Analysis of Structure and Cost in an American Longitudinal Study of Alzheimer's Disease
Received: 16-Dec-2017 / Accepted Date: 26-Dec-2017 / Published Date: 31-Dec-2017 DOI: 10.4172/2161-0460.1000411
Abstract
Objective: The purpose of this analysis is to understand the structure and change in costs for an established longitudinal study of Alzheimer’s disease with fixed enrollment.
Methods: The examination begins with a discussion of the design of the consortium based study and the types of data collected by the researchers. Financial statements (2005 to 2017) are analyzed and forward projections are confirmed using linear regression. Funding is broken down by institution, with looks at per patient and personnel costs.
Results: The rate of change for the costs is highly variable but correlated between institutions. Personnel costs are a critical driving factor. Per patient costs are noted to vary significantly between research institutions. The experiment will not be able to continue in its present form unless costs are brought to equilibrium with available funding. Sources of funding will need to consider opportunity costs, growth rates, and concurrent obligations as they evaluate projects.
Conclusion: The longitudinal study is currently the most effective study design for progressive diseases. Funding for research does not align with the demonstrated need.
Keywords: Consortium lead longitudinal study; Alzheimer’s disease; Clinical study design; Financing and costs; Healthcare policy
Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disorder characterized by cognitive, behavioral, and physical impairment. It is progressive in nature with increased onset of behavioral and medical symptoms over a series of disease stages [1]. Notable symptoms include memory loss, disorientation, agitation coupled with reduced strength and balance [2]. AD is the primary cause of dementia which primarily affects aging demographics, estimated at 25 million patients worldwide, particularly 10% of the population over 65 and 50% of the population over 85 [3]. A multitude of factors have been correlated differently with AD as well, from education to race to gender [4-6]. There is also a high comorbidity with other illnesses due to the advanced age of the average patient. Recent progress has suggested a variety of potential causes; from the genetic perspective, the causes may be rare mutations and RNA damage while from the protein aggregation perspective amyloid β and tau peptides may also be indicative [7-9]. Despite the identification of potential contributing factors, the exact causes of AD have yet to be determined and as a result the diagnostic procedure is still subjectively grouped into possible, probable, and definite. There are currently no cures or mitigating therapies [10].
In 1985, the National Institutes of Health’s (NIH) National Institute on Aging (NIA) originally outlined the necessity of and protocol for long term studies of AD with standardized procedures and controls. The goal was comparability across studies and the benefits would include consensus diagnostic criteria, standard assessments, and characterization of collected data [11]. Historically, the conditions for this longitudinal design have been widely adopted as the standard. Longitudinal studies require the systematic collection of data from the same population over a period to assess trends [12]. As a result, the functional elements of the research tend to remain fixed. These studies are often conducted across institutions to maximize the demographic diversity [13]. One of the most effective research design structures is using a centralized consortium. A tissue bank and data collection center can thus be set up as a central resource. This design was first demonstrated by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) in 1986 and has been subsequently reproduced and refined [14].
Funding from government and foundation grants is an alternative to profit seeking research and development. Grant money is the preferred method of funding for early stage medical research, population public health studies, and other non-revenue promising work. Renewable grants require repeated requests with detailed uses of funds [15]. Funding for AD research has historically been disproportionately low. Alzheimer’s is the most financially costly disease in the United States at $214 billion per annum. The direct cost of a patient with AD is estimated at $47,581, while the indirect cost $173,932 per incidence. The discounted present values for the direct and indirect costs over time are $536 billion and $1.75 trillion [16]. Federal funding for AD research in 2015 was $566 million compared to $5.4 billion for cancer and $1.2 billion for heart disease [17-20]. This paper examines the design and funding for a consortium’s study into AD over time.
Methods
The consortium for this study coordinated six public and private medical research institutions across the state, a tissue bank, and a data collection center closely following the design outlined by the NIH. Of these six locations, one maintained an exclusively Spanish speaking cohort. The institutions are geographically and administratively separate from one another, in different metropolitan regions. The study was established in 2005 with enrollment growing incrementally before stabilizing in 2011.
The data collected by the consortium includes demographic information about patients, complete medical histories, as well as extensive biomarker testing. The diagnosis was classified as Alzheimer’s disease, Mild Cognitive Impairment or Normal Cognition. Demographically the patients are broken down by gender and ethnicity, including Hispanic and non-Hispanic designation. This study featured 62% women and 38% men, and 36% Hispanics and 64% non-Hispanics. Patient history is taken with emphasis on family, education, and health. There are then a series of assessments of physical, behavioral, cognitive function. The cognitive function assessments must be administered by a neuropsychologist or neurologist. The tests utilized include mini- mental state exam (MMSE), Wechsler Adult Intelligence Scale (WAIS), Wechsler Memory Scale (WMS), Boston Naming Test (BNT), Fluency, Intelligence Quotient (IQ), and CERAD exams. Finally, a blood sample is taken for biomarker and genetic testing. A total of 72 biomarkers are measured. The nature of some of the data collected is labor intensive and requires specialists.
Participation in the study is incentivized but voluntary. Each participant is rewarded a $100 stipend for their contributions. Participant turnover is corrected by replacing subjects to maintain the size of the cohort consistently over time. The collected data is databased on and available to scientists by proposal.
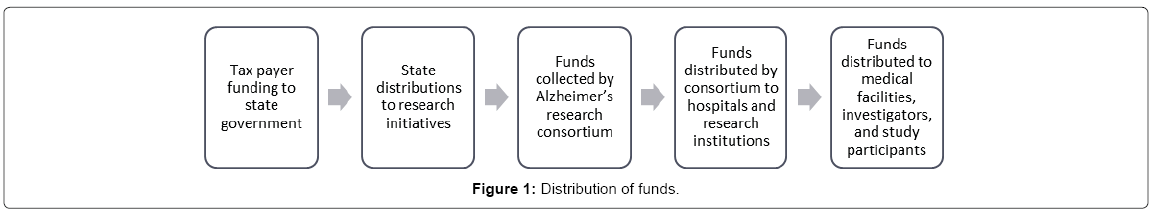
The funding data was collected from the source of funding, which allowed for the analysis of the relative uses of funds in between research institutions. Figure 1 details the multi stage process that takes place from collection to utilization.
Funding requisitions were documented in the form of Memorandums of Understanding (MOU). The requests occurred per biennium with an initial MOU and additional addendums. The requests begin with the projects founding in 2005 and continue into 2017. The years 2017 to 2023 were projected out to estimate the cost of maintaining the fixed enrollment study. The analysis of previous biennial period’s arithmetic mean rate of growth after project stabilization was used to project out forward and estimate future costs of maintaining the cohort [21]. The rate of growth in costs was high in the inceptual stages as the cohort size was increasing. The first two periods were therefore neglected in the forecasting basis. The costs were broken down in several ways for a more meticulous analysis. Per patient cost was examined in relation to the overall cost which included fixed operations. The costs were also broken down across different institutions.
The data analysis was done entirely in Microsoft’s Excel suite.
Results
Table 1 demonstrates the lower early enrollment that grew and stabilized at the final cohort size. This total cost includes the data collection center and tissue bank in addition to the costs associated with maintain the cohort. The special project costs involved grant funding from the organization to investigators using consortium data for research.
| Year | Milestone | Cohort Budget | Tissue Bank | Data Center | Special Projects | Care | Scientific Manager | Administrative | Total Operational |
|---|---|---|---|---|---|---|---|---|---|
| 2005-2007 | 1200 | $972,481 | $276,000 | $200,693 | $326,000 | $0 | $0 | $0 | $1,775,174 |
| 2007-2009 | 1846 | $2,313,104 | $586,618 | $308,992 | $586,618 | $0 | $0 | $0 | $3,795,332 |
| 2009-2011 | 3030 | $4,414,703 | $625,232 | $625,380 | $605,776 | $0 | $0 | $0 | $6,271,091 |
| 2011-2013 | 3458 | $3,591,087 | $351,937 | $538,980 | $159,985 | $0 | $0 | $0 | $4,641,989 |
| 2013-2015 | 3460 | $4,658,901 | $381,329 | $591,451 | $2,748,599 | $0 | $0 | $0 | $8,380,280 |
| 2015-2017 | 3460 | $5,334,064 | $495,149 | $705,169 | $0 | $400,000 | $219,636 | $250,000 | $7,404,017 |
| 2017-2019 | 3460 | $5,936,588 | $495,148 | $705,169 | $0 | $400,000 | $219,636 | $250,000 | $8,006,541 |
| 2019-2021 | 3460 | $6,633,635 | $495,149 | $705,169 | $0 | $400,000 | $219,636 | $250,000 | $8,703,588 |
| 2021-2023 | 3460 | $7,441,044 | $495,148 | $705,169 | $0 | $400,000 | $219,636 | $250,000 | $9,510,997 |
Table 1: Cohort size with distributions and projections.
The average growth rate of the three relevant biennial periods suggests that costs will continue to grow at between 11% and 12%. One of the periods showed a decrease in costs while every other period showed an increase. A notable observation is that the costs did not always grow as demonstrated by the down period in 2011-2013 suggesting that costs are not necessarily increasing but are unpredictable. The model was assessed using a linear regression which yielded an R-squared value of 0.9335, firmly affirming the model. The projected terminal growth rate was widely variable across institution. The institutions with cohorts that were established at the in 2005 at the beginning of the study period grew at average rates of 19.12%, 14.45%, 17.35% and 11.06%. The institution that established its cohort in 2009 grew at a rate of 3.43%. The final study was established so late that there was insufficient back data for forward projections. The continued increase in cost is substantially greater than inflation (2.1%) [22].
From here an analysis of the breakdown of costs is necessary. The overall cost of the project is heavily driven by the personnel budget, and can separately consider as a substantial subset of the whole. Table 2 also breaks down just the personnel cost revealing both periods of increase and decrease. The change can first be attributed to the changing research processes throughout the study. Despite efforts to maintain the design of the study over time, researchers felt compelled to consider other potential contributing factors. Testing for chronic inflammatory or autoimmune conditions, and the corresponding medication history, for instance, was added in 2013. The budget reveals a corresponding increase in costs for that period. This is compared with the overall cost of the experiment which continues to grow even as the cohort size stabilizes. The overall cost includes both the per patient cost of the study as well as that of maintaining a tissue bank and data center.
| Year | Cohort Budget | Change | Rate of Change | Per Patient Cost | Change in Per Patient Cost |
|---|---|---|---|---|---|
| 2005-2007 | $972,481 | - | - | $810 | - |
| 2007-2009 | $2,313,104 | $1,340,623 | 138% | $1,253 | $443 |
| 2009-2011 | $4,414,703 | $2,101,599 | 91% | $1,457 | $204 |
| 2011-2013 | $3,591,087 | -$823,616 | -19% | $1,038 | -$419 |
| 2013-2015 | $4,658,901 | $1,067,814 | 30% | $1,347 | $308 |
| 2015-2017 | $5,334,064 | $675,163 | 14% | $1,542 | $195 |
| 2017-2019 | $5,936,588 | $602,524 | 11% | $1,716 | $174 |
| 2019-2021 | $6,633,635 | $697,047 | 12% | $1,917 | $201 |
| 2021-2023 | $7,441,044 | $807,409 | 12% | $2,151 | $233 |
| Year | Personnel Budget | Change | Rate of Change | Per Patient Cost | Change in Per Patient Cost |
| 2005-2007 | $686,699 | - | - | $572 | - |
| 2007-2009 | $1,768,323 | $1,081,623 | 158% | $958 | $386 |
| 2009-2011 | $3,203,744 | $1,435,421 | 81% | $1,057 | $99 |
| 2011-2013 | $2,594,614 | -$609,130 | -19% | $750 | -$307 |
| 2013-2015 | $3,200,165 | $605,551 | 23% | $925 | $175 |
| 2015-2017 | $3,700,851 | $500,686 | 16% | $1,070 | $145 |
| 2017-2019 | $4,112,185 | $411,334 | 11% | $1,188 | $119 |
| 2019-2021 | $4,587,632 | $475,447 | 12% | $1,326 | $137 |
| 2021-2023 | $5,137,846 | $550,214 | 12% | $1,485 | $159 |
Table 2: Overall costs and projections.
An example of the change in personnel costs is visible in Table 3 as the change in staff between two consecutive biennial periods for one institution. The percent effort represents the amount of total professional time the contributor is providing for this particular project which is then related to their compensation. The issues with tracking the cost of personnel and specifically of individual personnel are several folds. First, the market value of skilled and specialized labor is highly variable, sensitive to market conditions. The opportunity costs of partial commitments to the project are especially variable. Secondly the availability of specialized clinical professionals will be highly inconsistent across a geographic region where both dense urban centers and more rural areas need to be represented. Thirdly, there is a degree of informality in the roles of researchers which makes tracking at a deliverable level difficult. This can best be observed by the transition from two physicians at a combined 75% effort to one at 30% while maintaining roughly the same budgeted amount.
| Biennial Period 2009-2011 | Biennial Period 2011-2013 | ||||
|---|---|---|---|---|---|
| Personnel by Category | Personnel by Category | ||||
| MOU 1 | % Effort | Budgeted | MOU 1 | % Effort | Budgeted |
| Principal Investigator | 30 | $ 100,956.79 | Principal Investigator | 30 | $ 100,956.79 |
| Physician | 30 | $ 61,982.00 | Physician | 30 | $ 61,982.00 |
| Psychologist/Outreach | Psychologist/Outreach | ||||
| Coordinator | 25 | $ 30,652.32 | Coordinator | 25 | $ 30,652.32 |
| Neuropsychologist | 10 | $ 6,489.00 | Administrative Assistant | 10 | $ 6,489.00 |
| Epidemiologist/Biomarkers | 10 | $ 14,388.79 | Data Manager | 10 | $ 14,388.79 |
| 10 | $ 17,446.49 | Geneticist 10 $ 17,446.49 Coordinator, | |||
| Data Manager | 25 | $ 21,347.27 | Appointments & | ||
| Coordinator, | Scheduling | 10 | $ 17,446.49 | ||
| Appointments & | |||||
| Scheduling | 20 | $ 9,488.98 | Medical Assistant II | 25 | $ 21,347.27 |
| Medical Assistant II | 30 | $ 13,092.02 | Coordiantor | 20 | $ 9,488.98 |
| Coordinator | 80 | $ 37,871.04 | Data Entry Operator II | 30 | $ 13,092.02 |
| Registered Nurse II | 10 | $ 5,075.43 | Database Analyst | 80 | $ 37,871.04 |
| Psychometrician | 5 | $ 2,162.64 | Psychometrician | 10 | $ 5,075.43 |
| Data Entry Operator II | 10 | $ 2,957.13 | Backup Coordinator | 5 | $ 2,162.64 |
| Clinical Support | 20 | $ 13,130.85 | Total Effort | 285 | $ 320,952.77 |
| Database Analyst | 25 | $ 12,620.85 | |||
| Backup Coordinator | 25 | $ 14,613.64 | |||
| Total Effort | 365 | $ 364,275.24 | |||
| ADDENDUM 1 | % Effort | Budgeted | ADDENDUM 1 | % Effort | Budgeted |
| Principal Investigator | 30 | $ 96,869.98 | Principal Investigator | 30 | $ 100,956.79 |
| Physician | 50 | $ 46,356.00 | Physician | 30 | $ 61,982.00 |
| Psychologist/Outreach | Psychologist/Outreach | ||||
| Coordinator | 25 | $ 28,943.00 | Coordinator | 25 | $ 30,652.32 |
| Physician | 25 | $ 15,978.00 | Administrative Assistant | 10 | $ 6,489.00 |
| Neuropsychologist | 10 | $ 7,336.59 | Data Manager | 10 | $ 14,388.79 |
| Epidemiologist/Biomarkers | 10 | $ 13,659.60 | Coordinator, | ||
| Clinical Support | 20 | $ 12,817.19 | Appointments & | ||
| Data Manager | 25 | $ 20,868.30 | Scheduling | 10 | $ 17,446.49 |
| Scheduling | 20 | $ 9,174.95 | Medical Assistant II | 25 | $ 21,347.27 |
| Medical Assistant II | 30 | $ 12,773.40 | Coordiantor | 20 | $ 9,488.98 |
| Coordinator | 80 | $ 41,913.60 | Data Entry Operator II | 30 | $ 13,092.02 |
| Registered Nurse II | 10 | $ 4,900.59 | Database Analyst | 80 | $ 37,871.04 |
| Psychometrician | 5 | $ 2,371.80 | Psychometrician | 10 | $ 5,075.43 |
| Data Entry Operator II | 10 | $ 3,402.56 | Backup Coordinator | 5 | $ 2,162.64 |
| Database Analyst | 25 | $ 12,275.69 | Total Effort | 285 | $ 320,952.77 |
| Backup Coordinator | 25 | $ 14,342.55 | |||
| Total Effort | 400 | $ 343,983.80 |
Table 3: Biennial personnel breakdown.
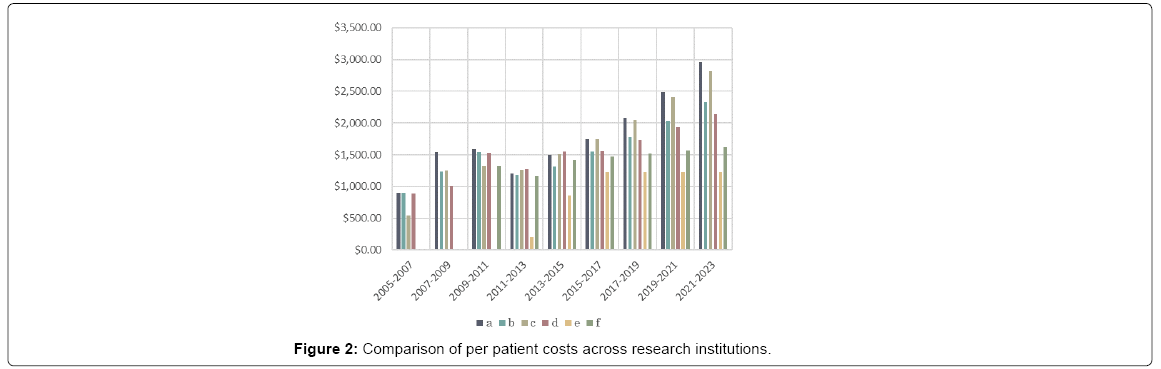
The costs are also better understood by breaking the total into the amount utilized by each individual institution. All the institutions independently outlined their funding needs in their requests. Although the rate of change over time was quite variable, the relative requisitions were quite similar across the institutions every period. A correlation matrix of the four initial centers showed the lowest to be .912 between a and d, the average was .958. This suggests that the factors that contribute to funding needs are likely broader economic trends. Figure 2 shows that addition of new research centers later in the study and how the per patient cost at those institutions remains lower than the ones with the established study. The total final enrollment varied across institutions, at 640, 600 460, 460, 600, 700, respectively prompting consideration that economies of scale could also play a role in the difference. However, that seems unlikely given that the study with the second highest enrollment was the most expensive.
Discussion
Limitations
One of the limitations of this analysis is that the approach was from the broader source of funding perspective rather than a more granular period by period look at individual personnel and functional costs. An entire separate analysis could be conducted from the individual research institutions perspective for a more comprehensive understanding of their needs and the challenges of operating a longitudinal study. The projection model lacked a second order sensitivity analysis due to the incompleteness of financials available. The driving external factors affecting the rate of change in costs remain speculative.
Scientific implications
The design of this experiment has been empirically validated by repeated successful implementation based on the model outlined by the NIH and demonstrated by CERAD. Furthermore, it is currently the standard for population studies of AD. This study design has already yielded hundreds of publications with corresponding citations in journals worldwide [23]. By nature, the value of longitudinal study continues to increase with its lifespan.
If the funding remains fixed, the ability of this experiment to continue full services will be affected. By calculating the incremental requirements for the upcoming period, if the budget does not increase proportionately an estimated 391 patients will have to be cut form the cohort. Removal of patients will affect the statistical significance of the study. The lower n for the study increases the required p value for the t test to overcome the null hypothesis. This will be especially destabilizing to the delicate demographic composition of the study. The alternative to removing patients from the study is to limit the variables being monitored. The costliest of these being the labor intensive cognitive assessments or the laboratory testing based biomarker analyses. Limiting the variables under consideration would inevitably dilute the scientific value of the study.
Financial implications
The growth rate of costs has a multitude of drivers including procedural and structural changes over the period. The variability in the rate of change between the periods, demonstrated by both periods of increase and decrease, leads to the conclusion that the financial needs of scientific study are more than anything unpredictable, especially when the study is labor intensive.
To provide some context, the per patient cost of conducting a study is far lower than the cost of caring for a patient, in 2007, the end of the first biennial period of study, the mean annual total cost was $23,400 in mild, $56,800 in moderate and $71,400 in severe cases of AD [24]. This consideration becomes crucial when government sources of funding are also responsible for other geriatric services such as Medicare, where the financial burden of care will fall on them one way or the other.
While increased funding will inevitably ease the burden on researchers, measures must also be taken to reduce costs by identifying key cost drivers. The increase in costs presents a compounding effect; they are increasing at an increasing rate which is fundamentally unsustainable. The analysis of this study shows a discrepancy between institutions in per patient costs. Increased internal governance has shown success in improving efficiency in clinical trials [25]. Maintaining longitudinal studies is the only way to monitor the progression of AD at this early point in the understanding of the disease.
Policy implications
Longitudinal medical studies, like most types of early stage research, are grant based. The bureaucratic process of funding approval requires some up-front assurances on the total amounts required. Studies that will need to anticipate growing costs may have a harder time getting traction for the initial set up. When grants are provided from a governmental authority there is an issue of opportunity costs with research budgeting and more general medical budgeting. Considering that most states already provide baseline assistance for geriatric care a potential solution could be integrating data collection into pre-existing care facilities.
Conclusion
Analysis into the cost of medical research studies, and specifically longitudinal studies with recurring costs has been limited. This work can help the directors of present and future studies understand their funding needs. It also creates a frame of reference for the providers of funding. More analysis in this area will help contextualize these results, especially if comparisons can be made to studies of different sizes and geographies [26,27].
At this stage in the understanding of AD, monitoring symptoms and progression against a control group is what will allow the development of effective therapies in the future. Longitudinal studies collect data that forms the foundation for the understanding of a progressive disease. This is a particularly difficult type of study design because of the immense time commitment and resource dedication that is required. However, these studies provide a knowledge bank for any scientist to be able to withdraw from to conduct independent study of the disease. When considered against the alternative, the cost burden of care, the cost of research pales.
The rate of growth of funding and the demonstrated need for funds are not aligned. On one hand grant based funds are limited and likely too low, on the other hand the costs show the tendency to increase. It is imperative that steps be taken to make the maintenance of cohorts financially stable.
References
- Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, et al. (2001) Measuring Alzheimer’s disease progression with transition probabilities: Estimates from CERAD. Neurology 57: 957-964.
- Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, et al. (1996) Longitudinal assessment of symptoms of depression, agitation and psychosis in 181 patients with Alzheimer's disease. Am J Psychiatry 153: 1438-1443.
- Evans DA (1989) Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA 262: 2551-2556.
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, et al. (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria and endoplasmic reticulum: Implications for Alzheimer’s disease. J Cell Biol 143: 777-794.
- Katzman R (1993) Education and the prevalence of dementia and Alzheimer’s disease. Neurology 43: 13-20.
- Tang MX, Maestre G, Tsai WY, Liu XH, Feng L, et al. (1996) Effect of age, ethnicity and head injury on the association between APOE genotypes and Alzheimer’s disease. Ann N Y Acad Sci 802: 6-15.
- Munoz DG, Feldman H (2000) Causes of Alzheimer's disease. CMAJ 162: 65-72.
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, et al. (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 46: 860-866.
- Zhang M, Katzman R, Salmon D, Jin H, Cai G, et al. (1990) The prevalence of dementia and Alzheimer’s disease in shanghai, china: Impact of age, gender and education. Ann Neurol 27: 428-437.
- Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314: 777-781.
- Khachaturian ZS (1985) Diagnosis of Alzheimer's disease. Arch Neurol 42: 1097-1105.
- Khachaturian ZS (2005) Diagnosis of Alzheimer's disease: two decades of progress. Alzheimers Dement 1: 93-98.
- Branstetter LG, Sakakibara M (2002) When do research consortia work well and why? Evidence from Japanese panel data. Am Econ Rev 92: 143-159.
- Moms JC, Heyman A, Mohs RC, Hughes JP, Belle GV, et al. (1989) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 39: 1159-1159.
- Jaffe AB (2002) Building programme evaluation into the design of public research-support programmes. Oxf Rev Econ Policy 18: 22-34.
- Ernst RL, Hay JW (1994) The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health 84: 1261-1264.
- Lowin A, Knapp M, Mccrone P (2001) Alzheimer's disease in the UK: Comparative evidence on cost of illness and volume of health services research funding. Int J Geriatr Psychiatry 16: 1143-1148.
- Reid TR (2015) Alzheimer’s research funding lags other diseases- Dementia.
- Callahan CM (1995) Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med 122: 422.
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 3: 186-191.
- Thompson SG, Barber JA (2000) How should cost data in pragmatic randomised trials be analysed? BMJ 320: 1197-1200.
- Anon (2017) Current US Inflation Rates: 2006-2017. US Inflation Calculator.
- Fillenbaum GG, Belle GV, Morris JC, Mohs RC, Mirra SS, et al. (2008) Consortium to establish a registry for Alzheimer’s disease (CERAD): The first twenty years. Alzheimer's Dement 4: 96-109.
- Mesterton J, Wimo A, By A, Langworth S, Winblad B, et al. (2010) Cross sectional observational study on the societal costs of Alzheimer’s Disease. Curr Alzheimer Res 7: 358-367.
- Sertkaya A, Wong HH, Jessup A, Beleche T (2016) Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials 13: 117-126.
- Geldmacher DS (2008) Cost-effectiveness of drug therapies for Alzheimer’s disease: A brief review. Neuropsychiatr Dis Treat 4: 549-555.
- Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, et al. (2016) Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: A longitudinal study. Lancet Neurol 15: 1044-1053.
Citation: Prabhakaran G, Bakshi R (2017) Analysis of Structure and Cost in an American Longitudinal Study of Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 8: 411. DOI: 10.4172/2161-0460.1000411
Copyright: ©2017 Prabhakaran G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6860
- [From(publication date): 0-2018 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 5925
- PDF downloads: 935