Research Article Open Access
Agronomic and Physicochemical Evaluation of Sweet Potato [Ipomoea batatas (L.) Lam.] Collections in Ethiopia
| Solomon Ali*, Wassu Mohammed and Beneberu Shimelis | |
| Department of Plant Science, Debre Markos University, Ethiopia | |
| Corresponding Author : | Solomon Ali Department of Plant Science Debre Markos University, Ethiopia Tel: +251914662007 E-mail: ethiotrust@gmail.com |
| Received: February 12, 2015Accepted: June 16, 2015 Published: June 23, 2015 | |
| Citation:Ali S, Mohammed W, Shimelis B (2015) Agronomic and Physicochemical Evaluation of Sweet Potato [Ipomoea batatas (L.) Lam.] Collections in Ethiopia. Adv Crop Sci Tech 3: 172. doi:10.4172/2329-8863.1000172 | |
| Copyright: ©2015 Ali S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The productivity of sweet potato [Ipomoea batatas (L.) Lam.] is mainly dependant on the acquisition accessions which posses desirable traits and development of high yielding varieties with desired quality attributes. For this purpose, Haramaya University collected 116 sweet potato accessions from International and National sources to develop varieties for eastern Ethiopia; however, the accessions characterization and documentation were not exhaustively done to support the improvement program. Therefore, this study was conducted during 2012/2013 cropping season to characterize, evaluate, and documenting of agronomic and physicochemical attributes of sweet potato accessions at Haramaya. Augmented design consisting of 114 entries/tests and two checks were used. Varied number of accessions recorded significantly higher values than the mean of the checks for days to physiological maturity, above ground fresh biomass, storage root fresh weight, total storage root yield, marketable storage root yield, reducing sugar, total sugar, and total starch content, pH, dry matter content, total soluble solid, specific gravity and peel content. Tis-9465-7 had the highest storage root fresh weight yield; marketable storage root yield and total storage root yield and Koka-12 and CN-2069-7 exhibited significantly highest values than mean of the checks for days to physiological maturity and above ground fresh biomass, respectively. CN-1752-14, CN-2056-8 and Tis-80/043-1 for reducing sugar, pH and total soluble solid, respectively, exhibited significantly highest values, while CN-1752-15 recorded the highest total sugar and total starch content. Korojo had significantly highest values for specific gravity, dry matter. Tis-82/0602 were exhibited that the lowest in peel content. Elliptic shape (27.19%) and horizontal constriction (45.62%) defect were dominant in the accessions. Most of the accession had white skin color (22.6%) while 21.92% accessions had creamy flesh color.
| Keywords |
| Sweet potatoes; Proximate analysis; Reducing sugar; Total starch content |
| Introduction |
| The sweet potato[Ipomoea batatas (L.) Lam.] is a dicotyledonous plant which belongs to the family Convolvulaceae. It is a tuberous root crop important for food security and cultivated in over 100 developing countries and ranks among the five most important food crops in over than 50 of those countries. Over 95% of the global sweet potato production is in developing countries. In Ethiopia, sweet potato has been cultivated for the last several years and over 95 percent of the crop is produced in the Southwest, eastern and southern parts, where it has remained for many years as one of the major subsistence crops especially in the periods of drought [1,2]. |
| Sweet potato is cultivated in Ethiopia mostly for human consumption. It ranks third after Enset [Ensete ventricosum (Wele) Cheesman] and Potato (Solanum tuberosum L.) as the most important root crops produced in the countries. Sweet potato covers about 81000 hectares of land in Ethiopia with an average national yield of about <9 t/ha on farm and 25-36 t/ha on research centers [3]. Conservation of genetic diversity within a crop species is the basis of all variety improvement. However, if the improved variety replaces traditional farmers’ varieties, as it often does, the result may still be genetic erosion. Therefore, collecting and conserving farmers’ varieties is an essential activity as equal to improving and disseminating new varieties. Haramaya University has released and made recommendation for cultivation two sweet potato varieties namely; Barkume andAdu for eastern part of the country. Moreover, there were 114 accession was maintained in Ethiopia and the two released varieties were also maintained for years which were obtained from International and local sources. However, extensive agronomic and physicochemical attributes has not been carried out to identify which accession(s) attributed what and potentially used for which purpose(s). This necessitates studying and documenting the agronomic and physicochemical attributes of these accessions. Therefore, this research was initiated with the objective of characterization, evaluation and documenting of agronomic and physicochemical attributes of sweet potato accessions in Ethiopia |
| Materials and Methods |
| Sweet potato accessions were grown using unreplicated plot under rainfed conditions during the year 2012/2013 main cropping season at Haramaya, Ethiopia research field. |
| Description of the experimental materials |
| One hundred fourteen (114) sweet potato accessions and two released varieties (Adu and Barkume) were used in this study. The accessions were collected from eastern Ethiopia, other regions of the country and International Research Canters. The two varieties, Adu and Barkume were released for eastern Ethiopia for cultivation by Haramaya University in 2007 after fulfilling the requirements set by the National Variety Release Committee. The accessions were planted at Haramaya University research field using augmented design in 2012/13 main growing season (Table 1). |
| Experimental design and procedure |
| The Accessions were tested in augmented block design with 19 replications. Each replication contained 6 accessions and 2 checks. Each check was appearing once in each block. The checks were replicated 19 times and 114 entries/tests were not replicated. Hundred cm and 30 cm was maintained between rows and plant, respectively. Twelve holes per plot were prepared and one vine cutting was planted in each hole of the ridge and the size of each plot was 3.3 m × 7 m (23.1 m2). |
| Phenological and growth related traits |
| The following parameters were recorded from 10 plants in each plot left the two plants grown at both ends of each row/plot as border plant. Days to physiological maturity, Number of branches per plant, Vine length (cm) were determined using a standard procedure. Days to physiological maturity was recorded on plot basis. |
| Yield and yield components |
| Root fresh weight (g/plant), Above ground fresh biomass yield (g/plant), Above ground dry biomass (g/plant), Average number of storage roots per plant, Average mass of storage root (g/plant), Marketable storage roots number/plant, Unmarketable storage roots number/plant, Total storage root yield (t/ha), Marketable storage root yield (t/ha) and Unmarketable storage root yield (t/ha): |
| Storage root physical attributes |
| The following storage root physical attributes were recorded as per [4] descriptor for the crop, Storage root shape, Storage root defects, Storage root skin color and Storage root flesh color |
| Chemical attributes of storage roots |
| Chemical attributes of sweet potato accessions storage roots were measured through the following parameters and procedures. Sugar analysis, reducing sugar, total starch content, pH, total soluble solid, specific gravity, moisture content, peel content and dry matter were determined using a standard format |
| Statistical analysis |
| The data were subjected to analysis of variance using the Statistical package for augmented design (SPAD) software [5]. Means that differ significantly were separated using critical difference in each category. |
| Results and Discussion |
| Analysis of variance was computed for 22 phenological, growths, yield, yield components, physical and chemical attributes of sweet potato accessions and are presented in Table 2. The result revealed that the presence of highly significant differences (P<0.01) among accessions for reducing sugar, total sugar, total starch content, pH, total soluble solid, days to physiological maturity and specific gravity while significant differences (P<0.5) was observed for root fresh weight, total storage root yield, marketable storage root yield, dry matter content and peel content. However, non-significant differences among accessions was observed for number of branches per plant, vine length, above ground dry biomass yield, average number of storage roots per plant, average mass of storage roots, marketable storage root number/ plant, unmarketable storage root number/plant and moisture content. |
| As the results are presented in Table 2, there were highly significant (P<0.01) differences between the control (check) varieties for days to physiological maturity, storage root fresh weight, dry peel content, reducing sugar, total sugar, total starch content and total soluble solid while significant (P<0.5) differences were observed for above ground fresh biomass yield, above ground dry biomass yield, marketable storage root yield, dry matter. However, non-significant differences between the check varieties was observed for number of branches per plant, vine length, average number of storage root, marketable storage root number, unmarketable storage root number, average mass of storage root, total storage root yield, pH, moisture content and specific gravity |
| Analysis of variance also exhibited highly significant (P<0.01) differences among tests for days to physiological maturity, total storage root yield, reducing sugar, total sugar, total starch content, pH and specific gravity. Likewise significant (P<0.5) differences were exhibited among test entries for dry matter content, peel content and total soluble solid. However, number of branch per plant, vine length, above ground fresh biomass, above ground dry biomass, storage root fresh biomass, average number of storage root, marketable storage root number, unmarketable storage root number, average mass of storage root, marketable storage root yield, unmarketable storage root yield and moisture content were found to be non significant. The result in Table 2 revealed that the presence of highly significant (P<0.01) differences among test versus control for days to physiological maturity, storage root fresh weight, total storage root yield, marketable storage root yield, total starch content, pH and moisture content while significant differences (P<0.5) was observed for average mass storage root, reducing sugar and total sugar. However, non-significant differences among test versus control were observed for number of branch per plant, vine length, above ground fresh biomass, above ground dry biomass, average number of storage root, marketable storage root number, unmarketable storage root number, unmarketable storage root yield, dry matter content, peel content, total soluble solid and specific gravity. |
| Generally, it was observed significant differences among entries, among test versus control/check varieties, between check varieties of sweet potato studied for considerable number of traits which can be exploited in breeding program or that will allow breeders to select entries for desirable trait(s) that they wish to improve [6]. Describes two basic principles for plant breeding, ‘selection for yield’ and ‘defect elimination’. Therefore, the basic philosophies behind plant breeding programme are to develop cultivars with better yield potential and quality attributes as well as to develop cultivars that have genetic resistance against production hazards that can prevent a cultivar from expressing its yield potential [7]. Based on these philosophies the sweet potato breeding programme may relies on improvement of storage roots yield and improved the quality of the storage roots as per the end use and the observed differences among entries may allow the breeders to use accessions for different objectives. |
| Variation is the occurrence of difference among individuals due to difference in their genetic composition and/or the environment in which they are raised [8,9]. If the character expression of two individuals could be measured in an environment exactly identical for both, difference in expression would result from genetic control and hence such variation is called genetic variation [8]. The presence of variation in the germplasm for the trait of interest is, therefore, very important. Therefore, information generated in this study on variation of accessions can be utilized by the breeders since the observed variability greatly helps in formulating sound crop breeding and improvement program [10] (Table 2). |
| Phonological and growth traits |
| Days to physiological maturity: The data in Table 2 showed that day to physiological maturity among accessions, between check/ control varieties and among test versus control was highly significantly (P<0.001). Accessions registered for physiological maturity ranged from 199 to 111 days. Koka-12 and CN-2059-4 accessions were late maturing than most of the accessions and checks, respectively. However, when early maturing is considered as desirable trait, Korojo-2 and Tis- 9065-5 accessions were earlier than most of the accessions and check varieties. Sixteen accessions mature earlier than checks. |
| These accessions had the advantage of earliness than others. Particularly, Korojo-2 (111 days) and Tis- 9065-5 (120 days) significantly matured earlier than checks while Arebaminch, Koka-12, CN-2059-4 were significantly late maturing than both checks |
| The observed differences of maturity among accessions as well as checks and entries or test accessions may be mainly attributed to the genetic constitution of the new entries as well as check varieties since all accessions were tested in one location with similar management. This suggestion might be supported by Zhang et al., [11] who reported that physiological maturity is genetically controlled trait in sweet potato. In agreement with this study result Teshome et al. [12] reported that four sweet potato varieties tested at Adamitulu was between 114 and 124 days for days to physiological maturity [13]. |
| Number of branches per plant and vine length: Non- significant differences were observed among accession, control, tests and tests versus control for number of branches per plant (Table 2). |
| The number of branches per plant was ranged from 20.447 and 1.47. The largest number of branches per plant was recorded on CN-2065- 15 and CN-2054-7 than most of the accessions and checks. However, Korojo-2 and Tis-82/0602-6 exhibited the lowest number of branches per plant than the rest of the accessions. The observed differences of branch number among accessions as well as checks and accessions may be mainly attributed by the genetic constitution of the new entries as well as check varieties. This suggestion might be supported by Juo and Mukhtar et al. [14,15] who reported that branch number is genetically controlled trait in sweet potato. |
| Similar to the result for number of branches per plant, it was observed that non- significant differences for vine length among accession, control, tests and tests versus control (Table 2). The mean of the check varieties for vine length was 58.487 cm while the mean of accessions was 67.729 cm. The length of vine was ranged between 3.62 and 138.862 cm. Nefissie and CN-2065-5A were exhibited the longest vine than most of the accessions including check while Tis-9465-1 (3.62 cm) and Tis-9465-8 (7.112 cm) was recorded the shortest from the entire accessions. [15] Mukhtar reported that the difference among accession, checks and checks and accession was due to difference in genetic constitution. |
| Mean performances of accessions for yield and yield components |
| Above ground fresh and dry biomass yield: Analysis of variance results presented in Table 2 revealed that the presence of significant (P<0.05) difference between checks/among control for above ground fresh and dry biomass but non- significant differences were observed among accessions, tests and test versus control. Though, statistically non- significant differences were observed among accessions, the variation among accessions was too large which ranged from 265 to 5060 g for above ground fresh biomass. The mean of accessions for above ground fresh biomass was 1875.88 g, while the mean of checks was 1365 g. Accession CN-2059-7 and Koka-9 were registered highest above ground fresh biomass than most of the accessions and checks. However, Neffsie and CN-2065-1 accessions were exhibited the lowest above ground fresh biomass yield than others. |
| The observed differences of above ground fresh biomass among accessions as well as checks and accessions may be mainly attributed by the genetic constitution of the new entries as well as check varieties. This statement is in agreement with Chowdhury and Mukhtar [15,16] who reported that above ground fresh biomass yield is genetically controlled trait in sweet potato. In agreement with this study result [12] reported that the above ground fresh biomass yield of sweet potato varieties were in the range between 429.23 to 2516 g, the mean of accession for above ground dry biomass yield was 952.3 g and mean of checks was 785.5 g. The range for above ground dry biomass was between 210 and 2145 g. Accession Abadiro and Koka-12 were found to be superior for above ground dry biomass yield than most of accessions. On the other hand, CN-1753-8 and Tis-8250-8A were inferior for above ground dry biomass yield since the accessions exhibited the lowest values from all accessions including checks. |
| Storage root fresh weight and average mass of storage root: Analysis of variance showed that there were significant (P<0.05) differences among accessions and the presence of highly significant (P<0.01) differences between check varieties/among control and control versus tests but differences among tests were not significant for storage root fresh weight (Table 2). The mean storage roots fresh weight of accessions and checks were 484.51 and 486.09 g, respectively. The range for storage root fresh weight was between 74.94 to 854.26 g.Tis-9068-7 and Koka-12 were recorded the highest fresh weight than most of the accessions (mean storage root fresh weight) and checks. Likewise, the adjusted means of CN-1754-6 and Nefissie were the lowest storage roots fresh weight from most of accessions. Janssens [17] reported that the difference among accessions for storage roots weights was due to differences in genetic constitution of genotypes tested [18], ewthwaite, Kenneth and Richardson reported that the weight of fresh storage roots of sweet potatoes were ranged from 210 to 716 g and 280 to 1520 g, respectively, which the results are in agreement with the present finding. |
| Non- significant differences were observed among accessions and tests for average mass of storage roots but it was evident that the presence of significant (P<0.05) differences for test versus control (Table 2). The mean of the accessions for average mass of storage root was 140.05 g while the mean of checks was 168.5 g. Accessions for average mass of storage roots ranged between 18 to 318 g.Tis-9060-8 and Tis-82/0602-12 were exhibited the highest average mass of storage root of all entries as well as the mean of checks while CN-1753-13 and CN-1753-14 ranked the first from the last. The observed differences of average mass of storage root among accessions as well as checks and accessions could be due to genetic constitution of the new entries as well as check varieties since all accessions were tested in one location with similar management. This suggestion might be supported by Mukhtar et al. [15] who reported that average mass of storage root is genetically controlled trait in sweet potato. |
| Marketable, unmarketable and total storage root number: Statistically non-significant differences were observed for marketable, unmarketable and total storage root number among tests, accessions and tests versus control (Table 2). However, the range for these parameters was too large. Accessions registered for total marketable storage roots number were 2 to 11, 0.695 to 5.395 and 0.816 to 6.316 marketable and unmarketable root number, respectively. CN-2065-16 and CN-2065-15 had the highest number of marketable storage root where as CN-2065-16 and CN-2065-15 were exhibited the highest number of unmarketable storage root and CN-2065-16 and Becale were recorded the highest number of total storage roots number. on the other hand accession CN-2054-7 and Tis-82/0602-1A were recorded the lowest number of marketable storage roots and Tis-8250-9 and Tis-8441-3 had the lowest unmarketable storage roots number while CN-1753-14 and CN-1753-13 were exhibited the lowest storage roots number from most of accessions. Marketable as well as unmarketable storage roots number was highly controlled by genetic constitution as well as environment [19]. There was a variation in storage roots number between cultivars. The observed differences among accessions in this study might be due to genetic differences since all accession as well as checks receive equal management and treatment. |
| Marketable, unmarketable and total storage root yield: Highly significant (P<0.01) difference was exhibit between test and control and also there was a significant (P<0.05) difference among accessions but the difference among tests was not significant (Table 2) for marketable storage root yield. The result in Table 2 revealed that non-significant differences among accessions, checks, tests and tests versus control for unmarketable storage root yield. The differences which were exhibited among tests and tests versus control were highly significant (P<0.01), but non-significant differences among accessions and between check varieties/ among control for total storage root yield. |
| The mean of accessions for marketable, unmarketable and total storage root yield was 8.143 t/ha, 3.9 t/ha and 12.025 t/ha while the mean of checks for marketable unmarketable and total storage roots yield were 11.77, 4.431 and 16.21 t/ha, respectively. |
| marketable storage root yields of Tis-9465-7 and Tis-82/0602- 12 were highest among test accessions including checks where as Unmarketable storage root yield of Koka-12 and Bacale exhibited the highest and total storage root yields of Tis-9465-7 and Koka-12 recorded the highest among test accessions and checks. However, Abadiro and Tis-82/0602-1B had low yield for marketable storage root yield and unmarketable storage root yields of Tis-9465-1 and CN-2065- 11 were the first from the last in other word those accessions that had lowest unmarketable yield also had highest yield among test accessions. Total storage root yields of Tis-9465-1 and Nefissie were the lowest |
| The range for marketable and unmarketable storage root yields were between 0.512 and 22.088 t/ha, 0.001 and 13.631 t/ha, respectively, and the yields of total storage roots ranged from 2.261 to 28.461 t/ha. Marketable storage root yield of sweet potato was inherited genetically [14]. The current result agree with Mwololo [13] who reported the total storage root yield of sweet potato collections was ranged between 10.3 and 32.22 t/ha. |
| Dry matter and moisture content: Analysis of variance showed that significant (P<0.05) differences among accessions, tests and control but there was non-significant difference among tests versus control for dry matter content while for moisture content it was observed highly significant (P<0.01) differences among test versus control but difference among accessions and control and tests were not significant (Table 2). |
| The mean of accessions were 24.878% and 8.85%, respectively, while the mean of checks were 28.42% and 8.8 %, for dry matter content and moisture content, respectively. Dry matter content was ranged from 13.275% to 40.215 % likewise the range of moisture content was between 1.003% and 16.698%. |
| CN-1753-18 (40.215%) and CN-1753-11 (39.535%) had the highest dry matter content among test accessions including checks. On the other hand, Abadiro and Tis-9468-7 had the lowest dry matter content. Tis-9468-7 and Tis-82/0602-6 were the first in moisture content. On contrary, Becale-B and Tis-8250-2 found to be the first from the last. |
| The observed differences of dry matter content and moisture content among accessions as well as checks may be mainly due to genetic constitution of the new entries as well as check varieties since all accessions were tested in one location with similar management. This suggestion might be supported by Dominguaz [20] who reported that dry matter content is genetically controlled trait in sweet potato. Catherine and Scott [21,22] reported that the dry matter percentage of different sweet potato varieties were between 13.4 and 29.2%, 25.23 and 41.11%, respectively. Tsakama, Fred, Bonsi and Loretan [23-26] also found that the dry matter in storage roots were ranged from 12.5 to 30.2, 29 to 39.07, 25 to 42 and 25.5 to 31.7%, respectively. As reported by Chen et al., [27] the dry matter percentages of Xushu18, Sushu2 and Sushu8 were 31.9%, 36.7% and 18.6%, respectively. The dry matter of Beauregard, White Star and skin of White Star variety were 17.54%, 17.89% and 18.97%, respectively [28]. |
| Specific gravity and peel content: As it was presented in Table 2, highly significant (P<0.01) differences was observed among tests for specific gravity and there was no significant differences among control and tests versus control. Differences among control was highly significant (P<0.01) and for accessions and tests were significant (P<0.05), but it was non-significant among accession and tests (Table 2) for peel content. The mean of accessions for specific gravity and peel content were 2.194 and 34.709, respectively while the mean of checks was 1.75 for specific gravity and 37.225 for peel content. The result showed that the range for specific gravity was between 0.046 and 42.334 and for peel content was 14.41 and 78.04. The result showed that specific gravity of CN-2054-1 and CN-1753-16 were the highest values among test entries and checks. Tis-70557-2 and Tis-8250-2 had the smallest among test accessions as well as checks. The difference observed between checks, accessions and checks and accession was due to differences in genetic constitution. This statement might be supported by Ruinard [29]. |
| Accession Korojo and Tis-82/0602-12 registered for peel content was the highest from most of accession including checks. Whereas accession Tis-82/0602-6 and Tis-9465-9 were the least from all new entries as well as checks. The difference observed between checks, accessions and checks and accession may be due to differences in genetic constitution. This statement might be supported by Surayia [26]. Who reported that genetic constitution of each accession contributed for difference of peel content. |
| Storage root shape, color and defect: Sweet potatoes are nutritious and have numerous health benefits. The orange-fleshed and whitefleshed cultivars are the most familiar to consumers. However, it is also possible to identify other cultivars with varying shape, defect, flesh and skin color. As general remark qualitative traits such as shape, color, defect is genetically controlled traits [30,31]. |
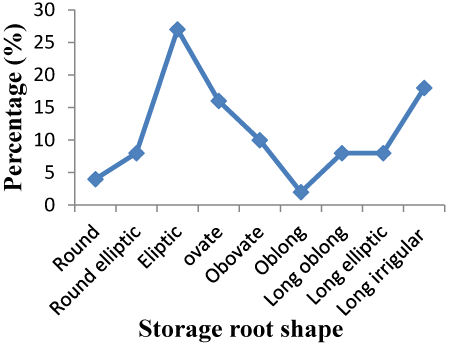
| Storage root shape: Figure 1 showed that the storage root shape of 27.19% of 114 sweet potato accessions was elliptic and 18% of accession was long irregular. Other accessions had also different storage root shape i.e., ovate (15.78%), obovate (9.65%), long elliptic (7.89%), long oblong (7.89%), round elliptic (7.89%), round (4.38%) and oblong (1.75%). The highest number of accessions was exhibited elliptic, however; only six accessions had oblong shape. Most of accessions had similar storage root shape of the checks (Figure 1). |
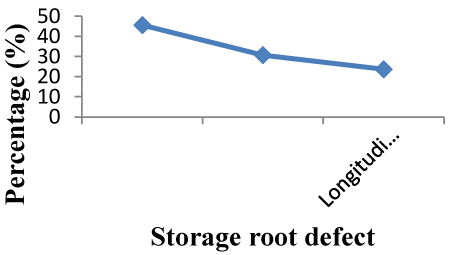
| Storage root defect: There was a variation between checks and accession this may be due to difference in genetic makeup of each accession. Storage root orientation was varied with varieties. Defects were different between accessions. The defect of both checks Adu and Berkume was Alligator-like skin. As it is presented in Figure 2, 45.62% of 114 test entries had a defect of Alligator-like skin which was similar to the check varieties. The defect 30.70% and 23.68% of 114 accessions were horizontal constriction and longitudinal grooves, respectively. Alligator-like skin sweet potato accessions were the highest percentage proportion from the entire accessions where as longitudinal grooves was showed the lowest percentage proportion (Figure 2). |
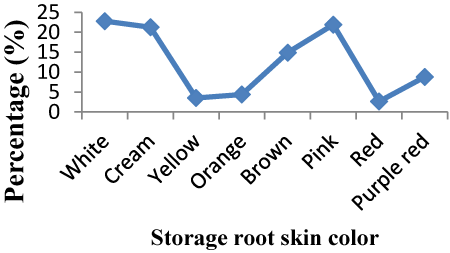
| Storage root skin color: Sweet potato skin color was different for check varieties which Adu had pink color where as Berkume was creamy. Majority of root skin color of accessions was white which accounts 22.8% followed by pink (21.92%). Variation of skin color was due to genetic difference. Storage root skin color of 21.25%, 14.9%, 8.77%, 4.38%, 3.51% and 2.63% of the accessions were cream, brown, purple red, orange, yellow and red, respectively. Pink color was the highest (22.8%) percentage proportion, however, red account the lowest (2.63%) percentage proportion (Figure 3). |
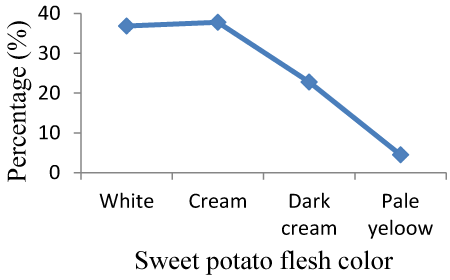
| Storage root flesh color: The flesh color of sweet potato accessions was different which may be due to genetic difference of accessions. Adu and Berkume exhibited cream and white flesh color, respectively. The flesh color of most of accessions was different. 37.79% of sweet potato collections exhibited cream color followed by white (36.84%). Dark cream was accounts 22.8% and 1.75% of accessions flesh color was pale yellow. Cream color was the highest flesh color percentage where as pale yellow exhibit lowest proportion (Figure 4). |
| Chemical attributes of collections |
| Reducing sugar: Highly significant (P<0.01) differences among accession, tests and control and significant (P<0.05) differences were observed among tests versus control for reducing sugar (Table 2). The mean of accession those registered for reducing sugar was 6.143 mg 100 g-1 while mean of check was 6.346 mg 100 g-1 and reducing sugar content was ranged from 2.576 to 10.331 mg 100 g-1.CN-1752-14 and CN-1752-9 was exhibited that the highest reducing sugar content from most of accession. Whereas Neffsie and Korojog-1 were registered the lowest reducing sugar which is consider as desirable. The difference here between accession, checks and check and accessions may be due to genetic differences for the trait which this statement was in agreement with Frankin [32] who reported that reported that reducing sugar of storage root was genetically controlled traits. |
| Hacineza and Picha [33,34] stated that the total reducing sugar in fresh sweet potato was 6.94 mg 100 g-1 and, 7.84 mg 100 g-1, respectively. Walter [35] reported that the concentration of reducing sugar of fresh fry type sweet potato ranges from 5.88 to 6.31 mg g-1similar result were also found by Loretan [26]. This work is in agreement with the findings of Ruinard [29] who reported that the reducing sugar concentration of four varieties was between 2.9 and 5.8 mg 100 g-1. |
| Total sugar: Analysis of variance in Table 2 showed that there was highly significance difference (P<0.01) among accessions, tests and control but among tests versus control the difference was statistically non-significant for total sugar content. The mean of accessions was 13.305 mg 100g-1 while the mean of checks was 13.603 mg 100 g-1. |
| The range for total sugar concentration was between 9.533 and 17.258 mg 100 g-1. Total sugar concentration of CN-1752-15 and CN-2059- 7 was found to be the highest among accessions. However, accession Tis-80/063-3 and Tis-9465-2 were exhibited the lowest. The observed differences of total sugar content among accessions as well as checks and accessions may be mainly attributed to the genetic differences of the entries as well as check varieties since all accessions were tested in one location with similar management. This suggestion might be supported by Frankin [32] who reported that total sugar concentration is genetically controlled trait in sweet potato. Andrade [36] reported that the concentration of total sugar of five sub Saharan Africa sweet potato collection was laid between 1.7 mg 100 g-1 to 27 mg 100-1 which this result was strongly agree with the present result. According to Onwueme [37] the range of recommended total sugar concentration was between 6.98 to 14.59 g 100 g-1 and this result strongly agree with the present finding. Average (11.2 mg 100 g-1) total sugar concentration of four sweet potato varieties was recorded by Hamed [38]. |
| Total starch content: There was highly significant (P<0.01) differences among accessions, tests and control and there was significant (P<0.05) differences among tests versus control for total starch content (Table 2). The mean of accessions for total starch content was 12.569 mg 100 g-1 while the mean of check was 12.923 mg 100 g-1. |
| Total starch content concentration was ranged from 1.167 to 16.402 mg 100 g-1. CN-1752-15 and CN-2059-7 had the highest concentration of total starch content among accessions and checks. WhereasTis-7035-7 and Tis-80/043-3 had the lowest total starch content. There was a difference in total starch concentration between accession, checks and checks and accession. The observed differences may be due to genetic differences among accessions. This suggestion is in agreement with [23]. |
| The present study results agrees with Ruinard [29] report that the total starch content of four sweet potato varieties was laid in the range between 13 mg 100 g-1 and 21 mg g-1. Similar results were also reported for Xushu18, Sushu2 and Sushu8 varieties by Chan [27]. |
| pH value and total soluble solid: There was highly significant (P<0.01) differences among accessions, tests and tests versus control but non-significance differences was observed between check varieties/ among control for pH (Table 2). Likewise, highly significant (P<0.01) differences were exhibited among accession and tests and difference among tests were significantly (P<0.05) different for TSS but there was non-significant difference among tests versus control (Table 2). The mean of accessions for pH and TSS was 6.203 and 12.138° brix, respectively, while the mean of checks was 6.06 (pH) and 12.637° brix (TSS). The pH and TSS values were ranged from 5.044 to 7.264 and 7.132 to 7.132° brix, respectively. The pH value of CN-2065-8 and Tis- 9465-8 were found to be the highest. However, Tis-82/0602-1A and Becale-1 had the lowest pH values. Kure [39] reported that the pH value of seven sweet potato varieties was ranged from 5.5 to 7.1. Aina and Woolfe et al. [40,41] reported that the range of pH in sweet potato varieties were ranged from 5 to 6.9 and 5.5 to 6.7. These findings are strongly agreed with the present findings. |
| Summary and Conclusion |
| Four accessions showed significantly delayed maturity than both checks mean. Koka-12 was found to be the latest maturing accession whereas Korojo was found to be early maturing. CN-2059-7 and CN- 1953-1 had the highest and the lowest above ground fresh biomass yield from all entries, respectively. Storage root fresh weights of Tis-9465-7 and CN-1953-7 were the maximum and minimum storage root fresh weight, respectively. Marketable storage root yields of three entries (Tis-9465-7, Tis-82/0602-12 and Tis-70357-7) were significantly highest yield among accessions including checks. Tis-9465-7 was high yielder whereas Tis-82(0602)-1B was the poor yielder. Total storage root yield of Tis-9465-7 was significantly highest while Neffissie storage root yield was the lowest. |
| The physical attributes of all accessions were evaluated considering shape, defect, skin and flesh color. Accessions had different shape of storage root. The shape of checks (Berkume and Adu) was laid between elliptic to long irregular. Elliptic storage root shape accounts the largest proportion of accession (27.19%) whereas the lowest proportion was oblong (1.75%). About 45.62% of entries storage root defect was horizontal constriction and 23.68% of accessions had longitudinal groove storage root defect. White storage root skin color was dominant which accounts 22.8% of entries where as only few accessions (2.5%) had red skin color. Many accessions (37.79%) had creamy storage root flesh color while only 1.75% of accessions had pale yellow color. |
| CN-1852-14 and Neffissie had the highest and the lowest content of reducing sugar, respectively. Concentration of total sugar was highest in CN-1752-15 and lowest in Tis-80/043-3. CN-1752-15 and Tis- 70357-7 exhibited the highest and the lowest content of total starch concentration, respectively. CN-2065-8 and Tis-80/043-1 had the highest pH and TSS, respectively, whereas Tis-82/0602-1A and CN- 1752-8 were found to be posses the lowest pH and TSS, respectively. CN-2054-1, CN-1753-18 and Korojo had the highest specific gravity, dry matter and peel content, respectively. Tis-8250-2, Tis- 9468-7 and Tis-82/0602-6 registered the lowest specific gravity, dry matter and peel content, respectively. |
References
-
<
- id="Reference_Titile_Link" value="1">Taboge E, Lema G, Dametew M, Belehu T (1992) Improvement Studies on Ensete and Sweet Potato. Pp. 63-74. In: Horticultural Research and Development in Ethiopia. Proc second. Natl. Hort. Workshop of Ethiopia, 13 Dec., Addis Ababa, Ethiopia.
- id="Reference_Titile_Link" value="2">AdhanomN,Tsedeke A, Emana G(1985) Research on Insect Pests of Roots and Tuber Crops: In: Abate T (ed.) A Review of Crop Protection Research in Ethiopia: Proceedings of the first Ethiopian Crop Protection Symposium, 4-7 February, Institute of Agricultural Research, Addis Ababa, Ethiopia.
- id="Reference_Titile_Link" value="3">CSA (Central statistical authority) (2011) Ethiopian Agricultural Sample Enumeration, 2009/10; statistical bulletin, 146.Addis Ababa.
- id="Reference_Titile_Link" value="4">Huaman Z (1991) IPGRI (International Plant Genetic Resources Institute)Descriptors of sweet potato, Italy. 25-56.
- id="Reference_Titile_Link" value="5">Abhishek R, Parsad R,Gupta VK (2010) Statistical package for augmented design (SPAD) I.A.S.R.I, Library Avenue, New delhi.
- id="Reference_Titile_Link" value="6">Donald CM(1968)the breeding of Cropideotype. Euphytica Journal 17: 385-403.
- id="Reference_Titile_Link" value="7">Acquaah G(2007) Principles of plant genetics and breeding. Black well publishing. 350 Main Street, Malden, MA 02148-5020, USA. 584.
- id="Reference_Titile_Link" value="8">Falconer DS(1990) Introduction to quantitative genetics.3rd ed. John Wiley and Sons, Inc. New York. 450.
- id="Reference_Titile_Link" value="9">Allard RW (1960) Principles of plant genetics and breeding. John Willy and Sons, Inc., New York. 663.
- id="Reference_Titile_Link" value="10">WelshRJ(1990) Fundamentals of plant genetics and breeding. John Wiley and Sons, New York
- id="Reference_Titile_Link" value="11">Zhang D, Ghislain M, Huaman Z, Golmirzaie A, Hijmans RJ (1998)RAPD variation in sweetpotato [Ipomoea batatas(L.) Lam] varieties from South America and Papua New Guinea. Genetic Resources and Crop Evolution45: 271-277.
- id="Reference_Titile_Link" value="12">Teshome A, Nigussie D, Yibekal A (2012) Sweet Potato Growth Parameters as Affected by Farmyard Manure and Phosphorus Appl ication at Adami Tulu, Central Rift Valley of Ethiopia.Agricultural Science Research Journal 2: 1-12.
- id="Reference_Titile_Link" value="13">Mwololo JK, MburuMKW, Muturi PW (2012)Performance of sweet potato varieties across environments in Kenya. International Journal of Agronomy and Agricultural Research 2:1-11.
- id="Reference_Titile_Link" value="14">Juo ASR (1983) Selected method for soil and plant analysis. Manual Series, NO.1. Ibadan, Nigeria, IITA.
- id="Reference_Titile_Link" value="15">Mukhtar AA, Tanimu B, Arunah UL,Babaji BA(2010)Evaluation of the Agronomic Characters of Sweet Potato Varieties Grown at Varying Levels of Organic and Inorganic Fertilizer, World Journal of Agricultural Sciences 6: 370-373.
- id="Reference_Titile_Link" value="16">Chowdhury SR, Naskar SK(1993) Screening of drought tolerant traits in sweet potato: role of relative water content. Orissa Journal of Horticulture 21:1-4.
- id="Reference_Titile_Link" value="17">Janssens MJJ (2001) Sweet Potatoes (Ipomoea batatas L) Lam. In:RaemaekersRH (ed.), Crop Production in the tropical. 205-220.
- id="Reference_Titile_Link" value="18">Lewthwaite SL, Triggs CM (2010)Sweet potato cultivar response to prolonged drought. The New Zealand Institute for Plant & Food Research Limited, Pukekohe Research Centre, 49 Cronin Road, RD1, Pukekohe, New Zealand. 03-11.
- id="Reference_Titile_Link" value="19">Huaman Z, Aguilar C, Ortiz R (1999) Selecting a Peruvian sweet potato core collection on basis of morphological, co-geographical, and disease and pest reaction data. Theoretical and Applied Genetics 98: 840-845.
- id="Reference_Titile_Link" value="20">Dominguaz PL(1976) Feeding of Sweet Potato in Monogastrics. Roots, Tuber and Plantains and Bananas in Animal Feeding. 217-233.
- id="Reference_Titile_Link" value="21">Catherine G, Fred T, Emmarold M, Alois K (2012) Characterization of Tanzanian elite sweet potato genotypes for sweet potato virus disease (SPVD) resistance and high dry matter content using simple sequence repeat (SSR) markers, African Journal of Biotechnology11: 9582-9590.
- id="Reference_Titile_Link" value="22">Scott GJR, Best R, Rosegrant M, Bokango M (2000) Roots and tubers in the global food system: A vision statement to the year 2020, Lima Peru: International Potato Centre111.
- id="Reference_Titile_Link" value="23">Tsakama M, Mwangwela AM, Manani TA, Mahungu NM (2010) Physicochemical and pasting properties of starch extracted from eleven sweet potato varieties. African Journal of Food Science and Technology1: 90-9.
- id="Reference_Titile_Link" value="24">Fred TEM, Alois (2008) Morphological and agronomical characterization of Sweet potato [Ipomoea batatas(L.) Lam.] germplasm collection from Tanzania.African Journal of Plant Science 2: 077-085.
- id="Reference_Titile_Link" value="25">Bonsi C, David PP,Mortley DG, Loretan PA, Hill WA (1994) Selecting Sweet Potato (Ipomoea Batatas) Cultivars for Hydroponic Production.Proceedings of the Tenth Symposium of the International Society for Tropical Root Crops, held in Salvador, Bahia, Brazil.
- id="Reference_Titile_Link" value="26">Loretan P, Bonsi CK, Hill WA, Ogbuehi CR, Mortley DG, et al. (1989) Sweet potato growth parameters, yield components and nutritive value for CELSS applications. SAE Tech Paper Ser 891571.
- id="Reference_Titile_Link" value="27">Chen Z, Schols HA, Voragen AGJ(2006) Physicochemical Properties of Starches Obtained from Three Varieties of Chinese Sweet Potatoes.Journal of Food Science 68:431-437.
- id="Reference_Titile_Link" value="28">Surayia Z, Sarwar M, Jonathan A, Khan MN, Butt MS (2006) Variation in Physio-Chemical Characteristics of Some Cultivars of Sweet Potato.Pak. J. Bot 38: 283-291.
- id="Reference_Titile_Link" value="29">RuinardJ (1976) Notes on Sweet Potato Research in West New Guinea sweet potato symposium, West Irian.
- id="Reference_Titile_Link" value="30">Tairo F (2006) Molecular resolution of genetic variability of major sweet potato viruses and improved diagnosis of potyviruses co-infecting sweet potato. Doctoral diss. Dept. of Plant Biology and Forestry Genetics, SLU. ActaUniversitatisagriculturaeSueciae 5.
- id="Reference_Titile_Link" value="31">Gichuki ST,Barenyiv M, Zhang D, Hermann M, Schmidt J, et al. (2003) Genetic diversity in sweetpotato [Ipomoea batatas (L.) Lam.] In relationship to geographic sources as assessed with RAPD markers. Genetic Resources and Crop Evolution 50: 429-437.
- id="Reference_Titile_Link" value="32">Franklin W,Martin (1988) Starch-Sugar Transformation on Sweet and Staple-Type Sweet Potatoes. Tropical Agriculture Research Station, Southern Region Agricultural Research Service, U.S. Department of Agriculture Mayaguez, Puerto-Rico
- id="Reference_Titile_Link" value="33">Hacineaza E, VasanthakaalamH, Ndirigwe J, Mukwanta
- C ( 2010) Comparative study on the ß-carotene content and its retention in yellow and orange fleshed Sweet Potato flours. Association for Strengthening Agricultural Research in Eastern and Central Africa 5-8.
- id="Reference_Titile_Link" value="34">Picha DH (1985)HPLC determination of sugars in raw and baked sweet potatoes. J. Food Sci 50:1189-1190.
- id="Reference_Titile_Link" value="35">Walter WMJr, Hoover MW (1986) Preparation, evaluation and analysis of French-fry-type product from sweet potatoes. J Food Sci 51: 967-970.
- id="Reference_Titile_Link" value="36">Andrade M, Barker I, Cole D, Dapaah H, Elliott H, et al. (2009) Unleashing the potential of sweetpotato in Sub-Saharan Africa: Current challenges and way forward. International Potato Centre (CIP), Lima, Peru. Working Paper: 16-18.
- id="Reference_Titile_Link" value="37">Onwueme IC (1991)the tropical tuber crops:Yams, Cassava, Sweet Potato and Cocoyams. John Wiley and Sons, Chichester, UK 189-191.
- id="Reference_Titile_Link" value="38">Hamed M, Siliha H, Sandy SK (1973) Preparation and chemical composition of sweet potato flour. Cereal and Bakery Products 50: 133-139.
- id="Reference_Titile_Link" value="39">Kure OA, Nwankwo L,Wyasu G (2012) Production and quality evaluation of garri-like product from sweet potatoes. J Nat Prod Plant Resour 2:318-321
- id="Reference_Titile_Link" value="40">Aina AJ, Falade KO, Akingbala JO, Titus P (2009) Physicochemical properties of twenty-one sweet potato cultivars. Int J Food Sci Techno 44: 1696-1704.
- id="Reference_Titile_Link" value="41">Woolfe JA (1992) Sweet potato, an untapped food resource. Cambridge University Press, Cambridge, UK: 643
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
<
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 19357
- [From(publication date):
August-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 13939
- PDF downloads : 5418
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
