Alzheimer’s Disease, Blood Sugar Levels and a Little Kown Antioxidant
Received: 31-May-2021 / Accepted Date: 14-Jun-2021 / Published Date: 21-Jun-2021 DOI: 10.4172/2161-1165.1000405
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease involving the progressive loss of memory and mental function along with brain atrophy. Approximately 5.8 million Americans age 65 and older are living with this disease, and deaths from Alzheimer’s disease have increased 146%. AD is characterized by the presence of neurofibrillary tangles containing tau and by the abnormal aggregation of Amyloid Plaques (Aβ) with prion-like protein mis-folding. Metabolic changes that occur in AD brains are thought to be indicative of faulty mitochondrial energy production related to the electron transport chain and oxidative.
Keywords: Alzheimer’s disease; Neurodegenerative disease; Amyloid plaques; Mitochondrial dysfunction
Introduction
One mechanism behind Aβ pathology is oxidative stress, a result of the production of the Reactive Oxygen Species (ROS) due to Aβ accumulation and subsequent to mitochondrial dysfunction [1-3].
Metabolic changes that occur in AD brains are thought to be indicative of faulty mitochondrial energy production related to the electron transport chain and oxidative phosphorylation [4-9]. AD brains exhibit increased mitochondrial oxidative stress, generating toxic ROS, and the Aβ peptides that accumulate in the brains are known to increase ROS production in neuronal cells [10-13]. ROS, superoxide and hydroxyl radicals, are produced as the consequence of partially reduced O2 at the end of the electron transport chain. Under normal conditions, these radicals are neutralized by cellular antioxidants. Unfortunately, if ROS concentration increases too rapidly in the cell, these antioxidant mechanisms cannot remove the excess ROS effectively. ROS then can oxidize proteins, lipids, and nucleic acids, disrupting cell homeostasis and increasing cell death [14-16].
Increases in ROS production are closely tied to hyperglycemia, a key pathology of untreated Diabetes Mellitus (DM) patients [6,17,18]. Hyperglycemia contributes to ROS production, oxidative stress, and cell death; pathologies observed in type 2 diabetes patients [19-21]. Aβ formation, a hallmark of AD, is strongly linked to hyperglycemia [22]. Patients with diabetes are at increased risk for AD, and certain physio-pathological traits, such as oxidative stress and mitochondrial dysfunction, could explain the comorbidity of AD and DM in many patients [23-25].
Unfortunately, AD isn’t just linked to hyperglycemia, but also hypoglycemia. An association between hypoglycemia and AD including patients with DM has long been documented [26,27]. In fact a single bought of hyperglycemia requiring a visit to the emergency room increases the risk of dementia development by 26%, a second by 80% and by the third time (or more) 94% [28].
Literature Review
Glucose is necessary for ATP production by the mitochondria and AD is classified as a mitochondriopathy, a disease where mitochondrial impairment is a hallmark. Mitochondria not only play a role in the maintenance of cell life, but also in cell death. Increases of Aβ in the brains of patients with AD have been closely tied to neurodegeneration by activating apoptotic death signals, specifically caspase pathways [29-32]. During apoptosis, or programmed cell death, the Bcl-2 regulatory proteins stimulate the release of molecules from the intermembrane space that activate caspase proteases to induce phagocytosis [33]. Mitochondria release cytochrome c, which complexes with apoptosis-protease-activating factor 1 (Apaf-1), dATP, and procaspase-9 to form the apoptosome, which activates caspase-9, an initiator caspase, to trigger the executioner caspase-8 [34,35]. Mitochondrial ROS production plays a further vital role in the cell death cascade. The upregulation of Tid 1, a gene that induces mitochondrial fragmentation and increased ROS production, in AD brains induces apoptosis and increased Aβ production [36]. Mitochondrial dysfunction therefore plays a key role in the pathogenesis of AD and DM, as well as the increased cell death for each condition.
One way to overcome the mitochondrial dysfunction is to increase the presence of antioxidants. Astaxanthin (ATX) is an antioxidant created by aquatic microorganisms that are consumed by marine organisms, including salmon, shrimp, lobster, and crayfish [37]. Substantial research has shown the neuroprotective potential of marine-sourced bio-compounds, including astaxanthin [38]. Unlike larger drugs, ATX can cross the blood-brain barrier and scavenge ROS in the brain, and ATX is able to accumulate in hippocampal cells as early as four hours after a single dose [37,39]. While hyperglycemia has been shown to compound the negative effects of Aβ on hippocampal cells, ATX has been shown to increase cell growth and return ATP usage and ROS production to normal levels even in AD and DM conditions [40].
Our research has examined the protective role of ATX under both hypoglycemic and hyperglycemic conditions. We have examined growth patterns, ATP production and ROS generation in hippocampal cell groups treated with either hypoglycemic or normal conditions with or without the presence of Amyloid Beta (Aβ) or ATX. We have also examined whether ATX is able to prevent cell death or merely promote cell growth. When hypoglycemic groups were treated with ATX, their growth patterns were either comparable to or increased under all hypoglycemic conditions. Likewise, cells treated with ATX in the presence of Aβ monomers also demonstrated an increase in their growth pattern over hypoglycemic cell groups treated with Aβ monomers and Aβ alone treated groups tended to have significantly less growth than controls (p<0.05). As would be expected, analysis of ATP production showed an overall low level of ATP in hypoglycemic treated groups as determined by ATP assays. Cells cultured with Aβ demonstrated low levels of average fluorescence output generated by ROS production using a MitoSox assay. Cells exposed to ATX actually produced higher to normal levels of ROS. Finally, cells grown with Aβ in the presence of ATX generally produced more ROS than Aβ groups. Thus, hypoglycemia does appear to compound the effects of Aβ monomers on hippocampal cells. Treatment of ATX demonstrates promise with increased cell growth that promotes the use of ATP by the cell, however, also the production of ROS.
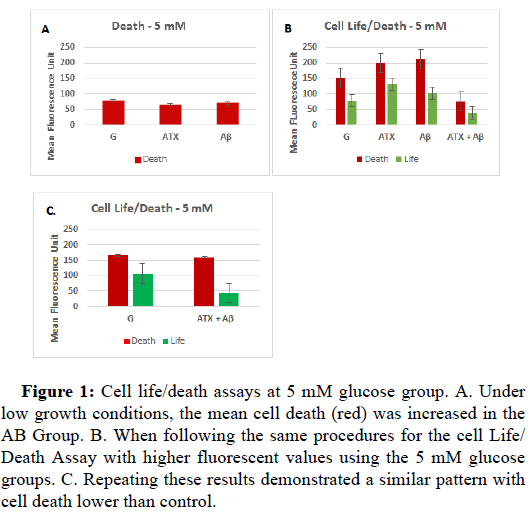
When examining cells under normal conditions, cells exposed to Aβ had increased cell death levels compared to those exposed to ATX (Figure 1A). As expected, when ATX treatment was given in the presence of Aβ an increase in cell life levels was observed as compared to the those exposed to Aβ alone, but also to those treated with ATX when initial cell growth conditions where low, though this was not the case when initial growth conditions were already higher (Figure 1B). The cell life levels were lowest for the cells treated with both ATX and Aβ groups under these conditions. In fact, under normal glucose conditions, cell life for groups treated with either ATX or Aβ is comparable to controls. The greatest difference is found in cell death levels under the high initial growth conditions. Cell death is increased in the Aβ groups while it is decreased in the group treated with both ATX and Aβ (Figures 1A-1C).
Figure 1: Cell life/death assays at 5 mM glucose group. A. Under low growth conditions, the mean cell death (red) was increased in the AB Group. B. When following the same procedures for the cell Life/ Death Assay with higher fluorescent values using the 5 mM glucose groups. C. Repeating these results demonstrated a similar pattern with cell death lower than control.
Discussion
When examining all the data, the AB and ATX values were significantly different (p<0.05). Cell life was significantly different between the glucose control and the ATX and AB groups (p<0.05). Cell life and cell death was also significantly different between the glucose control and the ATX+AB groups. Cell death was also significantly different between both the AB and the ATX groups as compared to the ATX+AB groups. , however, mean cell life decreased. The green fluorescence for the G and the ATX+AB groups are significantly different (p<0.05). Under hyperglycemic conditions, the results were consistent between the low and high growth conditions. Interestingly, cell death was higher in the ATX treated groups compared to the Aβ group. Likewise, cell life was also higher for ATX under the low initial growth conditions. Cell life levels were increased in the cells treated with both ATX and Aβ as compared to cells treated with ATX or Aβ alone under both the low and high cell growth conditions. Unfortunately, the combination of ATX and Aβ also presented increased cell death as compared to Aβ alone. Therefore under normal glucose conditions in the presence of Aβ, ATX may compensate by increasing cell growth. However, this doesn’t stop cell death. There is a decrease in cell death levels but in doing so, it also decreases cell life. For cells exposed to ATX under high growth conditions (Figure 1B), the GAβ have increased cell life compared to the GAβATX groups. While introducing these cells to GATX can help reduce cell death, it also decreases cell life. For cells in the hyperglycemic condition, ATX does increase cell life but it also increases cell death. This increase in cell life could relate to greater access to glucose, but as hyperglycemia increases mitochondrial dysfunction and oxidative stress in this condition, the increased access to growth materials and rapid growth parallels increased cell death. Similar to previous research of the 5 mM glucose groups, the 5 mM GAβ group exhibited the least growth, indicating low ROS production and low ATP production [40]. From this study, we discovered that the cells do grow but cell death far exceeds cell life.
When examining new cell growth to determine if a decrease in cell death actually occurred or if cell death was simply over powered by the formation of new cells, under normal glucose and Aβ, ATX did decrease cell death, but there was also decrease in cell life. For cells in the normal glucose condition, cells with ATX from the last two cell death assays (Figure 1B) show that the GAβ have increased cell life compared to the GAβATX groups. While introducing these cells to GATX can help reduce cell death, it also reduces cell life. For cells in the hyperglycemic condition, ATX does increase cell life but at the expense of cell death. This increase in cell life could relate to greater access to glucose, but as hyperglycemia increases mitochondrial dysfunction and oxidative stress in this condition, the increased access to growth materials and rapid growth parallels increased cell death. Similar to previous research done with 5 mM glucose groups, the 5 mM GAβ group exhibited the least growth, indicating low ROS production and low ATP production [40]. From this study, we discovered that cells grow but cell death far exceeds cell life.
ATX is the most effective at increasing life among cells exposed to the hyperglycemic condition (25 mM glucose), when they are exposed to Aβ. Cells exposed with ATX without Aβ may actually be undergoing more harm, especially in the hyperglycemic condition where cells have more access to glucose resources needed for growth. Research has shown that hyperglycemic groups demonstrated increased growth and ATP production due to increased availability of glucose but that this likewise leads to increased ROS. Under hyperglycemic conditions in the presence of Aβ, cells grew less as compared to much higher growth for observed with ATX present instead. This pattern was observed dominantly with repetition with only one exception. During the second set of cell death assays one set of cells grew similar to the control in the presence of Aβ however, with an increase in cell death observed. The overall set of cells displayed very low cell growth, and this variation could be due to low growth of the petri dish during the initial differentiation stages. Cell death observed in the hyperglycemic condition with Aβ supports previous findings of increased ROS in this group. Overall, however, ROS production was low, but under hyperglycemic conditions cells are producing more ATP as greater glucose is available [40].
Our research has shown that ATX does decrease neuronal cell death, but it also decreases cell life. It appears that ATX has the best effect for cells exposed to the hyperglycemic (25 mM glucose) condition when these cells are also exposed to Aβ. However, when cells are exposed to the increased glucose without the presence of Aβ and then treated with ATX, this leads to increased cell death. While cell death is increased in cells exposed to Aβ and the hyperglycemic solution, cell life is also increased, showing a promising result for using ATX to reduce cell death in cells exposed to Aβ in patients with hyperglycemia.
Conclusion
For this reason, ATX would be most effective for patients with both AD and DM, but may cause the most damage for patients suffering from hyperglycemia without AD because it seems to magnify the cell’s ability to use the more abundant glucose. Therefore, there is a link between blood glucose levels, Alzheimer’s disease and the usefulness of antioxidants. Simple changes in blood sugar alter ATP production and ROS production. The ability of an antioxidant to be beneficial depends on the cells production of oxidants which is tied to the health of the mitochondria within the cells.
Conflict of Interest/Disclosure Statement
The authors have no conflict of interest to report.
References
- Alzheimer’s Association (2020). 2020 Alzheimer’s disease Facts and Figures. Alzheimer’s and Dementia. 16, 3, 391.
- McAllister BB, Lacoursiere SG, Sutherland RJ, Mohajerani MH (2020) Intracerebral seeding of amyloid-β and taupathology in mice: factors underlying prion-like spreading and comparisons with α-synuclein. Neurosci Biobehav Rev 112:1-27.
- Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 7:1140–1149.
- Mariani E, Polidori MC, Cherubini A, Mecocci P (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 827: 65–75.
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, et al. (2010) Oxidative stress in Alzheimer disease: a possibility for Prevention. Neuropharmacology 59:290–294.
- Gu X, Sun J, Li S, Wu X, Li L (2013) Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Aβ production. Neurobiol Aging. 3:1069–1079.
- Toledo JB, Arnold M, Chang R, Rebecca A, Han X, et al. (2018) Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimers Dement 13:965-984.
- Cooper E, Ma M (2017) Alzheimer’s Disease: Clues from Traditional and Complementary Medicine. J Tradit Complement Med 7: 380-385.
- Perez Ortiz J, Swerdlow R (2019) Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176:3489-3507.
- Leuner K, Schütt T, Kurz C, Eckert S, Schiller C, et al. (2012) Mitochondrion-Derived Reactive Oxygen Species Lead to Enhanced Amyloid Beta Formation. Antioxid Redox Signal 16:1421-1433.
- Wang X, Wang W, Li L, Perry G, Lee H, et al. (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842:1240-1247.
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, et al. (2018) Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol 14:450-464.
- Huang H, Lou X, Hu B, Zhou Z, Che J, et al. (2019) A Comprehensive Study on the Generation of Reactive Oxygen Species in the Cu-AB-Catalyzed Redox Processes. Free Radic Biol Med 135:125-151.
- Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658.
- Brieger K, Schiavone S, Miller FJ, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659.
- Soto-Rojas L, de la Cruz-López F, Torres M, Viramontes-Pintos A, Cárdenas-Aguayo M, et al. (2015) Chapter 3: Neuroinflammation and Alterations of the Blood-Brain Barrier in Alzheimer’s Disease. In: Alzheimer ’s Disease: Challenges for the Future, Intech, London.
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031.
- Zuo L, Clanton TL (2005) Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol 289:C207–C216.
- Balaban R, Nemeto S, Finkel T (2005) Mitochondria, Oxidants, and Aging. Cell 120:483-495.
- LaRocca T, Sosunov S, Sharkelet N, Ten V, Ratner A (2016) Hyperglycemia Conditions Prime Cells for RIP1-Dpeendent Necroptosis. J Biol Chem 291:13753-13761.
- Ahmad W, Ijaz B, Shabbirt K, Ahmed F, Rehman S (2017) Oxidative Toxicity in Diabetes and Alzheimer’s Disease: mechanisms behind ROS/RNS Generation. J Biomed Sci 24:76.
- Kubis-Kubiak AM, Rorbach-Dolata A, Piwowar A (2019) Crucial players in Alzheimer’s disease and diabetes mellitus: friends or foes? Mech Ageing Dev 181:7-21.
- Chornenkyy Y, Wang WX, Wei A, Nelson PT (2019). Alzheimer's disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol 29:3-17.
- Madmoli M, Modheji Y, Rafi A, Feyzi R, Darabiyan P, et al. (2019) Diabetes and its predictive role in the incidence of Alzheimer's disease. J Med Sci 23:30-34.
- Fiore V, De Rosa A, Falasca P, Marci M, Guastamacchia E, et al. (2019) Focus on the Correlations between Alzheimer’s Disease and Type 2 Diabetes. Endocr Metab Immune Disord Drug Targets 19:571-579.
- Awasthi A, Matsunaga Y, Yamada T (2005) Amyloid-beta causes apoptosis of neuronal cells via caspase cascade, which can be prevented by amyloid-beta-derived short peptides. Exp Neurol 196:282–289.
- Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CMF (2006) An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol Dis 23:669–678.
- Ferreiro E, Oliveira CR, Pereira CMF (2008) The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 30:331–342.
- Agostinho P, Lopes JP, Velez Z, Oliveira CR (2008) Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int 52:1226–1233.
- Wang C, Youle RJ (2009) The role of mitochondria in apoptosis. Annu Rev Genet 43:95–118.
- Jimbo A, Fujita E, Kouroku Y, Ohnishi J, Inohara N, et al. (2003) ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp Cell Res 283:156-166.
- Zhang W, Liu F, Che, Z, Wu M, Tang Z, et al. (2019) ASB3 knockdown promotes mitochondrial apoptosis via activating the interdependent cleavage of Beclin1 and caspase-8 in hepatocellular carcinoma. Sci China Life Sci 62:1692-1702.
- Zhou Y, Song WM, Andhey PS, Tyler L, Kelly RM, et al. (2020) Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med 26:131–142.
- Galasso C, Orefice I, Pellone P, Cirtno P, Mtele R, et al. (2018) On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar Drugs 16:247.
- Bălașa AF, Chircov C, Grumezescu AM (2020) Marine Biocompounds for Neuroprotection—A Review. Mar Drugs 18:290.
- Manabe Y, Komatsy T, Seki S, Sugawara T (2018) Dietary Astaxanthin Can Accumulate in the Brain of Rats. Biosci Biotechnol Biochem 82:1433-1436.
- Griffith J, Northrup S, Cieslik E, Kelly-Worden M (2019) Hypoglycemia, Hyperglycemia and Astaxanthin: An in Vitro Alzheimer’s Disease Model. Advances in Alzheimer ’s Disease 8:27-38.
- Yaffe K, Falvey CM, Hamilton N, Tamara BH, Eleanor MS, et al. (2013) Association Between Hypoglycemia and Dementia in a Biracial Cohort of Older Adults With Diabetes Mellitus. JAMA Intern Med 173:1300–1306.
- Adolfsson R, Bucht G, Lithner F, Winblad B (1980) Hypoglycemia in Alzheimer's disease. Acta Med Scand 208:387-388.
- Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV (2009) Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301:1565–1572.
Citation: Kelly-Worden M, Cieslik E (2021) Alzheimer’s Disease, Blood Sugar Levels and a Little Kown Antioxidant. ECR 11:405 DOI: 10.4172/2161-1165.1000405
Copyright: © 2021 Kelly-Worden M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2831
- [From(publication date): 0-2021 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 1942
- PDF downloads: 889