A Novel Tool to Evaluate the Accuracy of Predicting Survival and Guiding Lung Transplantation in Cystic Fibrosis
Received: 25-May-2019 / Accepted Date: 08-Jun-2019 / Published Date: 17-Jun-2019 DOI: 10.4172/2161-1165.1000375
Abstract
Background: Effective transplantation recommendations in cystic fibrosis (CF) require accurate survival predictions, so that high-risk patients may be prioritized for transplantation. In practice, decisions about transplantation are made dynamically, using routinely updated assessments. We present a novel tool for evaluating risk prediction models that, unlike traditional methods, captures classification accuracy in identifying high-risk patients in a dynamic fashion.
Methods: Predicted risk is used as a score to rank incident deaths versus patients who survive, with the goal of ranking the deaths higher. The mean rank across deaths at a given time measures time-specific predictive accuracy; when assessed over time, it reflects time-varying accuracy.
Results: Applying this approach to CF Registry data on patients followed from 1993-2011 we show that traditional methods do not capture the performance of models used dynamically in the clinical setting. Previously proposed multivariate risk scores perform no better than forced expiratory volume in 1 second as a percentage of predicted normal (FEV1%) alone. Despite its value for survival prediction, FEV1% has a low sensitivity of 45% over time (for fixed specificity of 95%), leaving room for improvement in prediction. Finally, prediction accuracy with annually-updated FEV1% shows minor differences compared to FEV1% updated every 2 years, which may have clinical implications regarding the optimal frequency of updating clinical information.
Conclusions: It is imperative to continue to develop models that accurately predict survival in CF. Our proposed approach can serve as the basis for evaluating the predictive ability of these models by better accounting for their dynamic clinical use.
Keywords: Cystic fibrosis; Lung transplantation; Survival; Risk prediction; Classification accuracy
Introduction
Lung transplantation has been shown to improve survival for some cystic fibrosis (CF) patients whose disease is no longer amenable to more conventional medical therapies [1,2]. However, due to a shortage of donor lungs, a large number of wait-list patients die while awaiting transplantation employing the current allocation system in the US. In 2010-2012, the wait-list mortality rate was 15.4 per 100 wait-list years. Candidates aged 12-17 years had the highest wait-list mortality, at 19.7 deaths per 100 wait-list years, followed by those aged 18-34 years at approximately 18.5 deaths per 100 wait-list years [1].
Despite an increase in the rate of lung transplants over the past decade, wait-list mortality rates continue to rise [3]. Accurate predictions of mortality are necessary so that limited donor lungs may be prioritized to patients who are at the greatest risk of death without transplantation. The goal is to use a patient’s clinical characteristics to calculate the predicted risk of mortality within a specified time period and to rank or classify patients on the wait-list as those who are predicted to die soon versus those who are not.
Although forced expiratory volume in 1 second as a percentage of predicted normal (FEV1%) alone is the currently accepted measure of pulmonary function used for assessing patient prognosis and recommending lung transplantation, its prognostic accuracy has been questioned [4-6]. Several risk prediction (or prognostic) models combining FEV1% with other clinical factors have been proposed to improve survival prediction; however, it is unclear how accurate these models are, since their evaluation has failed to address classification accuracy and instead focused solely on measures of model fit or calibration. For example, Liou et al. developed the most well-known survival prediction model in CF that includes FEV1% and several additional clinical features [5]. They assessed model fit, and concluded that their model was more accurate than FEV1% alone for predicting 5-year mortality. However, only assessing how closely the model fits the observed data is not sufficient when the model is meant for classification [7-9]. While agreement between the predicted and actual numbers of deaths represents good model calibration, it does not necessarily translate to good classification accuracy [10].
Two papers have evaluated predicted versus actual mortality, Aaron et al. and Mayer-Hamblett et al. [9,11]. Aaron et al. compared predicted versus actual numbers of deaths and concluded that their model was accurate for prediction of one-year survival [11]. Mayer- Hamblett et al. however, addressed appropriate evaluation of their model using classification error rates, i.e. sensitivity and specificity, for classifying subjects based on 2-year mortality [9]. They showed that their multivariate model did not perform better than FEV1% alone, and that both are inadequate for use in practice. They concluded: “better clinical predictors of short-term mortality among patients with CF are needed [9] (P1550)”.
In addition to classification error rates, appropriate evaluation of a risk model must account for time-varying measurements. In practice, key clinical parameters included in the lung allocation score (LAS) are updated at routine care visits and then used to guide decisions regarding listing status in the US. Interestingly, the utility of updating markers has never been empirically evaluated. We assess time-varying accuracy using appropriate time-dependent classification error rates. Such evaluation could also help assess how often patient information such as FEV1% should be updated in practice before information becomes outdated and impacts accuracy.
Methods
Study cohort
We used the Cystic Fibrosis Foundation (CFF) National Patient Registry (CFFPR) data, which consists of all patients who agreed to participate in the CF Registry and who were seen in a CFF-accredited care center in the US from 1986 through 2012. The CFFPR obtains written and informed consent from each participant. This analysis was approved by the CFFPR oversight committee and was granted Institutional Review Board Exempt Status by the University of Washington Human Subjects Division (due to being anonymized data).
We selected the prevalent subjects in 1993 as our study cohort to match the analysis of Liou et al. [5]. Our study cohort consisted of 17,926 prevalent CF cases on January 1, 1993. We treated the measurements available on this date as baseline FEV1% measurements. 6,028 subjects were excluded due to missing baseline FEV1% measurements (of these, 3,507 were younger than 5.5 years at baseline). After exclusions, 11,254 subjects remained in the study cohort; 3,906 (35%) of these subjects were observed to die during the study period ending on December 31, 2011. 2,414 subjects were recipients of lung transplantation between 1993 and 2011; we censored these subjects at the time of transplantation.
For each subject, we had baseline demographic and diagnosis data and approximately annual measurements of FEV1%. Counting multiple observations per patient, we included 140,651 total annual records. Of these, 16,846 had missing FEV1% measurements. We imputed missing values by carrying forward the last non-missing value for that patient.
Risk models
FEV1% of predicted is a standard measure used in practice to guide recommendation for lung transplantation. We evaluated the following models: (i) a base model containing only FEV1% and (ii) a multivariate model consisting of FEV1%, age, gender, weight, presence of pancreatic sufficiency, Staphylococcus aureus infection and Burkholderia cepacia infection [5]. Predictions from Cox models were summarized into a single baseline risk score and a separate time-varying, updated risk score. For the baseline score, we used 10-fold cross-validation to protect against overfitting. For the time-varying score, we used baseline measurements as training data to develop the Cox model and predicted the score at follow-up times using updated values of FEV1%. We added flexibility to both models by using cubic splines to model continuous variables.
Evaluation of model accuracy
Diagnostic accuracy-classification error rates: The traditional diagnostic classification problem is based on a binary outcome, typically the presence or absence of disease. Mayer-Hamblett et al. assessed prognostic accuracy by defining a ‘yes/no’ outcome for death within two years from baseline [9]. Classification errors include false negatives, i.e. patients who were predicted by the risk score to survive for longer than two years were observed to die within two years, and conversely, false positives, i.e. healthier subjects who did survive beyond two years were predicted to die within two years. Minimizing these errors is equivalent to maximizing the sensitivity (or true positive fraction (TPF)) and specificity (or 1 - false positive fraction (FPF)), respectively. The Receiver Operating Characteristic (ROC) curve is a standard tool that plots TPF versus FPF for all possible risk score cutoffs [12-16]. The ROC curve is commonly summarized using the area under the ROC curve (AUC), ranging from 0.5 to 1.0, which indicate no discrimination to perfect discrimination. The AUC is also a concordance statistic and represents the probability that a randomly chosen subject who dies (case) has a higher marker value than a randomly chosen subject who survives (control).
Time-varying prognostic accuracy: Implicit in the use of traditional diagnostic TPF and FPF are current-status definitions of disease. Since we are interested in a setting where outcome status changes with time, precise definitions are necessary to include event timing in definitions of prognostic error rates. Time-dependent ROC curve methods that extend concepts of sensitivity and specificity and characterize prognostic accuracy for survival outcomes have been proposed in the statistical literature and now widely adopted in practice [17,18]. These innovative methods represent a dynamic tool for assessing longitudinal changes that may alter prognosis within a defined time period.
To evaluate both baseline and time-varying measurements, we use the incident-case definitions of Heagerty & Zheng, which are based on a binary classification of the risk set at any time t [18]. That is, among the patients who are still alive at time t, cases are defined as those who die at t and controls as those who survive beyond t. The sensitivity and specificity at time t are the error rates in classifying subjects at that time, and can be summarized using a time-dependent ROC curve. The time-dependent AUC, or AUC(t), is then defined as the area under the time-dependent ROC curve and interpreted as the probability that a randomly chosen case who dies at time t has a higher marker value than a randomly chosen control who survives beyond time t.
These definitions are appropriate for evaluating the performance of a baseline or time-varying marker in the CF lung transplantation setting, as interest lies in identifying patients who are at the highest risk of death in the near future, so that they may be given priority for limited donor organs. The recipient decision may be made at multiple time points as donor organs become available, but is always only applicable to those subjects who remain alive at those times.
We estimated the time-dependent AUC using a simple nonparametric rank-based approach [19]. The idea behind this approach is to compute for each risk set the binary concordance statistic using only the individual case and associated risk-set controls. For a fixed time t, we calculate a percentile for each case in the risk set relative to the controls in the risk set. A perfect marker would have the case value greater than 100% of risk set controls. The mean percentile at time t is calculated as the mean of the percentiles for all cases in a window around t. The summary curve, AUC(t), is then estimated as the local average of case percentiles. This nonparametric approach provides both a simple description for marker performance within each risk set and by smoothing individual case percentiles, a final summary curve over time characterizes how accuracy may be changing over time.
Although the AUC is a standard measure of accuracy, it summarizes the sensitivity of a risk score over the entire range of specificities from 0 to 100%. In contrast, clinical decisions are typically made based on a single risk score cut-off that has been shown to perform with high sensitivity and/or high specificity. Therefore, in evaluating a risk score ’ s performance and its impact for treatment decisions, it is important to also assess the sensitivity at a fixed high specificity. Using the above methods, we obtained a summary curve of sensitivity or TPF for a fixed specificity of 95% or FPF of 5%.
Finally, we assessed the time-varying prognostic accuracy of timevarying, or updated, risk scores using an extension of the approach of Saha-Chaudhuri & Heagerty [19] to accommodate time-varying markers. At any time t, the last measured value of the risk score was used as the current risk prediction
Stratification by risk group: We evaluated the performance of annually updated FEV1% measurements in subgroups defined by baseline FEV1% (FEV1% ≤ 30 versus FEV1% >30, based on the cut-off recommended by the International Society for Heart and Lung Transplantation [20]), baseline age (≤ 11 years, 12-17 years, and ≥ 18 years), gender, and F508 genotype.
Results
Cohort characteristics
Table 1 summarizes the characteristics of the study cohort. Patients with shorter survival also tended to be older at baseline, have lower FEV1% at baseline, be in lower weight and height percentiles, be slightly less likely to have Staphylococcus aureus infection and slightly more likely to have Burkholderia cepacia infection.
| Overall (n=11,254) | Died within 1 year (n=287) | Died 1-5 years (n=1,077) | Survived at least 5 years (n=9,146) | |
|---|---|---|---|---|
| Age on Dec 31, 1992, Mean (SD) | 18.0 (8.9) | 24.2 (9.6) | 22.0 (9.2) | 16.9 (8.5) |
| Sex, n (%) | ||||
| · Female | 5,187 (46%) | 144 (50%) | 529 (49%) | 4,185 (46%) |
| · Male | 6,067 (54%) | 143 (50%) | 548 (51%) | 4,961 (54%) |
| Race, n (%) | ||||
| · White | 10,858 (96%) | 281 (98%) | 1,031 (96%) | 8,819 (96%) |
| · African American | 320 (3%) | 6 (2%) | 40 (4%) | 259 (3%) |
| · Other | 76 (1%) | 0 (0%) | 6 (1%) | 68 (1%) |
| Genotype, n (%) | ||||
| · ΔF508 homozygous | 4,346 (39%) | 55 (19%) | 281 (26%) | 3,856 (42%) |
| · ΔF508 heterozygous | 3,139 (28%) | 31 (11%) | 165 (15%) | 2,819 (31%) |
| · Other | 883 (8%) | 11 (4%) | 63 (6%) | 742 (8%) |
| · Missing | 2,886 (26%) | 190 (66%) | 568 (53%) | 1,729 (19%) |
| FEV1%, Mean (SD) | 68.5 (28.0) | 32.4 (19.2) | 40.8 (20.1) | 74.5 (25.4) |
| Weight percentile, Mean (SD) | 28.5 (26.6) | 8.7 (16.1) | 12.4 (18.5) | 30.5 (26.6) |
| Height percentile, Mean (SD) | 29.3 (26.3) | 17.5 (23.0) | 19.1 (22.4) | 30.6 (26.5) |
| Staphylococcus aureus status, n (%) | ||||
| · Yes | ||||
| · No | 3,001 (27%) | 42 (15%) | 242 (22%) | 2,559 (28%) |
| · Not cultured | 7,014 (62%) | 234 (82%) | 793 (74%) | 5,468 (60%) |
| 1,239 (11%) | 11 (4%) | 42 (4%) | 1,119 (12%) | |
| Burkholderia cepacia status, n (%) | ||||
| · Yes | ||||
| · No | 346 (3%) | 33 (11%) | 88 (8%) | 195 (2%) |
| · Not cultured | 9,669 (86%) | 243 (85%) | 947 (88%) | 7,832 (86%) |
| 1,239 (11%) | 11 (4%) | 42 (4%) | 1,119 (12%) |
Table 1: Summary of baseline (1993) subject characteristics.
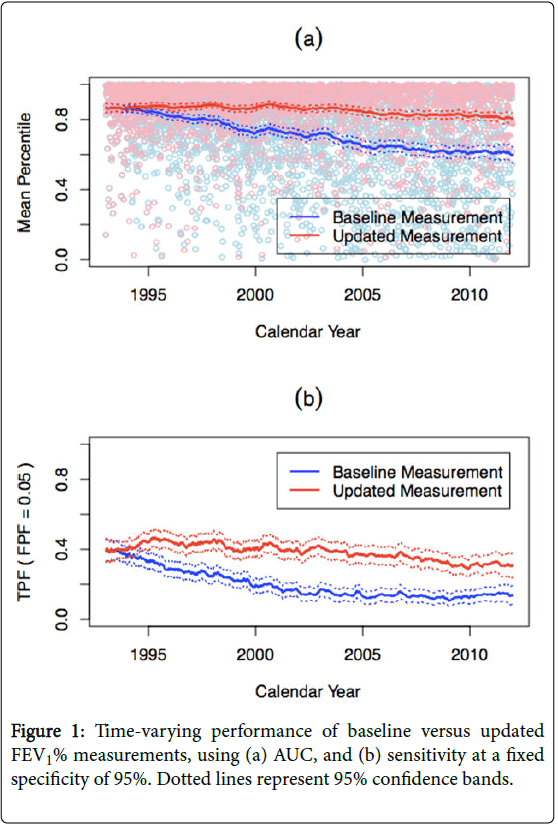
Assessment of time-varying prognostic accuracy of base model using baseline and updated measurements
Figure 1a shows the estimated AUC(t) or mean percentile for FEV1% baseline and annually updated measurements. Not surprisingly, the performance of a baseline FEV1% measurement declines over time, from 0.87 (95% CI (0.84, 0.88)) at baseline (1993) to 0.62 (95% CI (0.59, 0.65)) 20 years later (2012). In contrast, an annually updated FEV1% consistently maintains an AUC of approximately 0.90 over time. Although AUC=0.90 is typically considered to be excellent performance, it does not translate to FEV1% having adequate performance in this setting. 90th percentile means that out of 100 patients, the case marker value is higher than 90 control marker values; however, it also means that 9 other controls will be prioritized ahead of the case. Figure 1b shows the TPF for a fixed FPF of 5%. Again, an annually updated FEV1% has better performance than baseline FEV1%; however, a TPF of 45% is likely inadequate for clinical practice.
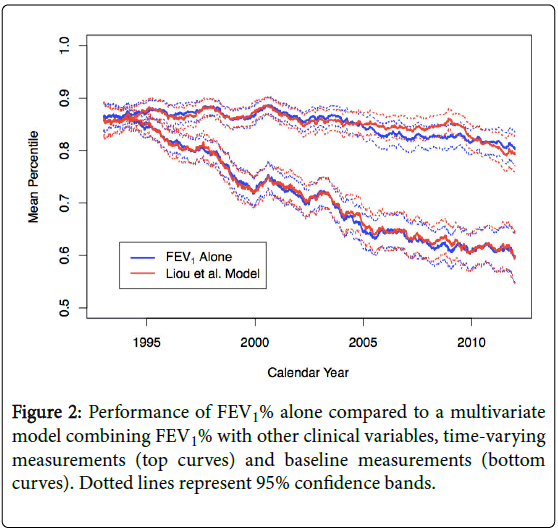
We also assessed the performance of a multivariate model that combined FEV1% with a number of clinical variables. We note that this model is very similar to Liou et al.’s model [5], which they proposed as a better predictor of mortality, with the exception that our model excluded diabetes mellitus and number of acute exacerbations, as we did not have data on these variables for this analysis. Figure 2 confirms the findings of Mayer-Hamblett et al. [9] and shows that adding clinical variables to the FEV1% base model does not improve performance beyond using FEV1% alone, using baseline or updated measurements. .
Finally, an assessment of the performance of annually updated FEV1% measurements compared to those updated every two years shows slightly worse performance of the latter in even years when the measurement is a year old. Performance in odd years is exactly the same, since FEV1% is always updated in those years (Table S1).
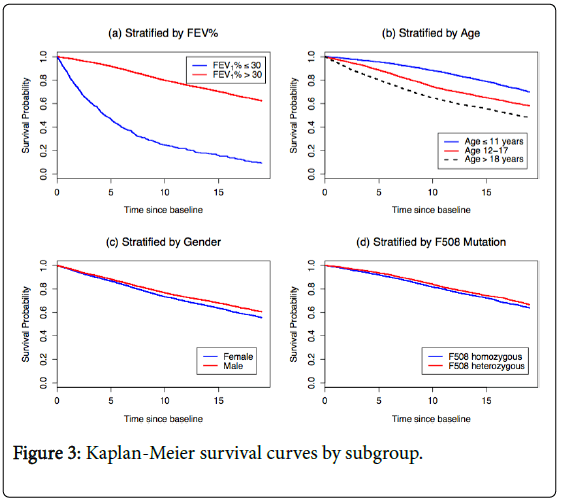
Stratification by risk group
Figure 3 presents Kaplan-Meier survival curves for subgroups defined by baseline FEV1%, baseline age, gender, and F508 genotype. We see a large gap in survival probabilities by baseline FEV1%. Patients with FEV1% ≤ 30 have poor prognosis, with an estimated 5-year survival probability of 35%, compared to 90% in the FEV1%>30 subgroup. The second panel shows worse survival with increasing age, with estimated 5-year survival probabilities of 95%, 87% and 76% in the ≤ 11 years, 12-17 years, and ≥18 years subgroups, respectively. Gender and F508 genotype have little impact on survival probabilities. This result regarding F508 genotype is likely due to the low rate of genotyping in this cohort (66% of the patients who died within one year were not genotyped).
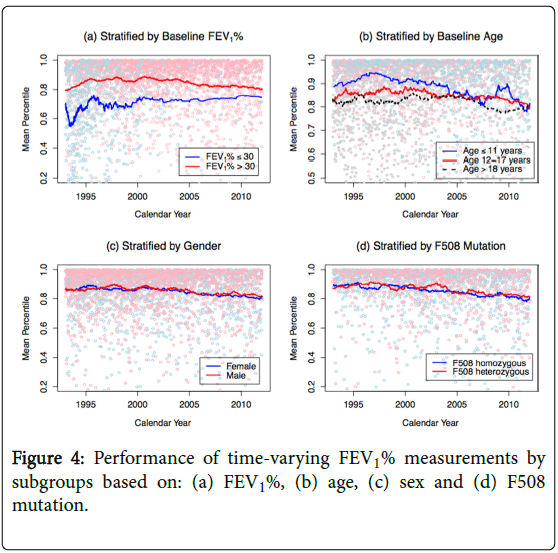
Figure 4 shows the predictive accuracy of FEV1% in the same subgroups. The performance of FEV1% in each subgroup seems to be largely determined by the prognosis in that subgroup. FEV1% has poorer predictive performance in subgroups with poorer survival, where it is likely that a patient’s prognosis is dominated by other factors that are not captured by FEV1%.
Discussion
Commonly used measures of model fit and calibration are insufficient for evaluating risk prediction models in CF when the goal is to prioritize patients for lung transplantation and when a patient’s clinical status is changing over time. Therefore, we presented an approach that uses time-dependent classification error rates that can be used to characterize the potential performance of a survival model, while accounting for the time-varying nature of the prediction itself.
In the CF setting, we found that updated measurements of FEV1% have consistent performance over time, whereas the performance of a baseline measurement declines over time. Thus, previously reported estimates of the accuracy of FEV1% alone do not capture its true performance in a clinical setting. It is clear that patient information should be updated over time to maintain classification accuracy; however, it is also evident that neither FEV1% alone nor existing multivariate models are adequate for use in practice.
Being able to evaluate a model’s time-varying accuracy may also help guide clinical practice and policy with regards to the frequency of updating patient information. A comparison of 1-year versus 2-year measurements of FEV1%, for example, showed minor differences in performance.
A limitation of the analysis is that it was done in an earlier cohort, in order to compare performance with Liou et al.’s model [5]. A more up-to-date cohort could change the results, but it would not take away from the key point – that time-varying approaches are better than the traditional approaches currently used in CF.
Conclusion
In this study we demonstrated that using a statistical evaluation approach that is closely tied to the clinical goal of using predicted risk as a score to rank patients as a function of time can significantly change the conclusions drawn about a risk prediction model ’ s performance. As new models are developed, perhaps incorporating novel biomarkers, the proposed approach could be used to accurately assess their predictive ability. As shown, standard methods may underestimate their performance by not capturing how these models will be used dynamically within the clinical setting. We note that our focus here is on risk prediction models, assuming that patients are added to a lung transplantation wait-list based on their expected benefit from transplantation. In practice, any risk prediction should be coupled with assessments of treatment benefit. It is imperative to continue to develop models that accurately predict survival in CF. Our proposed approach can serve as the basis for evaluating the predictive ability of these models by better accounting for their dynamic clinical use.
Acknowledgments
The authors thank the Cystic Fibrosis Foundation for allowing the use of the registry data.
Funding
This project was supported by the NIH (R37-CA218413, R01- HL072966, UL1TR000423, HL103965, R01-AI101307, P30- DK089507), the PhRMA Foundation, the Cystic Fibrosis Foundation, and the FDA (R01-FD003704).
References
- United Network for Organ Sharing (2012) OPTN/SRTR 2017 Annual Data Report: Preface. American Journal of Transplantation 19: 1-10.
- Whitehead B, Helms P, Goodwin M, Martin I, Scott JP, et al. (1991) Heart-lung transplantation for cystic fibrosis. 2: Outcome. Arch Dis Child 66: 1022-1017.
- Cystic Fibrosis Foundation (2013) Patient Registry 2013 Annual Data Report.
- Flume P (1998) Cystic fibrosis: when to consider lung transplantation? Chest 113: 1159-1161.
- Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, et al. (2001) Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 153: 345-352.
- Milla CE, Warwick WJ (1998) Risk of death in cystic fibrosis patients with severely compromised lung function. Chest 113: 1230-1234.
- Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd edition. Wiley: New York, NY.
- Kattan MW (2003) Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst 95: 634-635.
- Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML (2002) Developing cystic fibrosis lung transplant referral criteria using predictors of 2-Year mortality. Am J Respir Crit Care Med 166: 1550–1555.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, et al. (2010) Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 21: 128-138.
- Aaron SD, Stephenson AL, Cameron DW, Whitmore GA (2015) A statistical model to predict one-year risk of death in patients with cystic fibrosis. J Clin Epidemiol 68: 1336-1345.
- Green DM, Swets JA (1966) Signal Detection Theory and Psychophysics. John Wiley & Sons, New York.
- Hanley JA, McNeil BJ (1982) The meaning and use of the area under an ROC curve. Radiology 143: 29-36.
- Pepe MS (2003) The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press.
- Swets JA, Pickett RM (1982) Evaluation of Diagnostic Systems: Methods From Signal Detection Theory. Academic Press: New York.
- Metz CF (1978) Basic principles of ROC analysis. Semin Nucl Med 8: 283-298.
- Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56: 337-344.
- Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61: 92-105.
- Saha-Chaudhuri P, Heagerty PJ (2013) Non-parametric estimation of a time-dependent predictive accuracy curve. Biostatistics 14: 42–59.
- Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, et al. (2006) International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 25: 745-755.
Citation: Bansal A, Mayer-Hamblett N, Goss CG, Chan LN, Heagerty PJ (2019) A Novel Tool to Evaluate the Accuracy of Predicting Survival and Guiding Lung Transplantation in Cystic Fibrosis. Epidemiology (Sunnyvale) 9:375 DOI: 10.4172/2161-1165.1000375
Copyright: © 2019 Bansal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3457
- [From(publication date): 0-2019 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 2585
- PDF downloads: 872