Alzheimer’s Disease:Intracellular Beta Amyloid Completes the Irreversible Pathway from Spirochetes to Biofilms to Beta Amyloid to Hyperphosphorylated Tau Protein
Received: 08-Feb-2018 / Accepted Date: 27-Apr-2018 / Published Date: 30-Apr-2018 DOI: 10.4172/2314-7326.1000276
Abstract
In this histopathological study, we have identified beta amyloid (Aβ) intracellularly in hippocampal specimens of Alzheimer’s disease (AD) patients. This is a continuation of the same histopathological project in which we observed biofilms intracellularly in the same neuronal cells in the same brain samples. To demonstrate that these were intracellular biofilms, we utilized the same techniques that showed biofilms in senile plaques in AD, in occluded eccrine ducts in atopic dermatitis, and in tonsils of psoriasis patients. Lyme spirochetes have recently been cultured from AD brains, and those same cultivated organisms have been shown in vitro to make biofilms, beta amyloid precursor protein (AβPP), and Aβ. We believe these spirochetes (and others) make the in vivo biofilms, and we believe our finding of intracellular Aβ helps confirm the in vitro observations. The Aβ, in turn, has previously been shown to stimulate the production and accumulation of hyperphosphorylated tau protein which has been shown to result in axonal and dendritic disintegration. With neuronal cell deterioration, the biofilms, AβPP, Aβ, and neurofibrillary tangles that were once inside are now present outside the cells. Once in the tissue, biofilms lead to upregulation of Toll-like receptor 2 (TLR2) which by known pathways leads to further production of Aβ. Thus, the Aβ can be derived from two sources: one is the spirochetes themselves and the other is from the activation of the innate immune system. The two major components of AD (tau protein and Aβ) have consequently been shown to be created by the pathogenic spirochetes. The spirochetes themselves have been shown to be of Lyme disease and dental origin.
Keywords: Alzheimer’s disease (AD) patients; Hippocampal specimens; Intracellular biofilms
Introduction
Recently, we have observed intracellular biofilms within hippocampal neurons in Alzheimer’s disease (AD) brains [1]. These biomasses stained positively for periodic acid Schiff (PAS) and Congo red (CR) which stain extracellular (outside the microbe) polysaccharides (EPS) and amyloid respectively. The EPS make up the bulk of the biofilm while the amyloid forms its scaffolding [2]. Whereas Lyme (Borrelia burgdorferi) spirochetes have been cultivated from AD brains and dental spirochetes have been found by PCR in those same brains (25% Lyme, 75% dental), it seems apparent that those microbes are making the biofilms [3,4]. The dental organisms are well known for making biofilms (plaque) on teeth, and the Lyme organisms cultured from AD brains have also been shown to make biofilms [3,5]. That these microbes make biofilms is not in any way unusual: most organisms in nature live in biofilms as opposed to the planktonic state [6].
Presumably, the microbes are using their quorum sensing genes to make the biofilms because, once inside the cell, they are not subject to any environmental stresses that may be present outside the cell [7]. Such stresses include salt, water, hyperosmolality, elevated temperatures, among others [8]. The quorum sensing mechanism for biofilm formation is relatively prolonged as opposed to the various stressors which cause biofilms to be made much more rapidly [7]. This may be a factor in the slow development of AD [9].
Recently, as has been stated, Miklossy has cultivated Lyme spirochetes from AD brains; those organisms were then stressed, and they made biofilms in vitro. They also made beta amyloid precursor protein (βAPP) and beta amyloid (Aβ) [3]. This production from the microbes is very likely a major source of βAPP when the cells lyse (after the disintegration of the dendrites) and the material becomes extracellular [10,11]. The extracellular (outside the neuron) βAPP, in turn, can become a source for the Aβ which is a major contributor to AD.
Recent review of our AD specimens has shown Aβ in an intracellular location; this was present in all the AD specimens and in none of the controls. This is further confirmation of intracellular microbes and biofilms as well as further confirmation of Miklossy’s in vitro findings. We discuss the impact this intracellular Aβ has on tau protein in its role in the disease.
Materials and Methods
Hippocampal specimens (which had been previously examined) from 7 AD patients and 11 controls were stained with routine hematoxylin and eosin, PAS, CR, Aβ immunostain, CD 282 (Tolllike receptor 2 [TLR2]), and PAS combined with Aβ immunostain. These were reviewed by 4 pathologists; the specimens were not blinded because, by gross inspection of the hippocampal tissue on the microscopic slide, it was possible to identify The AD hippocampi.
Results
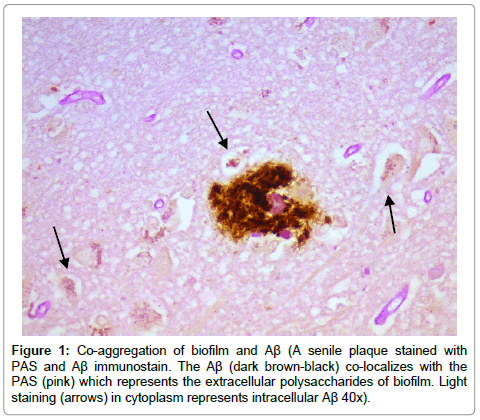
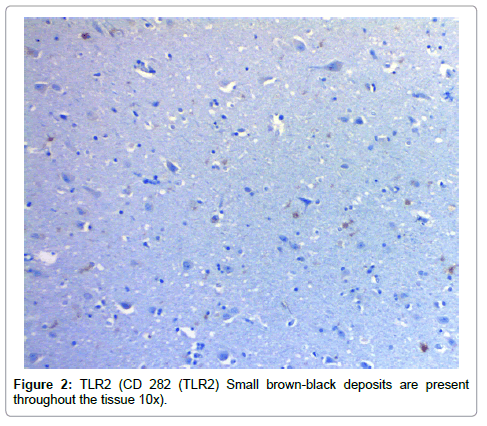
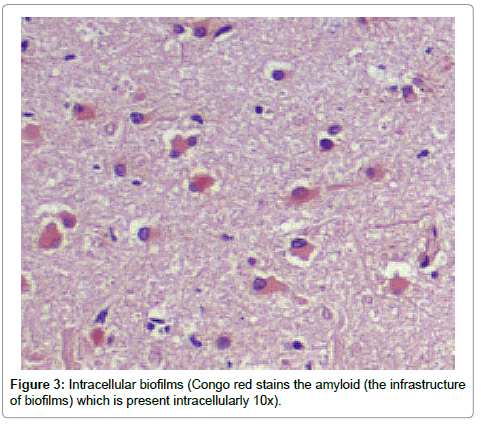
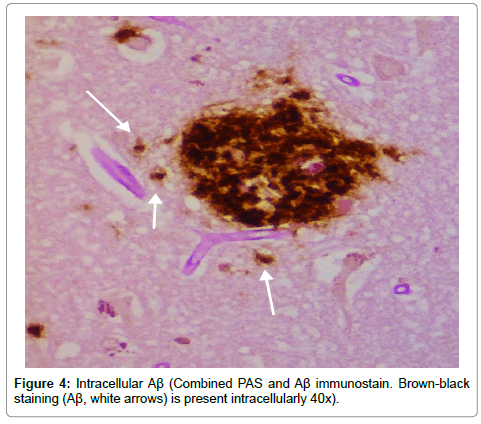
Results from the staining have been presented previously; recapitulation of those findings showed that the plaques (a signature finding in AD pathology) were composed of biofilms because of their staining with PAS and CR, and that Aβ co-localized with the plaques in addition to being present elsewhere. TLR2 was present extracellularly throughout the tissue. Intracellular biofilms were noted, and, most recently, intracellular Aβ was observed (Figures 1-4).
Discussion
These findings confirm many prior observations in the histopathological study of AD brains. First, and probably most important, the presence of Aβ inside the neurons (Figures 1-4) coupled with the prior observation of intracellular biofilms confirms the observations of Miklossy where Lyme spirochetes that were cultured from AD brains made biofilms, βAPP, and Aβ when stressed [3]. This shows that what took place in vitro occurs in vivo as well. It also confirms the presence of intracellular biofilms; and, in so doing, confirms the presence of the microbes (spirochetes) that make these biofilms.
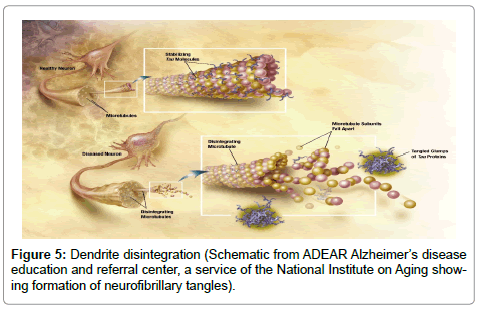
Next, the Aβ fibrils, which have been produced during the formation of the intracellular biofilms, have been shown to induce hyperphosphorylation of tau protein [10-12]. As the accumulation of hyperphosphorylated tau protein causes disintegration of neuronal axons and dendrites, the intracellular biofilm, βAPP, Aβ, and neurofibrillary tangles would leak into the surrounding tissue. The neurofibrillary tangles, senile plaques, and much of the neural tissue have also been shown to contain spirochetes [13] (Figures 5 and 6).
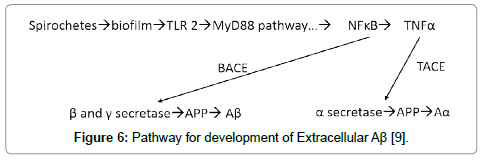
Figure 6: Pathway for development of Extracellular Aβ [9].
The presence of the biofilms in the tissue activates TLR2 (there are receptor sites for TLR2 on the biofilms) [14]. The observed TLR2 does not appear to be localized preferentially to peri-glial or peri-neuronal cells. While the biofilms are intracellular, the TLR2 does not recognize them and is dormant. Once the organisms with their biofilms are in the tissue (outside the neurons), the TLR2, as a first responder, tries to inactivate them by utilizing the MyD88 pathway which generates NFĸB and TNFα as agents of destruction [9]. The NFĸB, together with beta amyloid converting enzyme, catalyzes β and γ secretase which cleave βAPP into Aβ [9]. The Aβ thus arises not only from its production by the microbes in the biofilm, but also from the interaction of the innate immune system and the MyD88 pathway acting upon β and γ secretase. By these mechanisms, much, if not all, the Aβ, which interferes with the neurocircuitry, is produced. Moreover, the process seems selfcontained (Figure 7).
Conclusion
Last, this speaks to intervention at an early stage to kill the spirochetes that create this disease. Periodontal disease should be aggressively treated [15,16]. Penicillin, bactericidal to all known spirochetes, is fully capable of crossing the blood brain barrier and crossing the neuronal cell walls and killing these spirochetes, just as it kills the Treponema pallidum spirochetes in all except the last stage of syphilis [15]. It seems most reasonable to treat early Lyme disease and pre-dental exposures with this agent.
References
- Allen HB, Allawh R, Touati A, Katsetos C, Joshi SG (2018) Alzheimer’s Disease: The novel finding of Intracellular Biofilms. J Neuroinfect Dis 8: 247.
- Allen HB, Morales D, Jones K, Joshi S (2016) Alzheimer’s disease: A novel hypothesis integrating spirochetes, biofilm and the immune system. J Neuroinfect Dis 7: 200.
- Miklossy J (2016) Bacterial amyloid and DNA are important constituents of senile plaques: Further evidence of the spirochetal and biofilm nature of senile plaques. J Alzheimers Dis. 53 4: 1459-1473.
- Riviere GR, Riviere GH, Smith KS (2002) Molecular and immunological evidence of oral treponemes in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol 17: 113-118.
- Marsh PD (2006) Dental plaque as a biofilm and a microbial community–Implications for health and disease. 6: S14.
- Wimpenny JWT (2000) An overview of biofilms as functional communities. In: SGM symposium Volume 59: Community Structure and Cooperation in Biofilms. Allison DG (eds) 1-24, Cambridge University Press
- Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb 2: a012427.
- Johannes K, Knobloch K, Sabottke A (2001)Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: Differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183: 2624-2633
- Allen HB (2016) Alzheimer’s disease: Assessing the role of spirochetes, biofilms, the immune system, and beta amyloid with regard to potential treatment and prevention. J Alzheimers Dis. 53: 4 1271-1276
- Iqbal K, Alonso AC, Chen S, Chohan MO, El-Akkad E, et al. (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739:198-210
-  Zempel H, Thies E, Mandelkow E, Mandelkow EM (2010) Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30: 11938-11950
- Bloom GS (2014) Amyloid-β and Tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71: 505-508.
- Miklossy J (2015) Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci 7: 46
- Tukel C, Wilson RP, Nishimori M, Pezeshki M, Chromy BA, et al. (2009) Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 6: 45-53.
- Allen HB, Hannaway M, Joshi S (2015) Tertiary treponematosis. J Clin Exp Dermatol Res 6: 288.
- Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Brys M, et al. (2008) Alzheimer’s disease and peripheral infections: The possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis 13: 437-449.
Citation: Allen HB, Allawh RM, Cusack CA, Joshi SG (2018) Alzheimer’s Disease: Intracellular Beta Amyloid Completes the Irreversible Pathway from Spirochetes to Biofilms to Beta Amyloid to Hyperphosphorylated Tau Protein. J Neuroinfect Dis 9: 276. DOI: 10.4172/2314-7326.1000276
Copyright: © 2018 Allen HB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6981
- [From(publication date): 0-2018 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 5881
- PDF downloads: 1100