Review Article Open Access
A Review of the Biology and Ecological Interactions of Oncideres (Cerambycidae): Brazilian Wood Borers Species
Paulino-Neto HF*
Professor Pesquisador do Curso de Ciências Biológicas e responsável pelo Laboratório de Ecologia da Polinização, Evolução e Conservação (LEPEC) da Universidade do Estado de Minas Gerais (UEMG), Brasil.
- *Corresponding Author:
- Paulino-Neto HF
Professor Pesquisador do Curso de Ciências Biológicas
e responsável pelo Laboratório de Ecologia da Polinização, Evolução e Conservação (LEPEC)
Universidade do Estado de Minas Gerais (UEMG), Brasil
Tel: (035) 35296058
E-mail: hipolitopaulino@gmail.com
Received Date: December 22, 2016; Accepted Date: December 26, 2016; Published Date: December 30, 2016
Citation: Paulino-Neto HF (2016) A Review of the Biology and Ecological Interactions of Oncideres (Cerambycidae): Brazilian Wood Borers Species. J Ecosys Ecograph 6: 223. doi:10.4172/2157-7625.1000223
Copyright: © 2016 Paulino-Neto HF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
These insects have a cosmopolitan distribution and are especially abundant and diversified in the tropics. Many cerambycid species are considered pests in various parts of the world because of their ability to damage wood and wooden constructions. This damage frequently results in important economic losses because of the reduction in wood quality and commercial value. These insects can also alter the recruitment and age structure of host plant populations.
Keywords
Cerambycidae; Larvae; Insect-plant interation; Longevity; Mating ecology; Oncideres; Wood borers
Introduction
Distribution and importance
The Cerambycidae is one of the major insect groups, with about 35,000 species [1]. These insects have a cosmopolitan distribution and are especially abundant and diversified in the tropics [2,3]. Cerambycid larvae are woodborers and use living [4,5] or dead [5,6] trunks. Attack by these borers rapidly stresses host plants [7] and leads to their death in most cases [8]. Many cerambycid species are considered pests in various parts of the world because of their ability to damage wood and wooden constructions. This damage frequently results in important economic losses because of the reduction in wood quality and commercial value [3,6,9-11].
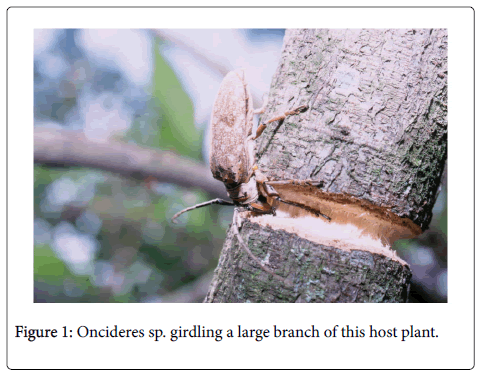
In addition to their ability to girdle and bore wood, the Cerambycidae can also cause death of the host plant indirectly by acting as vectors for nematodes [12], fungi and bacteria [8]. These insects can also alter the recruitment and age structure of host plant populations [13,14]. According to Romero et al. [14], 43% of host plants died after girdling by a cerambycid species and this resulted in an altered population structure in the next year, with a greater use of new host plants (Figure 1).
Biology
Habits
Most adult cerambycids are diurnal [5,15,16], but some species are active during the night [12,17]. The adults feed on the nectar and pollen of the host plants [6,16], as well as flowers [18-20], fruits, leaves, bark [5,18] and roots [18]. Matter et al. [19] reported that cerambycids use flowers as shelter against predators and for mating.
Longevity
The life-span of most adult cerambycids is less than three months [3,5,6,10,16,21,22], with the entire adult phase being devoted to reproduction [3,10,16,18,19,21,23,24].
Mating ecology
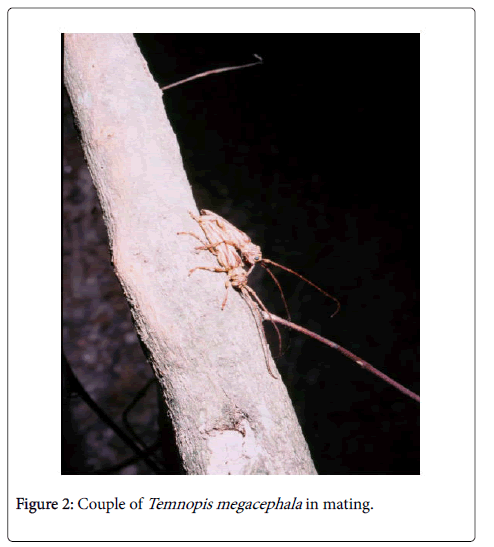
Cerambycids generally show no mating behavior since adult females apparently accept any male, perhaps because of the difficulty in encountering males [16]. Males search for females and also dispute them with other co-specific males through aggressive confrontations in which the winner expels or kills the weaker male and subsequently monopolizes females, food resources and oviposition sites [12,16]. When the new male wins the confrontation, it immediately copulates with the female many times between each egg-laying session. Sometimes the female can pull violently the male or the male can abandons the female and the host plant to practice other activities. In other cases, the male may abandon the female to mate with other females that are close by [12]. Mating with the same female may confer a reproductive advantage since females that copulate more than once have a larger number of fertile eggs and lay more eggs than females that mate only once [25] (Figure 2).
When the host plants are abundant and uniformly distributed, males have more difficulty in finding females because they need to move greater distances to locate them. Consequently, males defend their females even more aggressively than they do food resources. Cerambycids defend their host plants aggressively especially when the plants are disperse and/or limited, at which time the males congregate on a few plants. In addition, the high density favors the identification of potential partners. Thus, the mode that the food resources are distributed can influence the ecology of the mating systems and it also influences the life-span of cerambycids, which tends to be greater when the resources are widely distributed [16].
Another important factor in the reproduction of cerambycids is the diameter of the branches of the host plant on which the larvae will be develop since this parameter can directly affect the body weight of adults (adults that develop on stems or branches with larger diameters tend to be heavier) [18,26]. Reproductively, a greater body weight is extremely important since larger and heavier females produce larger eggs and lay more eggs than small females [25]. On the other hand, larval predation and parasitism are greater and offspring fitness is lower on girdled stems or branches with a small diameter. In contrast, stems or branches with a very large diameter can result in unnecessary energy expenditure; the extra time spent in a given activity does not result in any major advantage and the females are more exposed to natural enemies resulting in greater predation and less probability of concluding the activities necessary for oviposition [14]. Additionally, the sexual ratio, which in most species is 1:1 [22], can be altered by parental manipulation when cerambycids lay eggs that will yield females on thick stems or branches and males on thin stems or branches [23].
However, the diameter of the host-plant not is the only factor that affects the interaction between cerambycids and their host plants since these coleopterous also attack fallen, moribund or stressed trees [5-9,12,15,18,27-29]. The density of cerambycids is determined by the abundance of host plants [8,28,30] and these insects are particularly associated with plants that grow in forest clearings [28].
Introduction into a new habitat
When cerambycids and their host plants are introduced into a new habitat these insects may become important pests because of the absence of natural enemies [6,31]. Alternatively, the high level of physiological stress in the host plants may result in poor adaptation to the new habitat and this could make the plants more susceptible to attack by cerambycids and other herbivores [6,7,9].
Localization and use of the host plant
Most adult cerambycids locate their host plants mainly through scent [9,18,29,31] that is detected by sensillas in the antennas [18]. Host plants with characteristics that favor colonization tend to have a patchy distribution, which means that adult cerambycids must be able to disperse well and have a good ability to locate host plants [32]. The volatile scent compounds released by the host plants must attract specialist cerambycids and repel generalists, and at the same time stimulate feeding, mating and oviposition [3,9]. The initial attack by cerambycids may result in the host plants releasing additional volatile chemicals that are even more concentrated in order to facilitate their localization and to attract sexual partners [9,33] (Figure 3). When a host plant is located, the females of some species girdle suitable branches and lay their eggs on them [4,5,10,34,35]. In other species, the females do not girdle their hosts but bore into them [12] while still others use plants that have already been girdled by other cerambycid species [6].
Constraints in the colonization and use of host plants
Many plant species have a variety of constraints to their colonization and use as host plants by Cerambycidae, e.g., low nutritional quality [8,31,36], the presence of resin [8], the absence of compounds that stimulate oviposition, chemical and physical barriers that prevent the larvae from crossing the stem suber to reach the pith and its tissues rich in nutrients [19,36]. Several characteristics of the wood also limit infestation by borers, including the stem or branch diameter [4,13,14,36,37], hardness and the absence of stimuli for feeding [36]. According to Paulino-Neto [38] and Paulino-Neto et al. [4], cerambycids select branches for oviposition based on their diameter and the number of secondary branches so that host plants with a trunk diameter smaller or larger than the ideal diameter range are not used by these insects.
Plants can also defend themselves indirectly by attracting natural enemies of their herbivores [33]. In this case, the damages caused by herbivores causes the release of volatile chemicals that attract the predators of these herbivores. In some cases, the attraction of predators can be sufficiently efficient to eliminate 90% of the herbivores [33].
Environmental factors can also affect the level of infestation by cerambycids by influencing the rate of desiccation, the inner temperature of girdled branches and the accessibility to natural enemies [30]. Low temperatures increase the mortality ratio and reduce the emergence of adults and their body weight [10,22]. Among the various factors, excessive humidity is one of the most important because it reduces the egg eclosion ratio [8] and prevents larvae from reaching the pith [6,7,10,22]. Some species avoid excessive humidity by infesting stems on the side most exposed to direct sunlight [23].
Host plant selection
The choice of sites for egg laying is critical for woodborers because the larvae are unable to compensate the low nutritional quality of a host plant by moving elsewhere as do many other insect groups [25]. Consequently, adult females must select the host plants that provide the greatest fitness for their offspring [2,14,36].
The specificity of herbivores means that they must carefully assess several important characteristics of the potential host plant, including its diameter, age, height, vigor and nutritional quality to ensure successful colonization [4,5,14,34,38]. A close relationship between the site chosen for egg laying and subsequent larval development has been recorded for many herbivorous insects that use specific host plants [39]. The success rate is generally low in plants already infested or that have a high level of larval predation [8]. Williams [40] reported high usage of the more abundant host plants. Coulson [8] observed a lower rate of usage in more resistant host plants.
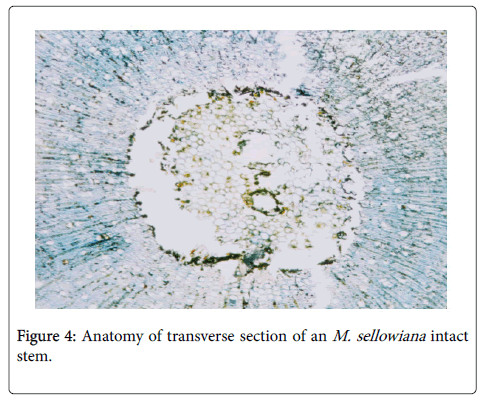
Plant families belonging to the order Myrtales have abundant parenchyma and phloem in the central pith [41] and some cerambycid borers choose these species as host-plants, as in the case of O. humeralis Thorms (Lamiinae) which uses Melastomataceae sp. [5] and other cerambycid species that girdle and bore plant species of other families, e.g., Myrtaceae that also belongs to Myrtales. Berkov [3] reported that cerambycids also prefer Lecythidaceae, which belongs to Ericales; according to Metcalfe et al. [41] these plants also have intraxillary phloem. Hence, the wood anatomy can be a very important factor in the choice of host plants by cerambycids (Figure 4). In this context, understanding how the choice of the host plant influences subsequent offspring performance is fundamental for studying the ecological relationships among herbivorous insects and their respective host plants [3,19,20,36].
Egg-laying behavior
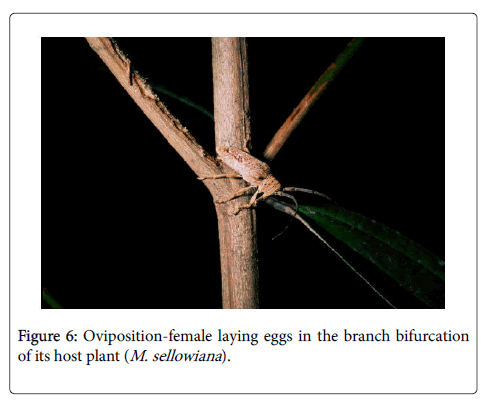
Once the host plant has been chosen, females can oviposit in cracks and bark crevices of the stems or branches [6,15,23]. More specialized behavior occurs in the genus Oncideres (subfamily Lamiinae), in which the females use their mandibles to girdle young plants and branches [5,35,37] and prepare the oviposition site by perforating and chewing a slit in the bark before introducing the ovipositor organs beneath the bark [5,24] (Figures 5 and 6).
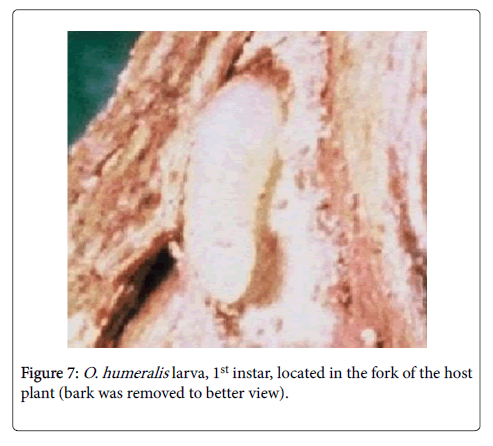
Paulino-Neto et al. [5] found that cerambycids can lay their eggs in branch forks because this region offers optimal conditions for the initial larval development (Figure 7). The branch forks contain parenchymal tissue and histological analysis has shown that these sites are the softest parts of the trunks and branches and provide an entrance for newly hatched larvae (Figures 8 and 9). Since the presence of forks in the selected branch is very important for its colonization and for successful larval development, females select branches by evaluating the number of secondary branches (ramifications) [4]. Subsequent observations have shown that other cerambycid species also lay eggs in branch forks. However there are many species of cerambycid that not lay their eggs exclusively in the forks. These species usually oviposite along the length of branch girdled [35]. The extent to which this behavior is common in the Cerambycidae remains to be determined.
Egg and larval period
The time between oviposition and eclosion of cerambycid eggs ranges from three to seven days [15], but may be as long as 25 days [5] (Figure 7). The first instar larvae feed on bark [6,32] and parenchymatous cells of the stem [5] (Figure 9), with the entire larval development occurring within girdled branches; last instar larvae construct their pupal chamber by obstructing the tunnel with frass (Figure 10). The galleries prepared by the larvae are used as shelter against predation and parasitism [5,6,8,22,31].
The duration of the larval period varies considerably from less than one year [6,22,31], with two offspring per year [6,21,22] to more than one year [5,10,23,24,42]. The length of the larval phase varies between males and females, and is generally greater for females since they start to feed later and therefore emerge after the males [23].
Larval competition
Various studies have shown that competition for food resources at high larval densities increases the mortality rate in Cerambycidae [8,9,22,26,37] and/or decreases the body weight of adults [22]. Larval cannibalism may occur when the competition is intense [9].
Natural enemies
The most important natural enemies of the Cerambycidae are parasitoid wasps such as the Braconidae [5,23,26,43], Encyrtidae, Eurytomidae [43] and Ichneumonidae [23,26,42,43] as well as the Megalyridae, Pteromalidae, tachinid dipterans [43], woodpeckers [24] and ants [5,11]. Cerambycidae larvae are also killed by fungi and other pathogenic microorganisms [8].
Biology of adults
Cerambycid adults emerge from the pupal chamber by chewing a circular exit hole through the bark [5,6,22,31] but can remain within their host plants until there are favorable environmental conditions for them to emerge [8]. As soon as the adults emerge, they fly to host plants where they feed on the bark of young branches [5,37]. Feeding on young branches is an important physiological necessity that allows the maturation of immature eggs in the emerging females [37]. The availability of food, e.g., nectar, pollen and sweet secretions, and favorable climatic conditions (temperature and humidity) increase adult longevity and female fecundity [44].
Specificity levels
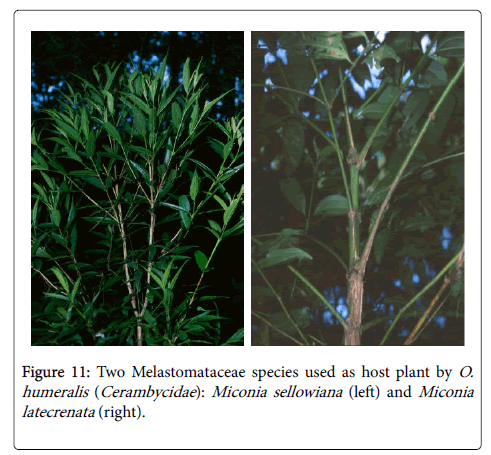
Cerambycids may show host plant specificity [2,15,45] by using plant species of the same genus or family [2,4-6,14,21,22,24,31,36,45] or may show no specificity [23,45] (Figure 11).
On the other hand, a single plant species may be colonized simultaneously by two cerambycid species [5,38] or many species may share the available resources by occupying different layers from the bark to the center of the pith or different parts of girdled stems or branches [5,46] (Figure 12). Monophagy is apparently uncommon in forests with a high diversity and, where observed, may represent an artifact of the sampling profile or protocol [2].
Figure 12: Two cerambycid species colonizing simultaneously Miconia latecrenata (Melastomataceae). O. humeralis (right) saws M. latecrenata and its larvae bores this girdled branch. T. megacephala (left), species, species smaller than O. humeralis , uses opportunistically this sawed branch to oviposit and its larvae bore the branch’s extremities, avoiding interspecific competition.
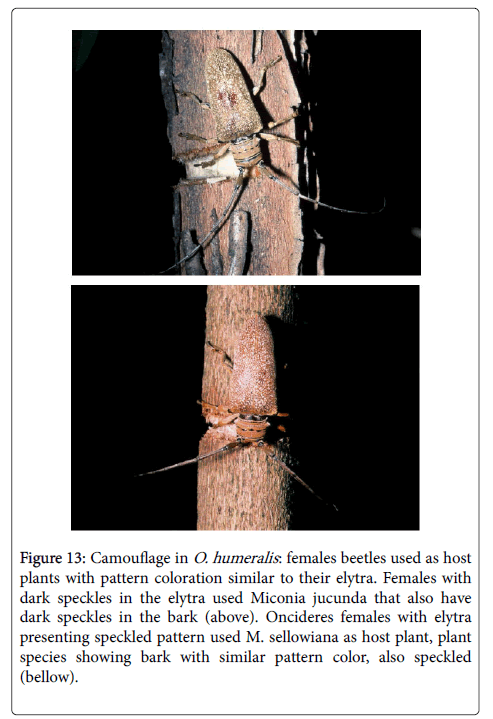
Camouflage
Paulino-Neto [38] and Paulino-Neto et al. [5] discussed the camouflage shown by O. humeralis in which the color pattern of these species elytra resembled that of the bark on the lower parts of the melastome branches used as host plants. Some adult females of this species had shown an interesting phenotypic variation in which their elytra have dark speckles. These females usually girdled Miconia jucunda Triana (Melastomataceae) in which the speckled pattern of the stem bark was very similar to that of these variant elytra. Female O. humeralis that had not dark speckles used other species of Melastomataceae in the study area (Figure 13).
Figure 13: Camouflage in O. humeralis : females beetles used as host plants with pattern coloration similar to their elytra. Females with dark speckles in the elytra used Miconia jucunda that also have dark speckles in the bark (above). Oncideres females with elytra presenting speckled pattern used M. sellowiana as host plant, plant species showing bark with similar pattern color, also speckled (bellow).
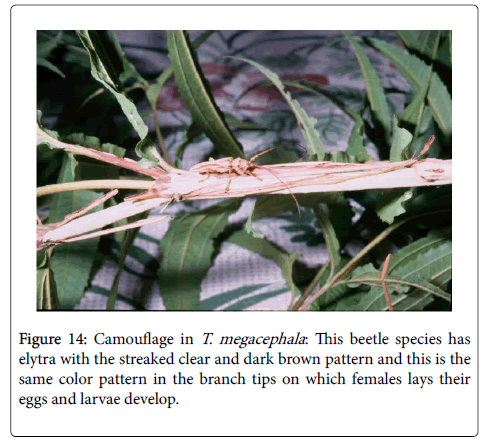
In a similar arrangement, the streaked clear and dark brown pattern of adult T. megacephala Germ (Lamiinae) blended well with the branch tips on which these insects occurred most commonly. The color patterns of these species probably represent a defense strategy to avoid or minimize their detection by visually predators such as birds and wasps. However, camouflage as a defense strategy needs be studied better in the two species mentioned above, as well as in other cerambycids (Figure 14).
Acknowledgement
H. F. Paulino-Neto thanks the “Universidade do Estado de Minas Gerais – UEMG, Unidade Passos for provide adequade working conditions and structure of the our laboratory "(Laboratório de Ecologia da Polinização, Evolução e Conservação - LEPEC)”. Paulino- Neto thanks Milene Souza Rodrigues Paulino (my wife) for unconditional support during all phases of this study and Otávio Rodrigues Paulino (my son) for the contagious joy and inspiration to always move on.
References
- Lawrence JF (1982) Coleoptera. In: Parker S (ed.) Synopsis and classification of living organisms. New York: McGraw Hill. pp: 482-553.
- Berkov A, Tavakilian G (1999) Host utilization of the Brazil nut family (Lecythidaceae) by sympatric wood-boring species of Palame (Coleoptera, Cerambycidae, Lamiinae, Acanthocinini). Biol J Linn Soc 67: 181-198.
- Berkov A, Meurer-Grimes B, Purzycki KL (2000) Do Lecythidaceae specialists (Coleóptera, Cerambycidae) shun fetid tree species? Biotropica 32: 440-451.
- Paulino-Neto HF, Romero GQ, Vasconcellos-Neto J (2005) Interactions between Oncidereshumeralis Thomson (Coleoptera: Cerambycidae) and Melastomataceae: Host-Plant Selection and Patterns of Host Use in South-East Brazil. NeotropEntomol 34: 7-14.
- Paulino-Neto HF, Vasconcellos-Neto J, Carmello-Guerreiro SM (2006) The biology of OncidereshumeralisThorms (Coleoptera: Cerambycidae: Lamiinae) and new Cerambycidae-Melastomataceae host-plant associations. Stud Neotrop Fauna Environ 41: 227-233.
- Hanks LM, Paine TD, Millar JG (1991a) Mechanisms of resistance in Eucalyptus against larvae of the eucalyptus longhorned borer (Coleopetra: Cerambycidae). Environ Entomol 20: 1583-1587.
- Chacaras C (1969) Biology and ecology of Phorachatasemipunctata F. (ColeopteraCerambycidae xylophage) eucalyptus pest in Tunisia, and methods of protection of stands. Ann Inst Nat Rech For Tunisia 2: 1-37.
- Coulson RN (1979) Population dynamics of bark beetles. Ann Rev Entomol 24: 417-447.
- Powell W (1978) Colonisation of twelve species of Eucalyptus by Phoracantasemipunctata (F.) (Coleopetra: Cerambycidae). Malawi. Bull Ent Res 68: 621-626.
- Cannon KF, Robinson WH (1982) An artificial diet for laboratory reasing of the old house borer, Hylotrupesbajulus (Coleoptera: Cerambycidae). Can Entomol 114: 739-742.
- Way MJ, Cammell ME, Paiva MR (1992) Studies on egg predation by ants (Hymenoptera: Formicidae) especially on the eucalyptus borer Phoracanthasemipunctata (Coleoptera: Cerambycidae). Portugal Bull Entomol Res 82: 425-432.
- Edwards OR, Linit MJ (1991) Oviposition behavior of Monochamuscarolinensis (Coleoptera: Cerambycidae) infested with the pinewood nematode. Ann EntomolSoc Am 84: 319-323.
- Caraglio Y, Nicolini E, Petronelli P (2001) Observations on the links between the architecture of a tree (DicoryniaguianensisAmshoff) and Cerambycidae activity in French Guiana. J Trop Ecol 17: 459-463.
- Romero GQ, Paulino-Neto HF, Vasconcellos-Neto J (2005) The effects of the wood-boring Oncidereshumeralis (Coleoptera, Cerambycidae) on the number and size structure of its host-plants in south-east Brazil. J Trop Ecol 21: 233-236.
- Papp RP, Samuelson GA (1981) Life history and ecology of Plagithmysusbilineatus, an endemic hawaiian borer associated with Ohialehua (Myrtaceae). Ann EntomolSoc Am 74: 387-391.
- Goldsmith SK (1987) The mating system and alternative reproductive behaviors of Dendrobiasmandibulares (Coleopetra: Cerambycidae). BehavEcolSociobiol 20: 111-115.
- Hanks LM, Millar JG, Paine TD (1990) Biology and ecology of the eucalyptus longhorned borer (Phoracanthasemipunctata F.) in southern California. In: Adams D, Rios J (eds.), Proceedings, 39th meeting of the California forest pest council, 14-115 November 1990. California Department of Forestry and Fire Protection, Sacramento, CA. pp: 12-16.
- Linsley EG (1959) The ecology of the Cerambycidae. Ann Rev of Entomol 4: 99-138.
- Matter SF, Landry JB, Greco AM, Lacourse CD (1999) Importance of floral phenology and florivory for Tetraopestetraphthalmus (Coloeptera: Cerambycidae): tests at the population and individual level. Environ Entomol 26: 1044-1051.
- Matter SF (1997) Population density and area: the role of between-and within-patch processes. Oecologia 110: 533-538.
- Pershing JC, Linit MJ (1986) Development and seasonal occurrence of monochamuscarolinensis (Coleoptera: Cerambycidae) in Missouri. Environ. Entomol 15: 251-253.
- Hanks LM, Millar JG, Paine TD (1991b) Evaluation of cold temperatures and density as mortality factors of the eucalyptus longhorned borer (Coleoptera: Cerambycidae) in California. Environ Entomol 20: 1653-1658.
- Starzyk JR, Partyka M (1993) Study on the morphology, biology and distribution of Obriumcantharinum (L.) (Col., Cerambycidae). J ApplEnt 116: 333-344.
- Solomon JD (1977) Biology and habits of oak branchs bores (Goes debilis). Ann EntomolSoc Am 70: 57-59.
- Lawrence WS (1990) Effects of body size and repeated matings on female Milkweed beetle (Coleopetra: Cerambycidae) reproductive success. Ann EntomolSoc Am 83: 1096-1100.
- Ikeda K (1979) Consumption and food utilization by individual larvae and the population of a wood borer PhymatodesmaakiKraatz (Coleoptera: Cerambycidae). Oecologia 40: 287-298.
- Hanks LM, Paine TD, Millar JG, Hom JL (1994) Variation among Eucalyptus species in resistance to eucalyptus longhorned borer in southern California. EntomolExpAppl 74: 185-194.
- Barbalat S (1996) Influence of logging on three families of wood - related beetles in the Gorges de I'Areuse. Rev Am Zool 103: 553-564.
- Fan J, Kang L, SUN J (2007) Role of Host Volatiles in Mate Location by the Japanese Pine Sawyer, Monochamusalternatus Hope (Coleoptera: Cerambycidae). ChemEcol 36: 58-63.
- Cole WE (1973) Crowding effects among single-age larvae of the mountain pine beetle, Dendroctonusponderosae. Environ Entomol 2: 285-293.
- Hanks LM, Paine TD, Millar JG (1993) Host species preference and larval performance in the wood-boring beetle Phoracanthasemipunctata F. Oecologia 95: 22-29.
- Haack RA, SlankyFJr (1987) Nutritional ecology of wood-feeding Coleoptera, Lepidoptera and Hymenoptera. In: SlankyFJr, Rodriguez JG (eds). Nutritional ecology of insects, mites, spiders and related invertebrates. John Wiley. New York.pp: 449-456.
- Kessler A, Baldwin T (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141-2144.
- Rice ME (1995) Branch girdling by Onciderescingulata (Coleoptera: Cerambycidae) and relative host quality of Persimmom, Hickory, and Elm. Ann EntomolSoc Am 88: 451-455.
- Paro CM, Arab A, Vasconcellos-Neto J (2014) Specialization of Atlantic rain forest twig-girdler beetles (Cerambycidae: Lamiinae: Onciderini): variation in host–plant use by microhabitat specialists. Arthropod-Plant Interactions 8: 557-569.
- Hanks LM, Millar JG, Paine TD (1995) Biological constraints on host-range expansion by the wood-boring beetle Phoracanthasemipunctata (Coleoptera: Cerambycidae). Ann EntomolSoc Am 88: 183-188.
- Rice ME (1989) Branch girdling and oviposition biology of Onciderespustulatus (Coleoptera: Cerambycidae) on Acacia farnesiana. Ann EntomolSoc Am 82: 181-186.
- Paulino-Neto HF (2004) Lumberjacks of nature. Science Today 35: 67-69.
- Myers JH, Monro J, Murry N (1981) Egg clumping, host plant selection and population regulation in Cactoblastiscactorum (Lepdoptera). Oecologia 51: 7-13.
- Williams KS (1983) The coevolution of Euphydryaschalcedona butterflies and their larval host plants. Oecologia 56: 323-329.
- Metcalfe CR, Chalk L (1950) Anatomy of dicotyledons. Part 1, Oxford: Clarendon Press. pp: 637-649.
- Di Iorio OR (1996) Cerambycidae y otrosColeoptera de LeguminosaecortadasporOncideresgermari (Lamiinae: Onciderini) en Argentina. Rev Biol Trop 44: 551-565.
- Austin AD, Quicke DLJ, Marsh PM (1994) The hymenopterous parasitoids of eucalypt longicorn beetles, Phoracantha spp. (Coleoptera: Cerambycidae). Aust Bull Entomol Res 84: 145-174.
- Hougardy E, Grégoire JC (2000) Spruce stands provide natural food sources to adult hymenopteran parasitoids of bark beetles. EntomolExpAppl 96: 253-263.
- Tavakilian G, Berkov A, Meurer-Grimes B, Mori S (1997) Neotropical tree species and their faunas of xylophagouslongicorns (Coleoptera: Cerambycidae) in French Guiana. Botan Rev 63: 303-355.
- Starzyk JR, Witowski Z (1986) Dependence of the Sex ratio of cerambycid beetles (Col. Cerambycidae) on the size of their host trees. J ApplEnt 101: 140-146.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 4669
- [From(publication date):
December-2016 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 3710
- PDF downloads : 959