Review Article Open Access
Antimicrobial Properties of Chili Peppers
| Morrine A Omolo, Zen-Zi Wong, Amanda K Mergen, Jennifer C Hastings, Nina C Le, Holly A Reiland, Kyle A Case and David J Baumler* | ||
| Department of Food Science and Nutrition, University of Minnesota-Twin Cities, St. Paul, MN, USA | ||
| Corresponding Author : | David J Baumler Department of Food Science and Nutrition University of Minnesota-Twin Cities St. Paul, Minnesota, USA Tel: 651-280-5895 E-mail: omolo004@umn.edu |
|
| Received March 28, 2014; Accepted May 27, 2014; Published June 06, 2014 | ||
| Citation: Omolo MA, Wong Z, Mergen AK, Hastings JC, Le NC, et al. (2014) Antimicrobial Properties of Chili Peppers. J Infect Dis Ther 2:145. doi:10.4172/2332-0877.1000145 | ||
| Copyright: © 2014 Omolo MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Chili peppers are used worldwide in foods for their pungent flavor, aroma, and to prolong food spoilage. With capsaicin contents ranging from zero to millions of Scoville heat units, the different varieties offer a wide range of options for people all over the world. In addition to their use in cuisines, chili peppers have been explored for their antimicrobial and antifungal properties. Consequently, research is underway to determine the potential for the application of chili pepper extracts in the food industry in place of artificial preservatives. As new antibiotic-resistant food borne pathogens emerge, the discovery of natural antimicrobials in chili peppers will be invaluable to food scientists. This review goes over some relevant research that has already been done in this area. In addition it lays the ground for the new research that is emerging testing new varieties of chili peppers for nutrient content, flavor profiles, and for antimicrobial activities against numerous human pathogens.
| Keywords | |
| Chili peppers; Chile peppers; Antimicrobial; Foodborne pathogen | |
| Introduction | |
| Human use of chili peppers dates back to prehistoric times. Preserved peppers have provided evidence that South Americans ate and grew aji, (chili in English), in 2500 B.C. The peppers became increasingly common and integrated into the diet of particular cultures. However, chili peppers and similar spices remained isolated in these cultures until the 13th century, when they became available to civilizations throughout the world [1]. The pungency of chili peppers is due to the accumulation of capsaicinoids (also known as capsaicinoids, a group of naturally produced compounds that are unique to the Capsicum genus [2,3]. The chili pepper is a member of the Solanaceae family. It is a diploid, facultative, self-pollinating crop, and closely related to potato, tomato, eggplant, tobacco and petunia. It is one of the oldest domesticated crops in the Western hemisphere, the most widely grown spice in the world, and is a major ingredient in most global cuisines [4]. Capsicum species are commonly grown in warm humid regions such as the tropics and subtropics and their fruits are mainly used in local cuisine. | |
| Chili peppers are widely used as spices in traditional Mexican foods. The flavor and pungent power of these peppers varies widely and so do their contents of capsaicin and its capsaicinoid analogs [2]. When eaten, many chili peppers evoke a sensation of heat and/or pain to the neurological systems in mammals, and these adverse effects can be overcome through the consumption of foods containing casein such as milk, cheese, or yogurt. Studies of the botanical pharmacopoeia of the indigenous Mayan inhabitants of Mesoamerica have shown that chili peppers (Capsicum species) are incorporated into a number of medicinal preparations. These preparations were applied for a variety of ailments including respiratory problems, bowel complaints, earaches, and sores. Early European observers noted the omnipresent nature of chili peppers in the Mayan diet, reporting that nothing was eaten without them. While typically regarded as a spice, the substantial role that chili peppers occupy in this culture’s diet may have important nutritional consequences for these people [5,6]. | |
| Chili peppers have a wide range of uses, including pharmaceutical, natural coloring agents and cosmetics, as an ornamental plant, and as the active ingredient in most defense repellants (i.e. pepper sprays) [4]. Capsaicin, a well-studied chemical component of the Capsicum species and one of the pungent capsaicinoids found in chili peppers, has already demonstrated a high degree of biological activity affecting the nervous, cardiovascular, and digestive systems [6]. Chemical analysis has demonstrated that Capsicum fruits contain relatively high concentrations of several essential nutrients, including vitamin C (up to 6 times the concentration of an orange) [6]. | |
| Strong consumer demand for safe and high-quality foods can be attributed in part to the wide spread availability and accessibility of quality health data and information. There are also new concerns about food safety due to increasing occurrences of new food-borne disease outbreaks caused by pathogenic microorganisms. This raises considerable challenges, particularly since there is increasing unease regarding the use of chemical preservatives and artificial antimicrobials to inactivate or inhibit growth of spoilage and pathogenic microorganisms [7]. In addition, currently available treatment options for food-borne pathogen infections have drugrelated side effects, bacterial resistance to antimicrobials, and in some cases no medical treatment exists for organisms such as Escherichia coli O157:H7. Therefore, newer treatments which are safe, cost effective, and simple to administer are urgently needed. In light of this, the use of nutritional agents is an attractive alternative to conventional therapeutics and warrants further investigation [3]. Consequently, natural antimicrobials, such as chili peppers, are receiving a good deal of attention for a number of microorganism-control issues [7]. Recent reports state that the Capsicum genus, among other plant genera, is a good source of antimicrobial and antifungal compounds [5]. | |
| Top 14 Food-borne Pathogens | |
| According to the U.S Food and Drug and Administration (FDA), there are several food-borne pathogens that are of concern and harmful to the general public, and are particularly harmful to pregnant women (Table 1) [8]. | |
| Aside from these 14, there are other well-known pathogens some of which are foodborne, including Bacillus cereus, Bacillus subtilis, Enterobacter aerogenes [6], Pseudomonas aeruginosa [6,9] and Helicobacter pylori [10] which seem to be of interest to research scientists. | |
| Species of the Genus Capsicum Presently Known | |
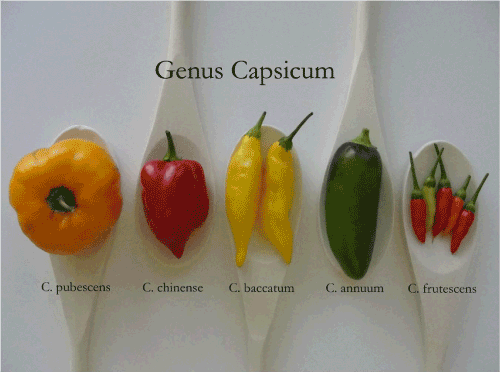
| Capsicum species are small perennial herbs native to tropical South America. The majority of researchers believe that this genus is comprised of more than 20 species. The 5 most common ones believed to be a result of domestication are C. annuum, C. baccatum, C. frutescens, C. chinense and C. pubescens [6], (Figure 1). | |
| The other species are exotic and not as widely distributed as these five. Below is a list of the other presently known species [11]. | |
| • Capsicum buforum | |
| • Capsicum campylopodium | |
| • Capsicum cardenasii | |
| • Capsicum ceratocalyx | |
| • Capsicum chacoense | |
| • Capsicum coccineum | |
| • Capsicum cornutum | |
| • Capsicum dimorphum | |
| • Capsicum dusenii | |
| • Capsicum eximium | |
| • Capsicum flexuosum | |
| • Capsicum friburgense | |
| • Capsicum galapagoense | |
| • Capsicum geminifolium | |
| • Capsicum havanense | |
| • Capsicum hookerianum | |
| • Capsicum hunzikerianum | |
| • Capsicum lanceolatum | |
| • Capsicum leptopodum | |
| • Capsicum lycianthoides | |
| • Capsicum minutiflorum | |
| • Capsicum mirabile | |
| • Capsicum mositicum | |
| • Capsicum parvifolium | |
| • Capsicum pereirae | |
| • Capsicum ramosissimum | |
| • Capsicum recurvatum | |
| • Capsicum rhomboideum | |
| • Capsicum schottianum | |
| • Capsicum scolnikianum | |
| • Capsicum spina-alba | |
| • Capsicum stramoniifolium | |
| • Capsicum tovarii | |
| • Capsicum villosum | |
| Studies on Antimicrobial Effects of Chili Pepperextracts on Some Foodborne and/or Human Pathogens | |
| Bacillus subtilis (not typically associated with foodborne illness) | |
| According to Jorge et al. [12], capsaicin (pure, purchased from Sigma Aldrich), had a strong inhibitory effect towards B. subtilis starting from 25 μg/ml (minimum concentration assayed). | |
| Escherichia coli | |
| Jorge et al. [12] determined that capsaicin (pure, purchased from Sigma Aldrich), at concentrations up to 200 or 300 μg/ml only retarded the growth of E. coli. | |
| Salmonella typhimurium | |
| Monica et al. [9] investigated the antimicrobial effect of Capsicum extract on S. typhimurium inoculated in minced beef. The minimum lethal concentration of the pepper extract was 1.5 ml/100 g of meat. The combination of sodium chloride and C. annum extract tested was not successful to eliminate Salmonella. This could be explained by the fact that Salmonella is tolerant to salt. The researchers proposed using a combination that had less salt and more pepper extract, because any more salt would be too much to eat. | |
| Pseudomonas aeruginosa | |
| In the same study, Monica et al. [9] investigated the antimicrobial effect of Capsicum extract on P. aeruginosa inoculated in minced beef. A reduction of P. aeruginosa growth was observed between 0.06-0.1 ml/ 100 g meat, with a bacteriostatic effect between 0.5-1.5 ml/100 g meat. As the extract concentration increased, a drastic bactericidal effect was observed, particularly between 4-5 ml/100 g meat. The combination of sodium chloride and C. annum extract tested eliminated P. aeruginosa after 3 days of storage. | |
| Staphylococcus aureus | |
| Nitin et al. [13] evaluated the possibility of capsaicin acting as an inhibitor of the NorA efflux pump of S. aureus. The minimum inhibitory concentration (MIC) of ciprofloxacin was reduced 2 to 4 fold in the presence of capsaicin. This reduction was more prominent for S. aureus SA-1199B (NorA overproducing) as compared with S. aureus SA-1199 (wild-type) up to 25 mg/L capsaicin. Beyond that, no concentration dependent effect was observed. S. aureus SA-K1758 (norA knockout) showed no reduction in the MIC of ciprofloxacin. Table 2 shows in vitro ciprofloxacin/ capsaicin combination studies. Table 3 shows postantibiotic effect (PAE) of ciprofloxacin alone and in combination with capsaicin against S. aureus SA-1199B after exposure of 2 h. Ciprofloxacin at 4 mg/L, at which no mutant was selected, was defined as the mutant prevention concentration (MPC). When tested in combination with capsaicin at 12.5 and 25 mg/L, the MPC of ciprofloxacin was reduced to 2 and 1 mg/L, respectively. The MPC of the combination was found to be lower than the Cmax of the ciprofloxacin (3-4 mg/L), indicating the clinical relevance of these combinations in restricting the selection of resistant mutants. Ethidium bromide fluoresces only when it is bound to nucleic acids inside cells. Only the control cells without capsaicin extruded ethidium bromide, resulting in a significant decrease in florescence over the assay period. In the presence of capsaicin, the loss of florescence was significantly reduced, reflecting a strong interference with ethidium bromide efflux by capsaicin [2]. Table 4 shows the mutation frequency of S. aureus ATCC 29213 [13]. | |
| Vibrio cholerae | |
| This study examines common spices to determine their inhibitory capacity against virulence expression of V. cholera (Table 5). Among them methanol extracts of red chili, sweet fennel and white pepper could substantially inhibit cholera toxin (CT) production (Table 6). As these species act against virulence expression rather than viability of V. cholerae, there is a lesser chance of developing resistance [14]. | |
| In a different study, Shruti et al. [15] determined that the methanol extract of red chili, and purified capsaicin could inhibit cholera toxin (CT) production in recently emerged V. cholerae O1 El Tor variant strains without affecting their viability. All 23 strains of V. cholerae used in the study (Table 7), were grown in the lab. Crude methanol extract of the red chili pepper was used (individual ingredients not isolated). Capsaicin was purchased from LKT laboratories Inc., MN. RNA isolation and real-time transcription-PCR (qRT-PCR) assay revealed that capsaicin effectively repressed the transcription of ctxA, tcpA, and toxA genes, but not the toxR and toxS genes. It enhanced the transcription of the gene hns (Table 8). | |
| Based on the experimental results, the researchers proposed a mechanism by which capsaicin and the red chili methanol extract represses the virulence genes of V. cholerae. Briefly, the activation of toxR, toxS, tcpP, and tcpH is caused by environmental factors such as pH, temperature, and osmolarity. This activation subsequently activates ctxAB and tcpA transcriptions via activation of transcriptional activator toxT. HN-S is a basal repressor of toxT, ctxAB and tcpA genes under nonpermissive conditions. In the presence of capsaicin, while ctxAB, tcpA, and toxT transcriptions were repressed, the transcription of hns was enhanced. Capsaicin may probably repress the virulence genes transcriptions in a direct manner or via modulation of the global regulator hns gene. The higher inhibitory impact of red chili methanol extract than capsaicin (43- and 23- fold respectively) indicates the possibility of other unidentified compound(s) in red chilis that can directly inhibit or synergistically act with capsaicin [15]. | |
| Helicobacter pylori | |
| In their experiment, Nicola et al. [3] determined that capsaicin inhibited growth of H. pylori strain LC-11 in a dose-dependent manner at concentrations above 10 μg/ml (ANOVA, P<0.05). This bactericidal effect was evident within 4 h of incubation. After 24 h, growth of the bacteria was completely inhibited. The effect of capsaicin was maximal at a concentration of 50 μg/ml. This bactericidal effect was not limited to H. pylori LC-11. Growth of LC-32 and LC-28 were inhibited to a similar extent at 500 μg/ml [3]. | |
| To examine the possible influence of pH on the bactericidal activity of capsaicin, the growth of H. pylori strain LC-11 was compared in broth culture at pH 4.5, 5.4, and 6.4 in the presence and absence of capsaicin. At each of these pH values, the growth of H. pylori was inhibited compared to bacterial growth in standard broth culture at pH 7.38. Capsaicin exerted a growth inhibitory effect of 92 ± 3.7% at pH 5.4 and 72 ± 11% at pH 6.4. At pH 4.5, bacterial growth did not differing the presence (93.5 ± 2.4%) and absence (88.4 ± 7.8%) of capsaicin [3]. | |
| Listeria monocytogenes | |
| Reverse-phase HPLC analysis was performed to determine the capsinoid-content of the pepper extracts of habanero, serrano, and pimiento chili peppers. Table 9 shows the HPLC profile of standard phenylpropanoid compounds, capsaicin, and dihydrocapsaicin from chili extracts, while Table 10 shows the content of some capsinoids in the habanero, serrano, and pimiento moron extracts (mg/ml) [2]. Lidia et al. do not specify what serotypes of the peppers they used. The following pictures show the most readily available varieties in the market (Figure 2). | |
| The capsinoid compositions of the three pepper extracts are different, and this may influence their antimicrobial effect. The concentration of capsaicin and capsaicinoids used in this study did not show an inhibitory effect on L. monocytogenes. Habanero which has the highest content of capsaicin was the least effective as a bacterial inhibitor. The pimiento morron extract, which contains m-coumeric acid and cinnamic acid but no capsaicin, showed a good inhibitory effect on the bacteria [2] (Table 11). | |
| Conclusion | |
| As more food scientists, consumers, and members of the medical field gain interest in chili peppers, it is certain that through ethnobotanical observations, Capsicum species harbor many economically significant benefits awaiting ‘discovery’ [6]. There are a variety of methods for testing the antimicrobial activities of chili peppers. These methods strongly affect the observed levels of inhibition. Various reasons may contribute in the differences between results, including inconsistency between analyzed plant materials [7]. | |
| In these experiments, crude extracts of chili peppers were used; no separation of pepper components was done, except by Lidia et al. [2]. Based on the data, it seems that capsaicin had a lesser antimicrobial effect compared to other components of chili pepper extracts. Therefore, future studies should try to determine what compounds in the chili pepper gives the spice its antimicrobial properties, and to do so purification of the extracts is necessary. Capsaicin gives chili peppers the ‘hot’ sensation, which some people might not like. It would, therefore, be beneficial if there is another substance in the pepper that could be used in the food industry as a preservative without the pungent taste and hotness. | |
| The studies examined herein were done in vitro. However, more tests need to be conducted to determine the antimicrobial effects of chili peppers in vivo, especially because such a large number of people eat peppers. This could be a potential means through which to minimize the effect of foodborne pathogens when there is an outbreak. David et al. [10] were unable to confirm the hypothesis that capsaicin has an inhibitory effect on H. pylori in vivo. They believe that natural substances and folk remedies should undergo testing in vivo before publication of the in vitro results to reduce the possibility of misinforming the public regarding the potential usefulness of these agents. | |
| Varied as these studies may be, they open the doors to greater research on chili peppers. The data already collected and methods of testing offer new directions for future experiments. To obtain more conclusive data, the number of pepper varieties used should be increased since hundreds of thousands of different types of chili pepper plants exist worldwide. The following picture shows some of the most common varieties, including many exotic types sourced from all over the world (Figure 3). | |
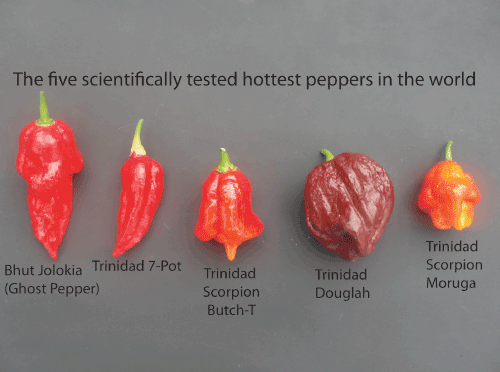
| For example the six hottest chili peppers in the world, Bhut Jolokia, Trinidad 7-pot, Trinidad Scorpion ButchT, Trinidad Doughlah, Trinidad Moruga Scorpion (shown in the next photo), and Carolina Reaper (not shown), have not been tested and may possessundiscovered antimicrobial compounds and activity [16]. | |
| Our lab will be working with over 700 varieties of chili peppers to determine the antimicrobial effects the extracts of leaves and fruits have on selected foodborne pathogens (Figure 4). These varieties will include peppers with and without capsaicin from all over the world. Also, purification of the extracts will be done to determine the most effective component of the extract for antibacterial usage. Finally, as mentioned earlier, peppers have vitamins and other nutrients. Our lab will carry out assays to determine the contents of vitamins A, C, E, and folic acid in many of these exotic types of chili peppers. This data will be useful when using peppers as an additive to value added foods. | |
References
- Mortensen JM, Mortensen JE (2009) The Power of Capsaicin. J Cont Ed11: 8-13.
- Dorantes L, Colmenero R, Hernandez H, Mota L, Jaramillo ME, et al. (2000) Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. International Journal of Food Microbiology 57: 125-128.
- Jones NL, Shabib S, Sherman PM (1997) Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS MicrobiolLett 146: 223-227.
- Kim S, Park M, Yeom SI, Kim YM, Lee JM, et al. (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature Genetics 46: 270-278.
- Seugill K, Minkyu P, Seon-In Y, Yong-Min K, Je ML, et al. (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature 1:1-10.
- Brito-Argáez L, Moguel-Salazar F, Zamudio F, González-Estrada T, Islas-Flores I (2009) Characterization of a Capsicum chinense Seed Peptide Fraction with Broad Antibacterial Activity. Asian Journal of Biochemistry 4: 77-87.
- Cichewicz RH, Thorpe PA (1996) The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J Ethnopharmacol 52: 61-70.
- Tajkarimi MM, Ibrahim SA, Cliver DO (2010) Antimicrobial herb and spice compounds in food.J Food Control 21:1199-1218.
- (2013) Food Safety for Moms-to-Be: Medical Professionals-Foodborne Pathogens
- Careaga M, Fernández E, Dorantes L, Mota L, Jaramillo ME, et al. (2003) Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J Food Microbiol83: 331-335.
- Graham DY, Anderson SY, Lang T (1999) Garlic or jalapeno peppers for treatment of Helicobacter pylori infection. AmJGastroenterol 94: 1200-1202.
- http://en.wikipedia.org/wiki/Capsicum
- Molina-Torres J, Garc├?┬▒├?┬üa-Chávez A, Ram├?┬▒├?┬ürez-Chávez E (1999) Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. J Ethnopharmacol 64: 241-248
- Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, et al. (2012) Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J AntimicrobChemother 67: 2401-2408.
- Yamasaki S, Asakura M, Neogi SB, Hinenoya A, Iwaoka E, et al. (2011) Inhibition of virulence potential of Vibrio cholerae by natural compounds. Indian JMed Res 133: 232-239.
- Chatterjee S, Asakura M, Chowdhury N, Neogi SB, Sugimoto N, et al. (2010) Capsaicin, a potential inhibitor of cholera toxin production in Vibrio cholerae. FEMS Mic robiolLett 306: 54-60.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 | Table 6 |
| Table 7 | Table 8 | Table 9 | Table 10 | Table 11 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 67555
- [From(publication date):
August-2014 - Aug 28, 2025] - Breakdown by view type
- HTML page views : 61010
- PDF downloads : 6545
