Adsorbers for Selective Removal of Extra Domain A-fibronectin from Patient Plasma
Received: 15-May-2017 / Accepted Date: 24-May-2017 / Published Date: 29-May-2017
Abstract
In patients with collagen diseases such as rheumatoid arthritis and systemic lupus erythematosus, immune complexes circulate in the blood deposits on tissues, causing inflammation, sclerosis, and deformable symptoms accompanying joint pain. These complexes form cryogels on cooling and can be removed by cryofiltration therapy. Fibronectin in the blood is a mediator of vascular inflammation and its levels are elevated in patients with fibromyalgia. In particular, the extra domain A containing FN (EDA-FN), which is important for cryogel formation, occurs in high concentration in the plasma of patients with rheumatoid arthritis. Gellan sulfate (GS) was developed to selectively remove EDA-FN from blood. Removal of EDA-FN with GS could be effective for suppressing inflammation caused by immune complex deposition such as in autoimmune disease and IgA nephropathy.
Keywords: Extra domain A-fibronectin; Cryogel; Gellan sulfate; Adsorber; Rheumatic arthritis
4993Introduction
In patients with collagen diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), immune complexes (ICs) circulating in the blood deposit on tissues, causing inflammation, sclerosis, nephropathy, and degenerative symptoms. ICs contain complement proteins and antibodies such as immunoglobulin G (IgG), IgA, and IgM, and the extracellular matrix binds to this complex. ICs deposit on joints, which activates neutrophils, causing inflammation and promoting tissue injury. ICs possess gelling properties (cryogel) [1-6] upon cooling. Circulating immune complexes (CICs) are mainly composed of the IgA complex [7,8].
Fibronectin (FN) is an extracellular matrix protein that is involved in cell adhesion, proliferation, and migration, whereas the extra domain A-containing fibronectin (EDA-FN) is a type of cellular FN. Small amounts of EDA-FN are involved in the formation of ICs, and its concentration in the blood of patients with RA is higher than those in healthy subjects [9,10].
Although the factors triggering the formation of ICs is unknown, a method of removing symptoms by therapeutic apheresis has been studied (cryofiltration) [11,12]. Therefore, selective EDA-FN removal from patient blood inhibits complex formation, reduces deposition, and may alleviate symptoms of collagen disease. Here, we reviewed materials that have been developed for adsorption removal therapy of EDA-FN although such therapy is still in the basic stage of research.
Cryogel and cryofiltration as therapeutic plasmapheresis
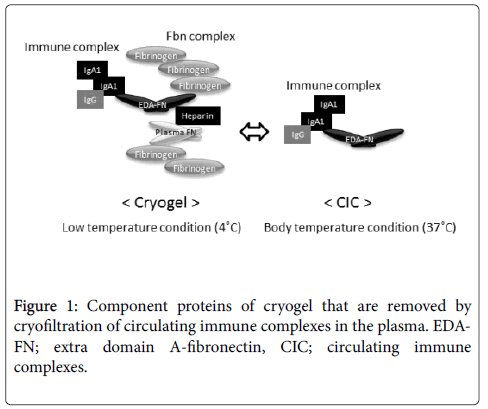
First, we would discuss the effect of removing EDA-FN cryogels from the blood of patients with collagen diseases. Cryogels (cryoprecipitate) contain ICs that are formed in the blood of patients [1-6] with collagen disease such as rheumatoid arthritis upon addition of heparin, an anticoagulant, and incubating it at low temperature (4°C). This cold insolubility is thermo-reversible and is noticeable in the blood of patients with diseases that are particularly accompanied by inflammation. Cryofiltration, which consists of plasma cooling and filtration with a porous polymer membrane, is a form of therapeutic plasmapheresis that attempts to remove cryogels from the plasma of affected patients [11,12]. Studies demonstrated that removal of cryogels using this therapy was effective in alleviating pain of patients with RA. Cryogel formation is affected by the addition of heparin to fibrinogen and fibronectin combined with low temperature, and is not caused by ICs itself. In addition, evidence shows that cellular FN is found in the cryogels derived from patients [9]. Investigations showed that EDA-FN levels were high in patients with RA. In addition, the EDA-FN plasma level corresponded with changes in joint pain. EDA-FN in cryogel was 51 times more than EDA-FN in plasma, and it was suggested that EDA-FN level was most efficiently reduced by cryofiltration [10]. In addition, the interaction between EDA-FN and heparin is more important than that with plasma FN for cryogelation under physical conditions; temperature-induced structure change and self-coagulation of fibrinogen were responsible for this interaction [13]. Plasma FN lacking EDA segments possess a looped compact conformation that can be stretched to an unfolded conformation [14,15]. In other words, the effective incorporation of EDA-FN in cryogels at low temperature is responsible for its therapeutic effect (Figure 1).
The excessive EDA-FN in patient plasma promotes cell proliferation around the tissues where ICs are deposited, which causes sclerosis associated with chronic inflammatory state and promotes organogenesis. Therefore, regulating the concentration of these complexes in the blood by various removal strategies should be effective.
Development of adsorbent materials for selective EDA-FN removal
Next, we would describe the development of a method for selective removal of EDA-FN, which is considered to trigger cryogel formation. Many proteins that form cryogels possess normal biological functions and do not appear to be associated with the cause of the disease. In other words, methods for the removal of ICs from the blood of patients with collagen diseases should aim to selectively remove EDA-FN (the primary cause of cryogelation and inflammation) to obtain the same effect as removal of EDA-FN by cryofiltration. For this purpose, use of adsorbents containing immobilized artificial ligands for EDA-FN would circumvent the cooling step after plasma separation that is required in the cryofiltration method.
During cryogel formation, heparin interacts strongly with FN. In particular, EDA-FN possesses an opened-structure site that allows easy binding of the heparin binding domain to heparin, which subsequently increases the affinity for heparin [16]. For this reason, heparin was used first as a ligand material for removal of the EDA-FN complex. However, side effects caused by the removal of heparin-binding proteins from blood, particularly blood coagulation factors such as antithrombin III (ATIII) and growth factors such as basic fibroblast growth factor (bFGF) was a matter of concern.
Therefore, a material with a more selective sulfated polysaccharide structure was studied. Sulfated cellulose [17], amino-cellulose [18], gellan sulfate (GS) [19,20] and similar molecules were examined. Studies showed that the binding of EDA-FN could be controlled by varying the sulfation position in the polysaccharide structure such as in cellulose and gellan. Among these, GS was selected as the most suitable adsorbent for EDA-FN removal because of its high affinity for EDA-FN and poor binding with other heparin-binding proteins such as ATIII in the plasma [19]. Gellan is a polysaccharide of microorganism origin, which is also used in food and is a novel material that can be modified chemically by introducing sulfate groups. GS binds to EDA-FN at a site that is different from the binding site of heparin, and it contains a chemically controllable binding position of the sulfate group.
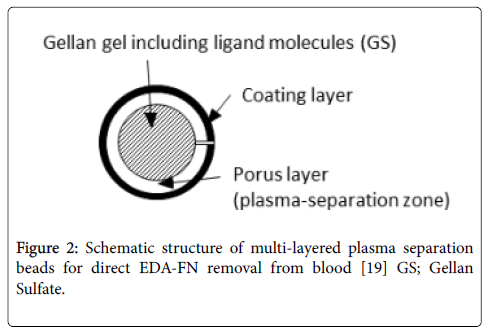
Furthermore, the blood adsorption system is generally used after plasma separation to avoid adhesion of platelets and white blood cells to the adsorbing material; however, a structure capable of direct blood adsorption has also been developed [20]. Here, we prepared a novel adsorber for the direct removal of EDA-FN from patient blood. The adsorber possesses plasma separation and EDA-FN trapping function, and is prepared by cross-linking GS and surface-coated gellan. The materials have a multi-layer structure, containing of a porous outer layer and an underlying GS phase. (Figure 2). The problems encountered with direct adsorbers, such as those regarding selectivity and plasma separation, have been solved using this material.
Figure 2: Schematic structure of multi-layered plasma separation beads for direct EDA-FN removal from blood [19] GS; Gellan Sulfate.
Significance of EDA-FN removal therapy
Finally, we discuss why the EDA-FN removal therapy is expected to successfully remove CICs based on the principles of antigen-antibody reaction. EDA-FN, a protein with normal physiological functions [21], is naturally expressed during development, cell proliferation, and inflammation associated with tissue repair [22,23]. In contrast, it is also highly expressed during metastasis and proliferation of cancer cells. It is a mediator of vascular inflammation [24,25] and its levels are elevated in patients with fibromyalgia syndrome [26]. Regulation of the blood concentration of EDA-FN by selective EDA-FN removal therapy using GS may be effective in such cases. In addition, CICs in patient blood, and not the large-sized cryogels, are deposited in the mesangial region of the renal glomeruli, which causes IgA nephropathy (IgAN) accompanied by cell proliferation and vascular destruction. A deposition phenomemon has been reported in patients with IgAN who contain high levels of circulating FN-IgA complexes [27,28]. Both IgA and EDA-FN concentrations are high in the blood of patients of IgAN, which correlated with the severity of the disease. Interestingly, both EDA-FN and IgA were adsorbed and removed effectively and simultaneously from the blood of patients with IgAN using GS [29], which demonstrates the applicability of the EDA-FN removal therapy.Finally, we discuss why the EDA-FN removal therapy is expected to successfully remove CICs based on the principles of antigen-antibody reaction. EDA-FN, a protein with normal physiological functions [21], is naturally expressed during development, cell proliferation, and inflammation associated with tissue repair [22,23]. In contrast, it is also highly expressed during metastasis and proliferation of cancer cells. It is a mediator of vascular inflammation [24,25] and its levels are elevated in patients with fibromyalgia syndrome [26]. Regulation of the blood concentration of EDA-FN by selective EDA-FN removal therapy using GS may be effective in such cases. In addition, CICs in patient blood, and not the large-sized cryogels, are deposited in the mesangial region of the renal glomeruli, which causes IgA nephropathy (IgAN) accompanied by cell proliferation and vascular destruction. A deposition phenomemon has been reported in patients with IgAN who contain high levels of circulating FN-IgA complexes [27,28]. Both IgA and EDA-FN concentrations are high in the blood of patients of IgAN, which correlated with the severity of the disease. Interestingly, both EDA-FN and IgA were adsorbed and removed effectively and simultaneously from the blood of patients with IgAN using GS [29], which demonstrates the applicability of the EDA-FN removal therapy.
Use of GS would prevent the loss of valuable heparin-binding proteins from blood while removing EDA-FN. However, use of GS in EDA-FN removal therapy is not without limitations. The use of heparin as an anticoagulant for plasmapheresis produces an inhibitory interaction between EDA-FN and ICs. Furthermore, the EDA-FN concentration temporarily increases upon addition of heparin [10]. Therefore, we developed a therapy that can positively remove the deposited EDA-FN via adsorption on GS. EDA-FN removal as a coping therapy for diseases in which EDA-FN is highly expressed is promising, but the future safety of GS and evaluation of its therapeutic effects are required.
Conclusion
EDA-FN removal therapy using GS may be effective in suppressing inflammation caused by IC deposition such as in autoimmune diseases and IgAN.
References
- Meltzer M, Franklin EC (1966) Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med 40: 828-836.
- Seligman M, Danon F, Basch A, Bernard J (1968) IgG myeloma cryoglobulin with antistreptolysin activity. Nature 220: 711-712.
- Christian CL, Hatfield WB, Chase PH (1963) Systemic lupus erythematosus. Cryoprecipitation of sera. J Clin Invest 42: 823-829.
- Hanauer LB, Christian CL (1967) Studies of cryoproteins in systemic lupus erythematosus. J Clin Invest 46: 400-408.
- Weisman M, Zvaifler N (1975) Cryoimmunoglobulinemia in rheumatoid arthritis. Significance in serum of patients with rheumatoid vasculitis. J Clin Invest 6: 725-739.
- Morrison PR, Edsall JT, Miller SG (1948) Preparation and properties of serum and plasma proteins XVIII. The separation of purified fibrinogen from fraction I of human plasma. J Am Chem Soc 70: 3103-3108.
- Novak J, Tomana M, Matousovic K, Brown R, Hall S, et al. (2005) IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504-513.
- Novak J, Vu HL, Novak L, Julian BA, Mestecky J, et al. (2002) Interactions of human mesangial cells with IgA and IgA-containing immune complexes. Kidney Int 62: 465-475.
- Umemoto R, Asakura S, Kariya Y, Yonekawa M, Kawamura A, et al. (1993) Cellular fibronectin in plasma: its implications in fibrinogen-associated cryoprecipitation and other related reactions. Blood Coagul Fibrinolysis 4: 127-131.
- Kawamura A, Yonekawa M, Takahashi M, Meguro J, Yanagida N, et al. (1994) Reduction of EDA(+) fiblonectin and its clinical importance on cryofiltration. Int J Artif Organs 17: 559-564.
- Malchesky PS, Asunuma Y, Zawicki I, Blumenstein M, Calabrese L, et al. (1980) On-line separation of macromolecules by membrane filtration with cryogelation. Artif Organs 4: 205-207.
- Nosé Y, Horiuchi T, Malchesky PS, Smith JW, Matsubara S, et al. (1982) Therapeutic cryogel removal in autoimmune disease: what is cryogel ? Therapeutic Plasmapheresis 2: 15-25.
- To WS, Midwood KS (2011) Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 4: 21
- Miyamoto K, Tokita M, Komai T, Miyashita K, Sakashita E (2001) Cryogelation in vitro. Int J Biol Macromol 28: 183-189.
- Hynes RO (1999) The dynamic dialogue between cells and matrices: Implications of fibronectin’s elasticity. Pro Natio Acad Sci USA 6: 2588–2590
- Miyamoto K, Kodera N, Umekawa H, Furuichi Y, Tokita M, et al. (2002) Specific interactions between cryogel components: role of extra domain A containing fibronectin in cryogelation. Int J Biol Macromol 30: 205-212.
- Miyamoto K, Shimizu M, Tokita M, Komai T (2002) Adhesion of 3-Carboxymethyl-Cellulose- 6-Sulfate to Extra Domain A- Containing Fibronectin: Development of Ligands for Cryogel Removal. J Artif Organs 5: 132-135.
- Miyamoto K, Uchiyama R, Tokita M, Yonekawa M, Kawamura A, et al. (2001) Development of novel polycationic adsorbent for cryogel removal. Int J Biol Macromol 29: 19-24.
- Miyamoto K, Asakawa Y, Arai Y, Shimizu T, Tokita M, et al. (2001) Preparation of gellan sulfate as an artificial ligand to removeal of extra domain A containing fibronectin. Int J Biol Macromol 28: 381-385.
- Miyamoto K, Sugihara K, Abe Y, Nobori T, Tokita M, et al. (2002) Novel plasma-separation dilayer gellan-gellan sulfate adsorber for direct removal of extra domein A containing fibronectin from the blood of rheumatoid arthritis patients. Int J Biol Macromol 30: 197-204.
- Schwarzhauer JE, Patel RS, Fonda D, Hynes RO (1987) Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J 6: 2573-2580.
- Peters JH, Maunder RJ, Woolf AD, Cochrane CG, Ginsberg MH (1989) Elvated plasma levels of ED1+("cellular") fibronectin in patients with vascular injury. J Lab Clin Med 113: 586-597.
- Lemanska-Perek A, Krzyzanowska-Golab D, Pupek M, Klimeczek P, Witkiewicz W, et al. (2016) Analysis of solble molecular fibronectin-fibrin complexes and eda-fibronectin concentration in plasma of patients with atherosclerosis. Inflamation 39: 1059-1097.
- Pay S, Calguneri M, Caliskaner Z, Dinc A, Apras S, et al. (2000) Evaluation of vascular injury with proinflammatory cytokines, thrombomodulin and fibronectin in patients with primary fibromyaliga. Nagoya J Med Sci 63: 115-122.
- Cederholm B, Wieslander J, Bygren P, Heinegard D (1988) Circulating complexes containing IgA and fibronecin in patients with primary IgA nephropathy. Proc Nati Acad Sci USA 85: 4865-4868.
- Davin JC, Li Vecchi M, Nagy J, Foidart JM, Foidart JB, et al. (1991) Evidence that the interaction between circulating IgA and fibronectin is a normal prosess enhanced in primary IgA nephropathy. J Clin Immunol 11: 78-94.
- Miyamoto K, Nomura S (2008) Adsorbent for blood purification and method for treating IgA nephropathy using the same. Japan Patent P4177702.
Citation: Miyamoto K, Hirukawa M, Komai T, Horiuchi T (2017) Adsorbers for Selective Removal of Extra Domain A-fibronectin from Patient Plasma. Fibrom Open Access 2: 122.
Copyright: © 2017 Miyamoto K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 4069
- [From(publication date): 0-2017 - Aug 18, 2025]
- Breakdown by view type
- HTML page views: 3171
- PDF downloads: 898