A Novel Classification for the Rational Management of Lateral Skullbase Osteomyelitis
Received: 24-Jan-2019 / Accepted Date: 19-Jun-2019 / Published Date: 26-Jun-2019 DOI: 10.4172/2161-119X.1000371
Abstract
Background: Skull base osteomyelitis (SBO) is a serious life threatening condition if not identified and managed early. Though the incidence has reduced due to wide use of antibiotics, the increase in other coexisting morbidities leads the patient to usually present with high rate of complications.
Methods: Our institute being a tertiary referral center encounters a spectrum of such patients. The spectrum of prequel to skull base osteomyelitis varies in a large way from infection (Bacterial/Fungal) to post radiation and so diagnosing a patient who comes with vague complaints become very important. This article is a retrospective case review of a variety of manifestations of SBO which were managed using a systematic protocol over a decade (2008- 2018) at our institute. We classified SBO into stages for deciding upon the most appropriate plan of management based on the severity of the disease.
Results: Optimal outcomes were achieved with minimal morbidity while following a step-wise management protocol whereby treatment was focused on multimodal therapy including intense diabetic control, appropriate antibiotic therapy and early surgical intervention for debridement of necrotic bone and replenishment of vascularity within the surgical cavity.
Conclusion: A rational approach to management of SBO is paramount since the presentations may be subtle and may vary upto life threatening complications. Following a systematic protocol as described here will lead to better long term outcomes.
Keywords: Skull base osteomyelitis; Malignant otitis externa; Necrotizing otitis externa; Facial reanimation; Temporoparietal flap
Introduction
Inflammatory disease of bone leading to destruction is referred to as osteomyelitis. When it affects the skull bone it can permeate into life threatening complications. But with the wide use of antibiotics Skullbase osteomyelitis (SBO) has now become a rare occurrence. It tends to occur in immunocompromized individuals where multidrug resistant organisms become virulent. Osteomyelitis of the temporal bone has various other synonyms like malignant otitis externa, necrotizing otitis externa, osteomyelitis of the mastoid, and osteitis of the base of the skull [1]. This work highlights the rational management protocol for this difficult to treat entity by proposing a novel classification.
Case Study
This is a retrospective study done at our Institute by auditing the medical records of patients who were diagnosed to have SBO over a decade from 2008 to 2018 (10 years). These patients were classified into different grades of SBO based on their severity of presentation as shown in Table 1.
| Classification | Symptoms | Clinical findings |
|---|---|---|
Mild (Confined to External Auditory canal but locally progressive) - akin to Malignant Otitis Externa (MOE) |
Ear pain & Ear discharge refractory to conventional MOE therapy | Discharge + Edema of the EAC Granulations |
| Moderate (Progressing but confined to the temporal bone) - akin to Necrotizing Otitis Externa (NOE) | Intense ear pain with blood-stained ear discharge + Facial nerve involvement |
Discharge ++ Erosion of the EAC/ Tympanic plate Granulations ++ Periostitis and seqestrum |
| Severe (Spreading beyond the confines of the temporal bone) - akin to progressive lateral Skullbase Osteomyelitis (SBO) |
Excruciating ear pain Temporoparietal cranialgia Sero-sanguineous ear discharge ("weeping-mastoid") Multiple lower cranial nerves palsies TM joint involvement Recurrent Acute Parotitis Peri-aural Lymphadenitis Labyrinthitis Profound deafness Intractable vertigo Meningeal irritation |
Discharge +++ Erosion of the EAC / Mastoid / TMJ Periosteitis with sequestrum & necrosis Granulations +++ TMJ / Mastoid or Parotid Abscess formation Dural / Sinus involvement Intra cranial involvement IJV / Sigmoid sinus / dural venous Thrombosis VII, IX, X, XI,XII cranial nerve palsy |
Table 1: Novel classification of skullbase osteomyelitis.
The demographic details of this cohort are given in Tables 2 and 3.
| Year | Mild SBO | Moderate SBO | Severe SBO | Total |
|---|---|---|---|---|
| 2008 | 32 | 7 | 4 | 43 |
| 2009 | 46 | 15 | 9 | 70 |
| 2010 | 57 | 21 | 7 | 85 |
| 2011 | 42 | 18 | 11 | 71 |
| 2012 | 37 | 20 | 7 | 64 |
| 2013 | 39 | 12 | 7 | 58 |
| 2014 | 51 | 22 | 6 | 79 |
| 2015 | 43 | 25 | 10 | 78 |
| 2016 | 54 | 21 | 9 | 84 |
| 2017 | 48 | 14 | 7 | 69 |
| 2018 | 45 | 27 | 14 | 86 |
| Total | 494 | 202 | 91 | 787 |
Table 2: Data of number with grades of SBO encountered at our institute.
| Year | Male | Female | Mean Age |
|---|---|---|---|
| 2008 | 32 | 11 | 61.3 |
| 2009 | 56 | 14 | 63.5 |
| 2010 | 65 | 20 | 66 |
| 2011 | 49 | 22 | 69 |
| 2012 | 46 | 18 | 62 |
| 2013 | 43 | 15 | 63 |
| 2014 | 69 | 10 | 66.2 |
| 2015 | 60 | 18 | 63.4 |
| 2016 | 63 | 21 | 66.7 |
| 2017 | 52 | 17 | 65 |
| 2018 | 63 | 23 | 68.1 |
| Total | 598 | 189 | 65.2 |
Table 3: Gender distribution of SBO at our institute.
Based on this decade of experience with such a large sample, an institutional protocol was developed in order to provide the most optimal management for this difficult entity based upon its severity
Review of Management Algorithms
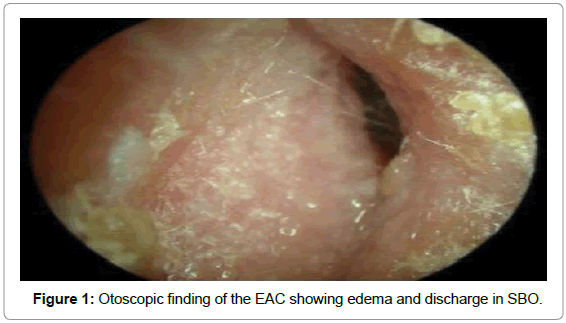
Once clinically suspected to have SBO, all patients underwent the following relevant investigations. Ear swab was taken for Gram stain and culture and sensitivity, which not only identified the organism involved but also helped in choosing the appropriate antibiotic for treatment [2,3]. Organisms commonly reported include P. aeruginosa, coagulase-positive Staphylococcus, Bacterioides spp, Streptococcus pneumoniae, Klebsiella pneumoniae, Aspergillus species and Mycobacterium tuberculosis (Figure 1).
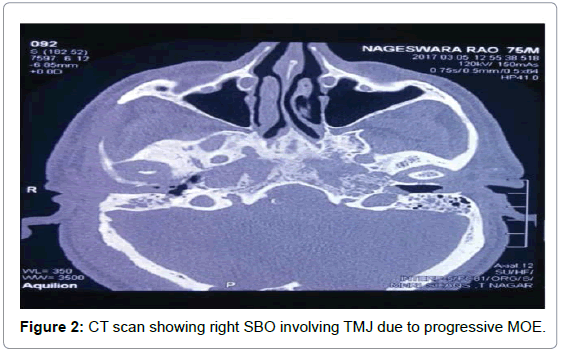
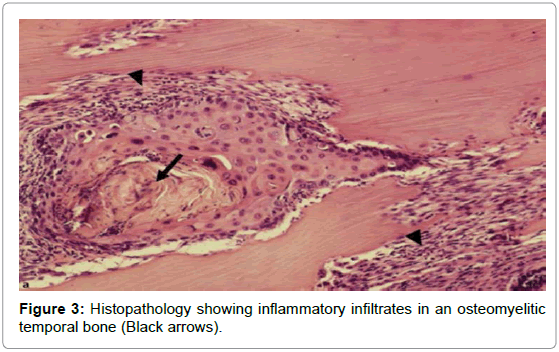
Total blood count, differential count, Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood sugar analysis, and enzyme-linked immunosorbent assay for Human immunodeficiency virus (HIV) were used to assess the patients general condition and monitor the severity of the infection [3]. High-resolution computed tomography (HRCT) of the temporal bone (axial and coronal sections) along with Magnetic resonance imaging (MRI) was done for determining the extent of involvement [4]. HRCT defined the location and extent of the disease (Figure 2) while MRI was useful to assess soft tissue planes especially in the setting of cranial neuropathy and progressive infection within the pneumatized temporal bone [5]. MRI was also important for assessing dural enhancement and intracranial complications. Highly sensitive but non-specific MRI findings of SBO included marrow T1 hypointensity and T2 hyperintensity [6]. Biopsies from the granulation tissues were done for histopathologic examination at the earliest (Figure 3) as it helped to categorically confirm SBO and also ruled out any early malignant change.
Based on the above investigations, as soon as the diagnosis of SBO is confirmed, our institutional protocol was to classify it into the grades as above and commence medical therapy at the earliest as an in-patient. Long-term antimicrobial therapy remained the mainstay of treatment.
Medical therapy
We followed a triple drug antibiotic regimen for broad spectrum cover while concurrently monitoring the culture and sensitivity of the ear discharge. The standard protocol was injection meropenem 1 g IV twice daily along with injection Piperacillin/Tazobactum 4.5 g IV twice daily and also injection levofloxacin 500mg once daily are given for 3 weeks, followed by oral antibiotics for 4 to 6 weeks. Aminoglycoside, β-lactamase antibiotics, third-generation cephalosporins like ceftazidime or an oral quinolone like ciprofloxacin were also given subsequently for several months in conjunction with our microbiologist’s recommendation. A prolonged course of low dose ciprofloxacin was recommended in a few cases upto 6 months to break relapsing cycles of osteomyelitic infection. Despite the reported efficacy of prolonged systemic antibiotic therapy, treatment failures did occur due to tissue hypoperfusion and hypoxia, as was the sequelae in the natural course of SBO, especially in fluctuant diabetic status due to microangiopathy.
Upon analyzing the symptoms among the cohort, we found that the main complaint for the patients and the best clinical prognosticator of treatment for the physician was the pain threshold. If patient recovers from SBO it was reflected upon the pain relief upon appropriate therapy and if there was a relapse then pain shooted up to indicate the same. Long term analgesics like Non-steroidal anti-inflammataory drugs (NSAID), opioids etc. were also needed under the care of the pain management specialists, initially as injectable and later as oral medications. Supplementary doses of gabapentin, piracetam, vinpositine and pregabalin were found to be helpful in relieving neuropathic cranialgia.
The entire prognosis of the patient usually depended upon strict diabetic control. Hence an in-house diabetologist was available to evaluate and manage these patients on a daily basis. The diabetic status was meticulously brought under control using a combination of dietary modifications and insulin injections using sliding scale and oral antiglycaemic drugs. Local treatment of the external auditory canal included meticulous cleaning and debridement on a daily basis until any granulation tissue resolved. All patients needed to be supplemented with a nutritious high-protein diet under the care of a nutritionist and dedicated nursing care for a good recovery.
Surgical algorithm
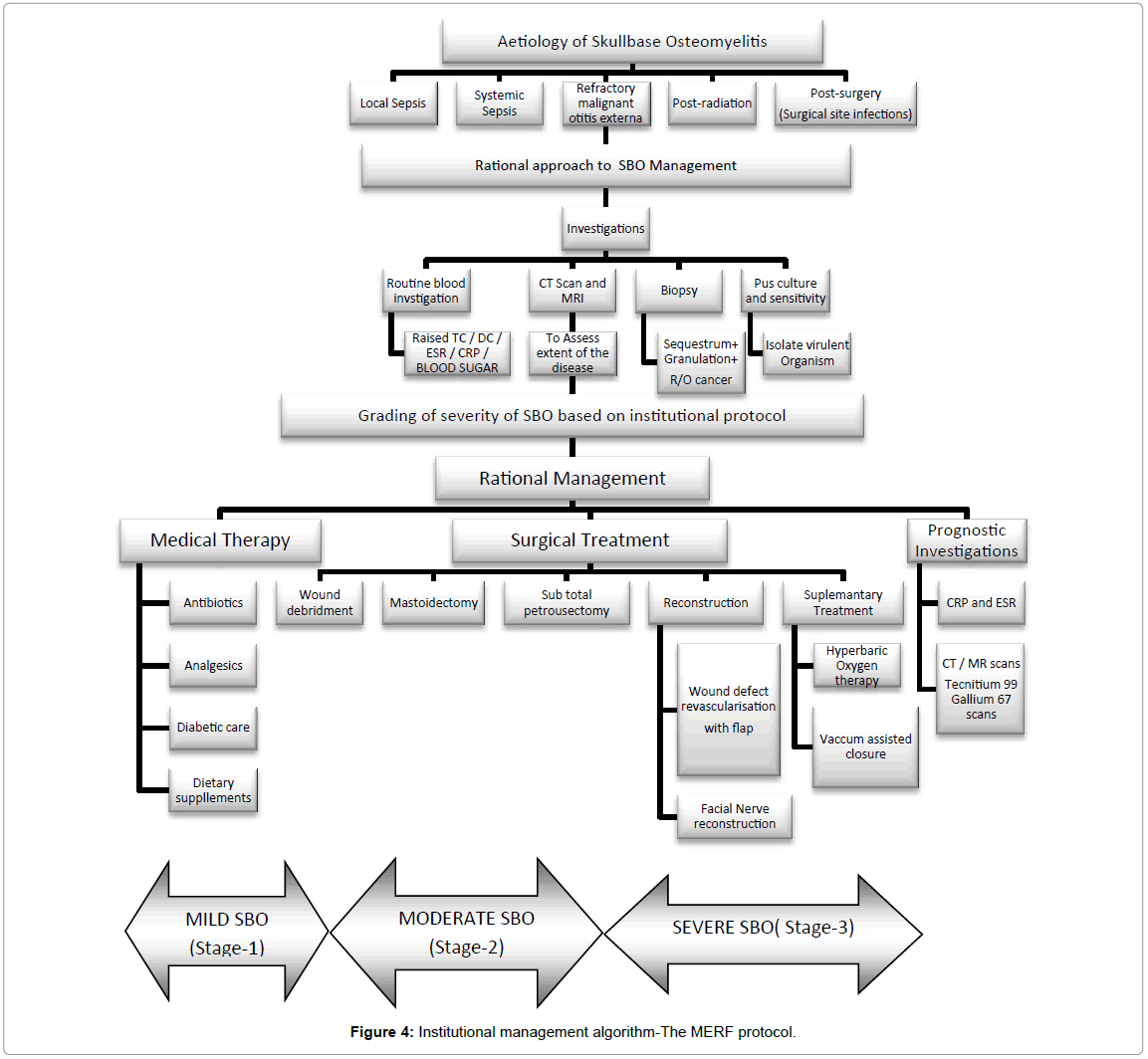
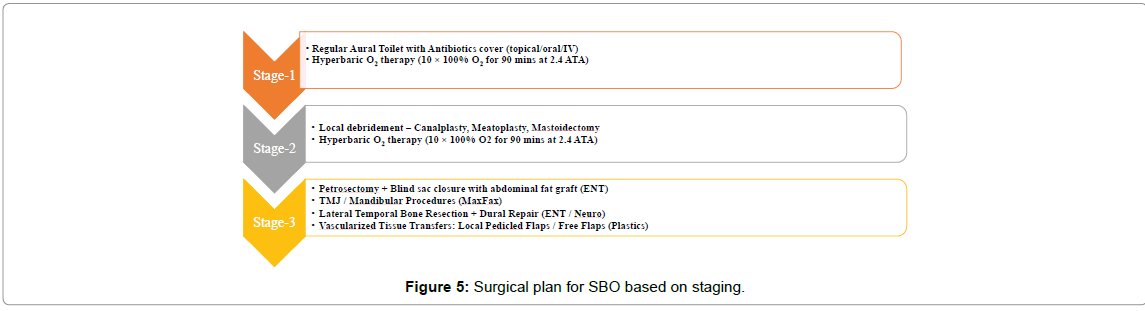
Surgery was chosen in the presence of severe unrelenting pain, detection of sequestration in HRCT, and in the presence of any complications as mentioned in Figure 4. Using the disease severity-Mild (Stage-I), Moderate (Stage-II) and Severe (Stage-III) as shown in Table 1 we had defined its appropriate surgical treatment in order to provide the most optimal outcome customized to each patient (Figure 5).
Stage-1 included repeated aural toiletting, pus drainage and localized cartilage debridement with skin preservation. The External auditory canal (EAC) was packed with steroid antibiotic ointment or 10% ichthammol glycerine packs for 4 to 8 days. The rationale in this stage included medical management with a triple drug regimen of IV antibiotics followed by oral antibiotics for 6 weeks with or without surgical management in more progressive cases as below. In stage-2, mastoidectomy was the treatment of choice when there was involvement of the middle ear cleft/facial paresis. Depending on the severity, either cortical or modified radical mastoidectomy with wide meatoplasty was performed. Necrotic cartilage, extensive granulations and bony sequestrum were indications for Modified radical mastectomy (MRM). A sub-total petrosectomy was done in cases of extensive granulations and sequestrations in the entire extent of the temporal bone but sparing the otic capsule. It included removal of all air cells of the temporal bone leaving behind the otic capsule and the petrous apex. The External auditory canal (EAC) was closed as a blind sac and the cavity obliterated with a rotated temporalis muscle flap [6,7].
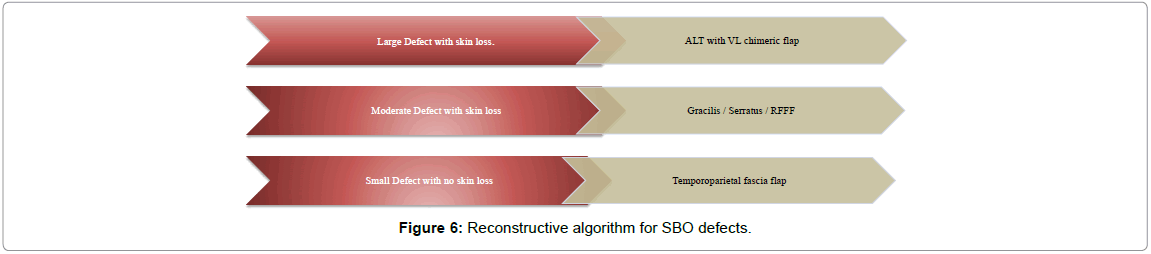
In our institution large temporal bone defects after clearing up SBO have been preferably reconstructed in a single-stage using vascularized muscle containing free flaps; with the Anterolateral thigh (ALT) flap, serratus and gracilis flap being used most often until adoption of a new method of harvesting; the chimeric Alanine aminotransferase (ALT). For smaller defects the Temporoparietal fascial flap (TPFF) as a musculocutaenous flap has been used [8,9] (Figure 6).
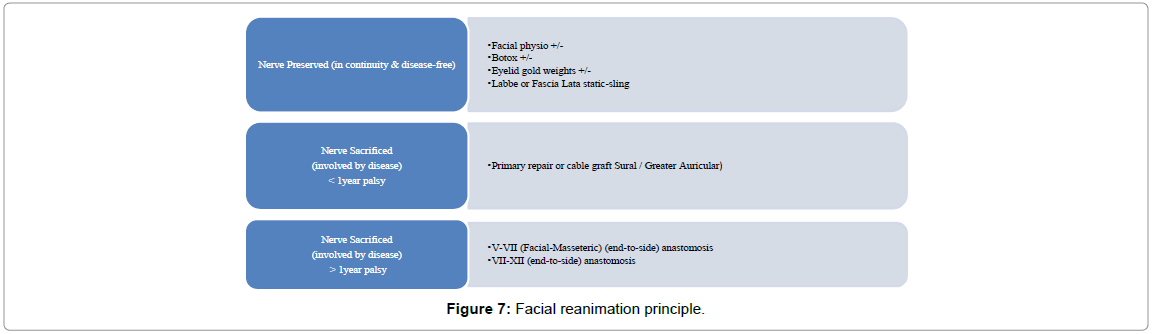
We also followed an institutional protocol for facial rehabilitation as defined below. In cases where the facial motor function was preserved, it was mandatory to preserve the nerve while dissecting in anatomical continuity in order to achieve grade-1 facial motor function postsurgery. In such cases even though there may be temporary paresis or conduction blocks in nerve activity, good results can be expected over time. A simultaneous Facial Nerve grafting and/or static re-animation have been implemented where the facial nerve had been sacrificed. Such patients required facial physiotherapy by a dedicated neurophysiology team with constant supportive care for a period of 6-9 months, during which the facial nerve function gradually improved. The aim in such patients was to maintain the facial muscle tone adequately while avoid wasting, synkinesis or mass movements by using appropriate methods as mentioned in (Figure 7).
Adjuvant treatment
Supplementry adjuvant therapies like Hyperbaric oxygen (HBO) therapy had also been used by us to treat a variety of infected, hypoperfused and hypoxic wounds. Leukocyte bactericidal capacity was substantially impaired at low oxygen tensions often observed in wounds. In these cases the use of hyperbaric oxygen increased wound partial pressure of oxygen levels, enhanced phagocytic oxidative killing of aerobic microorganisms and promoted neoangioneogenesis and osteoneogenesis [8,10]. HBO treatment consisted of 100% oxygen given for 90 minutes at 2.5 atm absolute pressure 5 days a week, 20 times as an adjuvant therapy. It was found to play a major role in preventing postoperative osteonecrosis [8].
Follow-up surveillance protocol
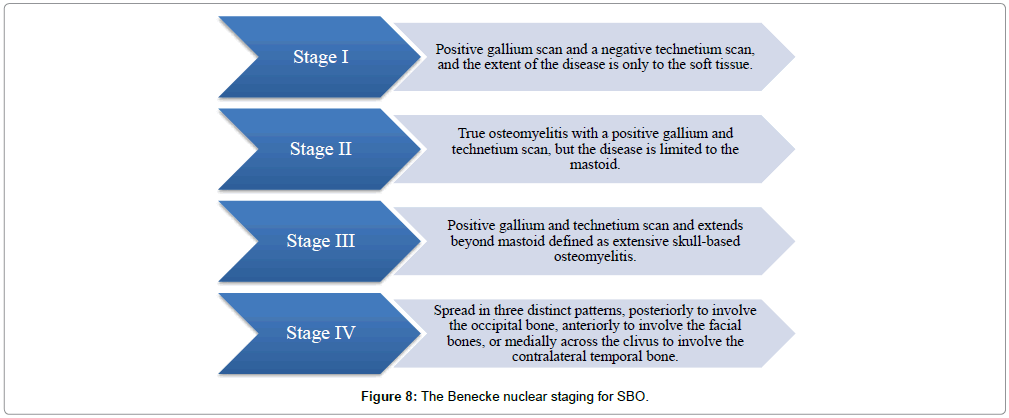
Based on nuclear imaging, Benecke in 1989 proposed the following staging system for osteomyelitis of the temporal bone originating from the EAC [7] (Figure 8). This was incorporated into the surveillance protocol of our institute. Technetium Tc 99m medronate methylene diphosphonate bone scanning and gallium citrate Ga 67 scintigraphy were used as specific prognosticators following treatment for SBO, as identified early recurrences and also assessed severity of inflammation post-treatment.
Results
A review of the overall results over a decade of treatment as per the algorithm defined here showed positive observations with satisfactory immediate and long term outcomes in our patients. A large cohort of 787 patients (598 males and 189 females; mean age=65.2 yrs; M:F ratio=3.6:1) had been treated among which 494/787 (62.7%) were having mild SBO, 202/787 (25.6%) had moderate SBO and 91/787 (11.5%) had severe SBO. Among the milder form of SBO in stage 1 & 2, all patients showed good recovery from disease in the immediate follow up period. Of these 12% had relapse over a range of next 5 to 11 months and had progressed onto higher stages of SBO. Their treatment was upgraded to higher stages as was necessary and they achieved remission in disease with appropriate management.
In stage 2 & 3, patients had surgical interventions of which 189/202 (94%) patients had tympanomastoidectomies for stage 2 SBO, while 69/91 (76%) patients had subtotal petrosectomies along with pedicled or free tissue transfer for their advanced stage 3 SBO. 35/91 (38.4%) patients required neurosurgical support while 27/91 (29.6%) patients had maxillofacial procedures. There was no failure of flaps in any of these cases due to the stringent monitoring and antiseptic protocols followed. 87 patients had facial nerve decompression in stages 2 & 3, while 43 patients had facial reanimation procedures in stage 3-27 primary repair, 13 cable grafts, and 7 neural anastomosis. Over 9 to 23 months of follow up of these cases, full recovery to HB grade 1 was achievable with primary repair, upto HB grade 2 in cable grafting and an improvement upto grade 3 in cases were neural anastomosis was performed. 88% of follow surveillance nuclear imaging scans showed no recurrence of disease, while 12% of cases showed disease progression as mentioned above, for which the management was upgraded as per the protocol. After completion of this upgraded therapy, the repeat nuclear imaging scans reported no further disease. At the time of this review currently, 605/787 (77%) of patients among the entire cohort were disease free, 47/787 (6%) patients had been lost for follow up while 135/787 (17%) patients had deceased due to ageing and other medical comorbidities. In summary we infer that our management strategy has proven to be efficacious in the long run as reflected by the overall outcomes as highlighted above. Therefore our institution, a tertiary referral centre for complex skull base diseases has now disseminated this protocol in the neighboring supported hospitals as standard of care. This has improved optimal services in the affiliated secondary care hospitals and has reduced the rate of complications of SBO and thereby onward referrals to our skull base unit.
Discussion
Skullbase osteomyelitis is most often a complication of Malignant otitis externa (MOE) secondary to pseudomonas aeruginosa infection [1]. It is usually common among elderly, diabetics and in patients with microvascular disease. Other predisposing conditions include arteriosclerosis, immunosuppression, chemotherapy, steroid use, and other immunodeficiency conditions [2]. SBO may also present without MOE and with other pathogens like fungi [3]. Diabetes mellitus is the most common predisposing factor in the development of temporal bone osteomyelitis secondary to MOE [1,4]. It is found that the diabetics ear had a higher average pH (alkaline pH), and it could provide a beneficial environment for bacterial overgrowth [5]. Other factors which also play a part in diabetic patients’ are microangiopathic changes and an altered immune response. They usually present with severe pain (temporal, parietal, postauricular or retro-orbital) foul smelling otorrhea, cellulitis, fever, woody induration of the pinna, cutaneous fistula, granulation tissue; intermittent facial twitching suggestive of facial canal dehiscence. Granulation tissue is seen within the auditory canal, once the bone gets completely involved it will lead to other cranial neuropathies indicating advanced disease. 6 Early diagnosis is very important, because MOE may not always be slow and gradual, but sometimes rapid and fulminant. Some patients present with complications like dural venous sinus thrombosis, meningitis cerebral infarction or abscess.
Fungal SBO is usually found in an immunocompromised host, prior antibiotic therapy, and steroid therapy. Fungal SBO has not been definitively associated with diabetes mellitus. Invasive fungal disease is usually caused by Aspergillus fumigatus and less commonly by Aspergillus flavus [4]. Central or atypical SBO is a potentially life threatening condition if not recognized early and treated. Atypical SBO does not begin with otitis externa [6]. These patients present with headaches initially and later may develop cranial abnormalities. Central or atypical SBO usually affect the sphenoid and occipital bone, often the clivus. It can present with a variety of cranial neuropathies, often lower cranial nerve neuropathies. Several life threatening complications can arise like cranial neuropathy, cavernous sinus thrombosis, and meningeal and brain parenchymal involvement. Cranial nerve involvement is commonly seen because of the proximity of the clivus to the brain stem, basal cisterns, cavernous sinuses, and skull base foramina [7].
Postoperative osteomyelitis is usually a complication of extensive surgery and wound infection. Patient usually present with high fever and marked local reaction and suppuration it may also cause fistula. Radiation for H & N malignancy may induce a spectrum of changes in the skull base and temporal bone which may lead to post radiation necrosis or Osteoradionecrosis (ORN) of temporal bone. It is a rare but feared complication. It may be limited to tympanic bone or extend diffusely to involve the lateral skull base [8,9].
SBO is thereby a life threatening disease if not diagnosed early and started on proper treatment. It can lead to grievous neurological morbidities and relapse in spite of maximal medical intervention. Greater awareness, new diagnostic methods, and better treatment for people with ready access to modern health care have led to a decrease in the rate of treatment failure in skull osteomyelitis. Early diagnosis, identification of the causative pathogens, prompt initiation of appropriate antimicrobial or surgical therapies, and continuation of therapy for an adequate period remain paramount while managing skull osteomyelitis [9].
A multidisciplinary approach with the ENT surgeon, diabetologist, neurologist, neurosurgeon, maxillofacial and plastic reconstructive surgeon working as a team is necessary to manage advanced SBO cases. A rehabilitative team of facial physiotherapists, occupational therapists, specialist nurses and speech & swallowing therapists need to give ample support for bringing back quality of life to these complex cases. Such a comprehensive service should be offered only in high volume tertiary referral care hospitals who are well versed in every aspect of management of this challenging disease [10].
The protocol as described here based on a decade of experience with complex SBO cases treated in our institute, was found to be comprehensive as it combined the staging based on severity along with the surgical options available for each stage as needed. As SBO tends to progress from one stage to another, a methodical step-wise surgical approach was recommended, while keeping the blood sugar levels under utmost control.
Accurate diagnosis of the condition with thorough ENT-head and neck examination is vital prior to commencement of therapy. The current staging of disease along with its complications, associated comorbidities and prior treatment which has been refractory should all be meticulously taken into consideration while planning appropriate medical treatment, wherein the emphasis on multidisciplinary inputs remain crucial. Long-term antimicrobial therapy remains the mainstay of treatment. Our institutional protocol was to follow a triple drug regimen for broad spectrum cover while concurrently monitoring culture and sensitivity of the discharge. This regimen is recommended for a period of 3 to 6 weeks, subsequent to which long term low dose oral antibiotic cover may be necessary for upto 3 months, until complete remission of the disease [10].
If the response to medical therapy is poor, early appropriate surgical intervention is necessary. Planning for surgery should include not only eradication of disease but preserving vital anatomy in its proximity and to provide adequate soft tissue cover and revascularization of the osteomyelitic bed. Treatment of chronic cranial osteomyelitis is difficult since we need to apply various reconstructive techniques for closure of osteomyelitis-related cranial defects. The dead space should be filled with an omental flap and titanium mesh plate can be used as a structural element to reconstruct. Cranial defects can also be reconstructed with split rib, split calvarial bone, biocompatible osteoconductive polymers, and by many other methods. The advantages of using an omental flap are that there is sufficient tissue volume to cover a large wound, it can be cut to any space, and it shapes relatively large blood vessels, making vascular anastomoses easier to perform. Titanium mesh plates make an excellent prosthesis in many ways, but carry the risk of exposure associated with thinning of overlying skin [10].
Oxygen tension plays an important role in the outcome of infection. Hence adjuvant Hyperbaric oxygen (HBO) therapy increases the oxygen tension in infected tissues, including bone, resulting in a direct bactericidal effect of HBO treatment on aerobic organisms such as Staph. aureus. HBO therapy improves host defenses and has been proven as a useful adjunct to antibiotics and surgery for the treatment of infectious wound complications after surgery in the irradiated head and neck. In radiation-injured tissues, HBO therapy facilitates the formation of new capillaries, thus improving tissue oxygen tensions and host defenses and improving osseointegration [9].
Conclusion
A rational management algorithm as described in our experience will help in achieving the best possible outcomes for complex patients presenting with SBO. Based on our decade of managing this difficult entity we recommend that these patients be categorized as per our staging into mild, moderate and severe SBO depending on their symptoms, complications and level of involvement of temporal bone. Based on this classification the treatment plan escalates step-wise appropriately from baseline medical management onto radical surgery with revascularization and reanimation as needed. We could achieve satisfactory outcomes among our patients over time while following such a management protocol.
Key Learning Points
1. Any elderly, diabetic/immunocompromised patient presenting with discharging ear with otalgia, headache and neural symptoms should be considered as SBO unless proved otherwise and treated as per above protocol.
2. SBO has to be classified depending on the aetiology; treatment has to be started based on it as it varies completely.
3. SBO presents like tumours of the temporal bone, the only investigation to differentiate is the histopathological examination.
4. Radiology scans (CT/MRI) can only be used to assess the extent of the lesion, but cannot confirm this diagnosis by excluding tumors.
5. Technetium 99m medronate methylene diphosphonate bone scanning, and gallium citrate Ga 67 scintigraphy can be used for prognosis of patients.
6. Drug regimen: To start on parental antibiotics: Inj Meropenem 1 g IV twice daily, Inj Piperiacillin Tazobactum 4.5 g IV twice daily and Inj Levofloxacin 500 mg IV once daily. Long term low dose oral ciprofloxacin is recommended as adjuvant in severe cases.
7. Diabetic control and Supportive care plays an important part in management.
8. Protocol for surgical management depends upon the extent of the disease.
9. Reconstruction of defects in vascularized tissue along with simultaneous facial nerve reanimation has to be done meticulously to achieve good results.
10. Systematic approach by a multidisciplinary team is the core requirement for SBO management.
Ethical Considerations
None.
Conflict of Interest
Nil.
Financial Disclosure
Nil.
References
- Asai S, Kamei Y, Torii S (2004) One-stage reconstruction of infected cranial defects using a titanium mesh plate enclosed in an omental flap. Ann Plast Surg 52: 144-147.
- Cohen D, Friedman P (1987) The diagnostic criteria of malignant external otitis. J Laryngol Otol 101: 216-221.
- Gherini SG, Brackmann DE, Bradley WG (1986) Magnetic resonance imaging and computerized tomography in malignant external otitis. Laryngoscope 96: 542-548.
- Mendelson MH, Gurtman A. Szabo S, Neibart E, Meyers BR, et al. (1983) Malignant external otitis: the role of computed tomography and radionuclides in evaluation. Radiol 149: 745-749.
- Neovius EB, Lind MG, Lind FG (1997) Hyperbaric oxygen therapy for wound complications after surgery in the irradiated head and neck: A review of the literature and a report of 15 consecutive patients. Head Neck 19: 315-322.
- Seabold JE, Simonson TM, Weber PC, Thompson BH, Harris KG, et al. (1995) Cranial osteomyelitis: diagnosis and follow-up with In-111 white blood cell and Tc-99m methylene diphosphonate bone SPECT, CT, and MR imaging. Radiol 196: 779-788.
- Tumeh SS, Aliabadi P, Weissman BN, McNeil BJ (1986) Chronic osteomyelitis: bone and gallium scan patterns associated with active disease. Radiol 158: 685-688.
- Pathak I, Bryce G (2000) Temporal bone necrosis: diagnosis, classification, and management. Otolaryngol Head Neck Surg 123: 252–257.
- Hao SP, Chen HC, Wei FC, Chen CY, Yeh AR, et al. (1999) Systematic management of osteoradionecrosis in the head and neck. Laryngoscope 109: 1327-1328.
- Raghunandhan S, Jonathan P, James B, Dimitrius E, Peter M, et al. (2017) Reconstruction for complex lateral skullbase defects: The Birmingham Approach. Conference proceedings of the British Skull Base Society meeting, Cardiff, Wales.
Citation: Rathnaraajan S, Devarasetty A, Panicker A, Gajapathy S, Sampathkumar R, et al. (2019) A Novel Classification for the Rational Management of Lateral Skullbase Osteomyelitis. Otolaryngol (Sunnyvale) 9: 371. DOI: 10.4172/2161-119X.1000371
Copyright: © 2019 Rathnaraajan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5152
- [From(publication date): 0-2019 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 4137
- PDF downloads: 1015