, Smith RW (2004) Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20°C in artificial groundwater. GeochimicaetCosmochimicaActa 68: 1701-1710.
- Shirakawa MRA, Cincotto MA, Atencio D, Gaylarde CC, John VM (2011) Effect of culture medium on biocalcification by Pseudomonas putida, Lysinibacillussphaericusand Bacillus subtilis. Brazilian Journal of Microbiology 42: 499-507.
- Neelamegam P, Jamaludeen AS, Ragendran A,Murugrananthan K (2010) Microcontroller-based system for estimate of calcium in serum samples. Biomed InstrumTechnol 44: 433-439.
- Bolsover SR and Spector I (1986) Measurements of calcium transients in the soma, neurite and growth cone of single cultu red neurons. J Neurosci 6: 1934-1940.
Citation: Shukla A, Cameotra SS (2016) A New Method for the Screening of Ureolytic Bacteria Inducing Calcium Carbonate Precipitation. J Biotechnol Biomater 6:248. DOI: 10.4172/2155-952X.1000248
Copyright: © 2016 Shukla A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13163
- [From(publication date): 12-2016 - Jun 30, 2025]
- Breakdown by view type
- HTML page views: 12031
- PDF downloads: 1132