Antibody Titers from Finisher Populations with Persistent/Latent PCV2 Infection Before, During and After the PCV2-SD Epizootic
Received: 24-Aug-2017 / Accepted Date: 01-Sep-2017 / Published Date: 14-Sep-2017 DOI: 10.4172/2161-1165.1000321
Abstract
Porcine circovirus type 2 (PCV2) diseases (PCVDs) have affected pig production worldwide over the last three decades. Since the advent of mass vaccination, manifestations of PCVDs have dramatically decreased. Nevertheless, persistent/latent PCV2 infections linger in the pig population.
Therefore, we investigated whether conclusions can be drawn regarding the health status of a population based on humoral responses to a persistent pathogen such as PCV2. We examined the Swiss finisher population because in this population, time points associated with major events have been well documented. We measured PCV2- specific antibody titers of finishers before the Swiss epizootic in 1996-97 and compared these titers with antibody titers in 2006, during the peak of the epizootic, and in 2011, three years after the start of the mass vaccination of piglets.
Two hundred serum samples from finishers were analyzed for each of these time periods, which correspond to before, during and after the Swiss epizootic. PCV2-specific IgG antibody titers were low to modestly positive during the pre-epizootic and post-epizootic periods. At the peak of the epizootic in 2006, almost all serum samples were positive, with higher average IgG concentrations than those detected in pre-epizootic and post-epizootic samples. Moreover, IgM antibodies were analyzed for 60 randomly chosen finishers in each time period. Of these 180 samples, only two serum samples from 2006 contained PCV2-specific IgM antibodies.
Mass vaccination against PCV2 reduced PCV2-specific antibody titers in pigs with persistent/latent infection to pre-epizootic levels. Our data show prevalence for and concentrations of IgG-specific PCV2 antibodies, which appear to be correlated with PCVD. This result is counterintuitive because one would typically expect higher antibody concentrations to be associated with less disease; a possible interpretation is that elevated concentrations of immature anti-PCV2 antibodies provided pigs with a certain level of protection against PCVDs.
Keywords: PCV2-specific IgG and IgM; Population study; Seroprevalence; Persistent/latent infections; Epizootic periods; Mass vaccination campaign
10646Short Communication
Porcine circovirus type 2 (PCV2), which is one of the most significant pathogens in pig production worldwide, is the causative agent of porcine circovirus diseases (PCVDs). PCV2 can be detected in both healthy and diseased pigs [1] and is found in the lymphatic system in most pigs [2,3].
We and others have demonstrated that PCV2 affects the maturation and differentiation of T cells [3-5]. We specifically identified T helper cells as being targeted by PCV2-infected cells in the thymus [4]. These T helper cells are essential for the proper maturation of high-affinity antibody-secreting centroblasts [6]. Overall, humoral responses to PCV2 do not correlate with the infection statuses of pigs [4,7,8].
This result is unsurprising due to the latent nature of PCV2, which is found in pig fetuses before immune competency [3,4]. In addition, current information regarding neutralizing antibodies and the infection status of pigs is controversial [7,8].
Moreover, the extent to which neutralizing capabilities of antibodies measured in vitro reflect immunologically effective humoral responses in pigs remains unclear.
Viral load reduction and a decreased proportion of infected pigs have been suggested as cornerstones for controlling clinical manifestations of PCVDs [9]. Vaccination against PCV2 prevents clinical manifestations of these diseases but does not eradicate PCV2 infection altogether [10].
Various studies have found elevated anti-PCV2 antibody concentrations after vaccination against PCV2 [7,11], significantly shorter and milder viremia [10], and a higher proportion of CD4(+) CD8(+) IFN(+) lymphocyte subsets in vaccinated piglets [12]. Nevertheless, elevated antibody titers that are due to vaccinations of piglets against PCV2 do not last until finisher stage [13].
Although PCVDs were only described in Switzerland in 2001 [14], PCV2 has been present in the Swiss pig population since at least 1979 [15]. In a prevalence study from 2001-2002, only four out of seventytwo (5%) wasting piglets from 26 different farms were diagnosed with PCV2-systemic disease (PCV2-SD) [16].
Moreover, 38 out of 57 (67%) piglets from 18 of 21 farms carried PCV2-specific antibodies [16]. Despite this high sero-prevalence, few cases of PCVDs were reported in Switzerland before 2003 [15]. The sudden onset of an epizootic correlated with a genetic shift from PCV2a group members to a specific PCV2b group member that we named PCV2b-CH [15].
Importantly, Switzerland is free of porcine reproductive and respiratory syndrome virus (PRRSV), enzootic pneumonia and actinobacillosis, which play roles in disease development in PCVDs [17].
With the introduction of piglet vaccination in Switzerland in 2008, the number of PCV2-SD cases decreased dramatically.
There were two aims of this study. First, we sought to gain a general understanding of how humoral responses appear in a persistently infected population under three different conditions. Second, we wished to understand the influence of mass vaccination in a persistently infected and disease-ridden population in terms of antibody responses against the pathogen.
To address these aims, we compared PCV2-specific antibody titers in the Swiss finisher population before (1996-97), during (2006) and after (2011) the epizootic. Notably, 2011 was three years after the advent of a mass vaccination program for piglets against PCV2.
Two hundred randomly selected finisher serum samples from each of the three time periods were obtained from the serum bank of the Institute of Virology and Immunology (IVI), Mittelhäusern, Switzerland, and were analyzed via competitive ELISA (SERELISA® PCV2 Ab Mono Blocking Systems, Synbiotics Corporation Europe SAS, Lyon) for the presence of PCV2-specific antibodies [11].
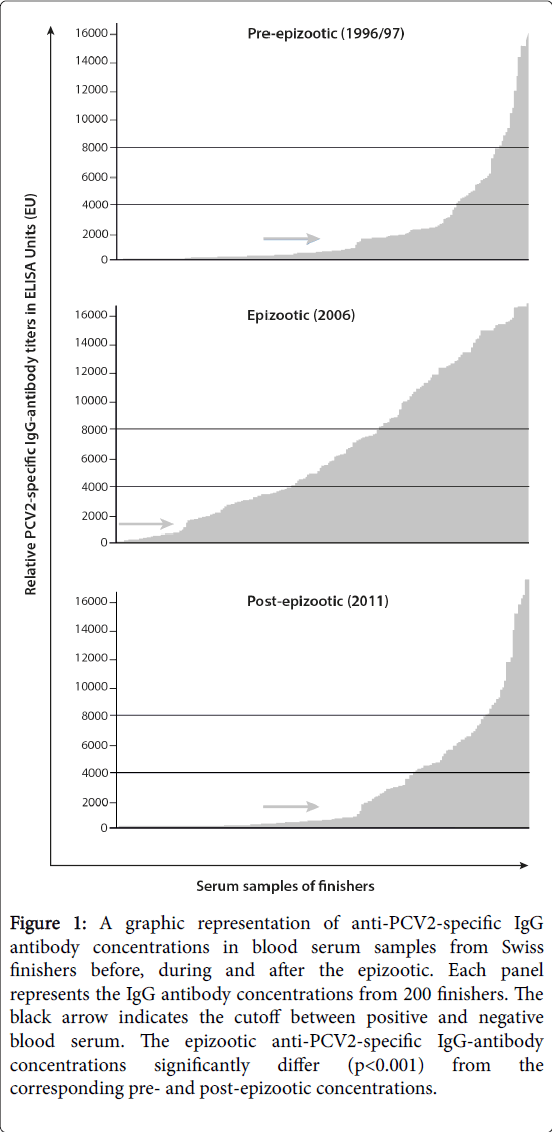
Categorical data were analyzed using chi2 tests or Fisher’s exact test. Titers Figure 1. Titers>4000 EU and titers>8000 EU were arbitrarily selected as references.
Figure 1: A graphic representation of anti-PCV2-specific IgG antibody concentrations in blood serum samples from Swiss finishers before, during and after the epizootic. Each panel represents the IgG antibody concentrations from 200 finishers. The black arrow indicates the cutoff between positive and negative blood serum. The epizootic anti-PCV2-specific IgG-antibody concentrations significantly differ (p< 0.001) from the corresponding pre- and post-epizootic concentrations.
Sixty pig serum samples from each time period were also tested for the presence of PCV2-specific IgM antibodies using a capture immunoenzymatic assay from Ingezim (called Circovirus IgG/IgM; Ingenasa, Madrid).
Prevalence and titers of PCV2-specific antibodies in samples from the three examined time periods are shown in Figure 1. Pre- and postepizootic PCV2-specific IgG antibody titers varied significantly from the corresponding epizootic titers. In particular, 57% of the samples from 1996-97 had a PCV2-specific antibody titer of less than 1000 EU and were classified as negative. During the pre-epizootic period, 18% and 7% of serum titers were >4000 EU and >8000 EU, respectively. From the pre-epizootic to the epizootic period, the proportion of positive titers (>1000 EU) rose from 43% to 84.5%; and 57% and 37% of the epizootic titers were >4000 EU and >8000 EU, respectively. Three years after the onset of mass vaccination of piglets, which reduced the number of PCVD cases, the proportion of serum titers that were positive for PCV2-specific IgG had decreased significantly from 84.5% to 40%; similarly dramatic decreases in titers>4000 EU (57% to 25%) and titers>8000 EU (37% to 10%) were also observed.
For all three reference points (>1000 EU, >4000 EU and >8000 EU), the pre- and post-epizootic anti-PCV2 IgG titers significantly differed from the epizootic anti-PCV2 IgG titers (p<0.001). No statistically significant differences between titers from the pre- and post-epizootic periods were detected (p=0.68). To analyze PCV2-specific IgM antibody titers, we randomly selected 60 serum samples from each of the three time periods; of these 180 samples, only 2 samples from the epizootic period contained PCV2-specific IgM antibodies.
Our population study is unique and interesting in several respects. We analyzed and compared the humoral immune response in pigs during three independent time periods to a pathogen that causes persistent/latent infections. Furthermore, this study provides insight by evaluating the humoral immune response at the end of the production cycle (in finishers). At that stage, individual immune systems are naturally normalized; that is, only serum samples from historically healthy individuals will be acquired from pigs in the slaughterhouse, and there is no distinguishable direct elevation of antibody titers in vaccinated piglets relative to non-vaccinated piglets at the finisher stage.
New PCV2 infections are uncommon (occurring in approximately 1% of individuals), based on the proportion of samples with PCV2- specific IgM antibodies. This observation supports the underlying hypothesis that PCV2 infections are persistent/latent. The IgM antibody isotype is typically an indicator of an early-stage acute infection; however, in cases of persistent or latent infection, class switching and antibody maturation may not occur in an appropriate manner due to a faulty T helper cell response [18]. We and others have previously shown that the porcine immune system mounts an inadequate T cell response to PCV2 [4,5]. It appears possible that only high virus infection pressure, as was observed during the epizootic, leads to the activation of sporadic, newly occurring naïve B cells that secrete PCV2-specific IgM antibodies without T helper cell support. Thus, we hypothesize that the infrequent occurrence of PCV2-specific IgM antibodies reflects a deregulated immune system due to inept T helper cell maturation [4].
In accordance with this reasoning, additional points of interest can be gleaned from our analysis of PCV2-specific IgG antibody responses to a persistent/latent virus during the three investigated time periods. One important consideration is that with the induction of immune tolerance [19], as is likely with porcine PCV2 infections, thymic negative selection of highly specific T cells dominates [3,4]; this selection affects the maturation of B cells and influences the maturation of antibodies specific to PCV2. The pre-epizootic and postepizootic PCV2-specific IgG antibody responses were statistically indistinguishable. In addition, in accordance with our prior findings [3], PCV2 vaccination did not eliminate the virus. During the PCVD epizootic, elevated virus infection pressure led to the more frequent activation of PCV2-specific plasma B cells; this phenomenon was reflected by a higher prevalence and higher average concentrations of PCV2-specific IgG antibodies than in the other investigated time periods. A previous investigation similar to this study reported the reverse correlation; in particular, a 22-fold median decrease in virus concentration resulted in a decrease from 78.8% to 19% in the proportion of pigs positive for PCV2-specific antibodies [20]. This prior finding supports our observation of a decrease in PCV2-specific IgG antibodies from the epizootic period to the post-epizootic period. Notably, this decrease occurred concomitantly with the implementation of the mass vaccination program. Thus, vaccination may induce the few anti-PCV2 specific T-cells that escape negative selection in the thymus, leading to the effective maturation of PCV2- specific centroblasts that produce an effective humoral response against this virus. This explanation is supported by several observations. First, the immune system of vaccinated pigs recognizes a large polypeptide fragment of the capsid protein (CP 43-233) that has strong virus-neutralizing activity [21]. Second, and by contrast, antibodies from diseased pigs are primarily directed against an immune-dominant oligopeptide of the capsid protein (CP 169-180) that lacks virus-neutralizing activity [22]. Taken together, these facts suggest the possibility that vaccination in pigs induces the production of more specific and mature anti-PCV2 antibodies than those found in non-vaccinated pigs.
In conclusion, the more IgG antibodies against a certain virus are present the worse is the health status of the persistent/latent-infected population since virus infection pressure dominates the humoral response outcome. And then again, higher concentrations of nonmatured anti-PCV2 antibodies apparently provided greater protection than a lack of anti-PCV2 antibodies in pigs during the epizootic period.
Conclusion
The age of menopause varies greatly in different parts of the world. When it occurs early, it limits the reproductive capacity of the woman but can also increase the occurrence of cardiovascular diseases. The results of our study show that the economic determinants of childhood and adulthood are the major determinants. There is an dire need to improve the living conditions during childhood and adulthood.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
We thank the Institute of Virology and Immunology (IVI), Mittelhäusern, Switzerland, for the generous gift of the finisher serum samples. We are also grateful to Roseline Weilenmann for technical assistance.
Author’s Contributions
TS, EB and XS designed and coordinated the study. PRV performed the experiment, and MH conducted statistical analysis. EB wrote the manuscript. All authors read and approved the final report.
References
- Segales J, Allan GM, Domingo M (2005) Porcine circovirus diseases. Anim Health Res Rev 6: 119-142.
- Blomström AL, Fossum C, Wallgren P, Berg M (2016) Viral Metagenomic Analysis Displays the Co-Infection Situation in Healthy and PMWS Affected Pigs. PLoS One 11: e0166863.
- Sydler T, Brägger S, Handke M, Hartnack S, Lewis FI, et al. (2016) Latent porcine circovirus type 2-infected domestic pigs: A potential infection model for the effective development of vaccines against latent or chronic virus induced diseases. Vaccine 34: 1047-1053.
- Klausmann S, Sydler T, Summerfield A, Lewis FI, Weilenmann R, et al. (2015) T-cell reprogramming through targeted CD4-coreceptor and T-cell receptor expression on maturing thymocytes by latent Circoviridae family member porcine circovirus type 2 cell infections in the thymus. Emerg Microbes Infect 4: e15.
- Stevenson LS, Gilpin DF, Douglas A, McNeilly F, McNair I, et al. (2007) T lymphocyte epitope mapping of porcine circovirus type 2. Viral Immunol 20: 389-398.
- Maddaly R, Pai G, Balaji S, Sivaramakrishnan P, Srinivasan L, et al. (2010) Receptors and signaling mechanisms for B-lymphocyte activation, proliferation and differentiation--insights from both in vivo and in vitro approaches. FEBS Lett 584: 4883-4894.
- Fort M, Olvera A, Sibila M, Segalés J, Mateu E (2007) Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet Microbiol 125: 244-255.
- Meerts P, Misinzo G, Lefebvre D, Nielsen J, Bøtner A, Kristensen CS, Nauwynck HJ (2006) Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res 2:6.
- Feng H, Blanco G, Segalés J, Sibila M (2014) Can Porcine circovirus type 2 (PCV2) infection be eradicated by mass vaccination. Vet Microbiol 172: 92-99.
- Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, et al. (2008) Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 26: 1063-1071.
- Kurmann J, Sydler T, Brugnera E, Buergi E, Haessig M, et al. (2011) Vaccination of dams increases antibody titer and improves growth parameters in finisher pigs subclinically infected with porcine circovirus type 2. Clin Vaccine Immunol 18: 1644-1649.
- Oh Y, Seo HW, Park C, Chae C (2014) Comparison of sow and/or piglet vaccination of 3 commercial porcine circovirus type 2 (PCV2) single-dose vaccines on pigs under experimental PCV2 challenge. Vet Microbiol 172: 371-380.
- Fachinger V, Bischoff R, Jedidia SB, Saalmüller A, Elbers K (2008) The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 26: 1488-1499.
- Borel N, Bürgi E, Kiupel M, Stevenson GW, Mittal SK, et al. (2001) Three cases of postweaning multisystemic wasting syndrome (PMWS) due to porcine circovirus type 2 (PCV 2) in Switzerland. Schweiz Arch Tierheilkd 143: 249-255.
- Wiederkehr DD, Sydler T, Buergi E, Haessig M, Zimmermann D, et al. (2009) A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet Microbiol 136: 27-35.
- Staebler S, Buergi E, Litzenberger B, McCullough K, McNair I, et al. (2004) Porcine circovirus as a possible cause of postweaning wasting in pigs in Switzerland. Schweiz Arch Tierheilkd 146: 461-468
- Sidler X, Eichhorn J, Geiser V, Bürgi E, Schüpbach G, et al. (2015) Lung and pleural lesions before and after implementation of a national eradication program against enzootic pneumonia and actinobacillosis as well as changes of slaughter carcass organs in slaughter pigs in Switzerland. Schweiz Arch Tierheilkd 157: 665-673.
- Qiao X, He B, Chiu A, Knowles DM, Chadburn A, et al. (2006) Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol 7: 302-310.
- Oldstone MB (2009) Anatomy of viral persistence. PLoS Pathog 5: e1000523.
- Puvanendiran S, Stone S, Yu W, Johnson CR, Abrahante J, et al. (2011) Absence of porcine circovirus type 1 (PCV1) and high prevalence of PCV 2 exposure and infection in swine finisher herds. Virus Res 157: 92-98.
- Trible BR, Kerrigan M, Crossland N, Potter M, Faaberg K, et al. (2011) Antibody recognition of porcine circovirus type 2 capsid protein epitopes after vaccination, infection, and disease. Clin Vaccine Immunol 18: 749-757.
- Trible BR, Suddith AW, Kerrigan MA, Cino-Ozuna AG, Hesse RA, et al. (2012) Recognition of the different structural forms of the capsid protein determines the outcome following infection with porcine circovirus type 2. J Virol 86: 13508-13514.
Citation: Vybiral PR, Sydler T, Haessig M, Sidler X, Brugnera E (2017) Antibody Titers from Finisher Populations with Persistent/Latent PCV2 Infection Before, During and After the PCV2-SD Epizootic. Epidemiology (Sunnyvale) 7: 321. DOI: 10.4172/2161-1165.1000321
Copyright: © 2017 Vybiral PR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3577
- [From(publication date): 0-2017 - Aug 23, 2025]
- Breakdown by view type
- HTML page views: 2693
- PDF downloads: 884