Application of Allele-Specific (AS-PCR) Marker for Identification of High-Molecular-Weight Glutenin Subunits (HMW-GS) at the GluB-1 Locus in Bread Wheat (Triticum aestivum L.)
Received: 13-Jul-2018 / Accepted Date: 04-Aug-2018 / Published Date: 11-Aug-2018 DOI: 10.4172/2329-8863.1000387
Keywords: HMW-GS; Triticum aestivum ; Chapatti making quality; AS-PCR marker-MAS
Introduction
Wheat is one of the major staple food crops providing 60% of calories and proteins to the human population. India occupies second position after China in the world with productivity of 87 million tones [1]. However, with increasing population size and growing demand for food grains there is a yield gap and urgent need to increase wheat production. The success of wheat breeding depends on several factors such as high yield potential, disease resistance and most crucially on the gluten protein fraction, which confers the visco-elastic properties that allows its dough to be processed into various (bread, pasta, noodles and chapatti) end products. Wheat consumption in India and neighboring Asian countries mainly takes place in the form of homemade chapattis (unleavened flat bread) and around 90% of total wheat produced is processed into Chapatti/roti as end product [2-4].
High molecular weight glutenin subunit (HMW-GS) plays a major role in determining the visco-elastic properties, thereby determining the quality of its end use products. Significant amount of work has been done to interpret the relation between protein and gluten contents, high molecular weight (HMW) glutenin subunit composition and chapatti making quality [5]. However, the information available on the role of protein fractions and composition on chapatti quality is conflicting and not very clear.
Ram and Nigam [6], earlier reported that equal amount of gliadin, glutenin and residue protein will give better chapatis. Payne Kolster and Vereijken [7] have reported 5+10, 1 and 2* as superior subunits which manifest better dough characteristics. Chapatti requires medium gluten strength which can be achieved by incorporation of HMW subunit 20 along with few other strong subunits at other Glu-1 loci [8-10]. It was found that GluB-1x20 together with GluA 1 null allele contributed significantly to the pliability of chapatti [11-13]. The high Glu-1 score is also correlated with good chapatti quality [12,14]. The Glu-1 quality score can be enhanced by assembling 2*, 20 in 5+10 or 2+12 subunit compositions through marker-assisted selection. The presence of HMW-GS 1Bx20 in varieties with good chapatti making quality such as C-306, C-273 suggests that subunit 20 alone or in combination with other subunits may provide a tool for screening of breeder’s material for chapatti quality. Hence, on the basis of these assumptions, allelic subunit compositions at the Glu-B1 locus are important in chapatti making quality and are important target in developing new lines with quality attributes in wheat breeding programs [15].
Previously, breeders have been selecting varieties with different allelic subunit composition and superior processing quality using various techniques like SDS and Zeleny sedimentation tests, mixographic evaluation, baking test and other methods. However, these methods are not useful for quality check at early stage of development as they are seed based destructive methods. They have found to give incorrect elucidation of the allelic differences [16] and not reliable. Therefore, it is essential to explore new methods which are inexpensive, rapid, reliable and require small amount of sample for analysis. PCR based DNA markers emerge as a new versatile tool in breeding which can easily overcome these constraints and make varietal selection more efficient, precise, rapid [17] and at an early stage of development.
Allele-specific (AS-PCR) markers employ primers designed from nucleotide sequences of the Glu-A1 , Glu-B1 and Glu-D1 genes. Primers used the complete coding region, including the signal peptide and designed from nucleotide sequences of respective genes. The amplification of the complete sequence of the gene is of interest in verifying the correspondence between protein size, as deduced from the coding region of the gene and that obtained by separating proteins using SDS-PAGE [18,19]. Therefore, this PCR approach could be very useful to infer that the obtained size of a HMW glutenin subunit band on agarose gel corresponds to its migration on SDS-PAGE, hence avoiding misleading results due to anomalous migration of few proteins [20]. These allele-specific primers can furthermore be designed from the conserved regions in wheat HMW-GS promoter and coding sequences. AS-PCR markers will also help in discriminating two subunits with same mobility on SDS-PAGE e.g., By 20* and by 20 [21].
Taking recourse to the above, the present study utilizes allelespecific primer analysis for distinguishing alleles at the Glu-B1 locus in 19 different bread wheat cultivars. The marker associated with 1Bx20 can be subsequently used in introgression of this subunit into cultivars with other desirable properties using marker assisted selection (MAS).
Materials and Methods
DNA extraction
DNA was extracted from leaf tissue (~0.5 g) using protocol described by Eswaran et al. [22]. The leaf tissue from nineteen cultivars with different glutenin allelic combinations (Table 1) were first ground in mortar and pestle with buffer containing 7 M urea, 100 mM Tris-Cl, 20 mM EDTA, 0.5 M sodium chloride and 0.1% β-mercaptoethanol. It was followed by extraction of proteins with phenol:chloroform:iso-amylalcohol.
| S No | Varieties/ Genotypes | Glu-B1 subunits | Band size (bp) |
|---|---|---|---|
| 1 | PBW-343 | 7 | 2373 |
| 2 | C-306 | 20 | ~2500 |
| 3 | PBW-226 | 7 | 2373 |
| 4 | Sonalika (SKA) | 7+8 | 2373 |
| 5 | WH-542 | 7+9 | 2373 |
| 6 | Chinese spring (CS) | 7+8 | 2373 |
| 7 | NIAW-34 | 7+9 | 2373 |
| 8 | NIAW-301 | 7+9 | 2373 |
| 9 | PBW-435 | 7+9 | 2373 |
| 10 | C-273 | 20 | ~2500 |
| 11 | C-518 | 20 | ~2500 |
| 12 | C-591 | 20 | ~2500 |
| 13 | Unnath C-306 | 20 | ~2500 |
| 14 | Sehore | 20 | ~2500 |
| 15 | WG-357 | 20 | ~2500 |
| 16 | Kalyan Sona | 17+18 | 2265 |
| 17 | NP-846 | 17+18 | 2265 |
| 18 | HI-385 | 17+18 | 2265 |
| 19 | NI-5439 | 17+18 | 2265 |
| 20 | N-5643 | 17+18 | 2265 |
| 21 | PBW-138 | 13+19 | No band |
Table 1: List of varieties/genotypes used in this study for allele-specific PCR (AS-PCR) marker analysis and composition of their Glu-B1 subunits.
The upper aqueous phase in both steps was transferred to new tube after centrifuging for 15 min at 15,000 rpm. DNA was precipitated with 0.1 volume of 3 M sodium acetate (pH 7.0) and 0.7 volume of isopropanol. Finally, the pellet was washed with 70% and 100% double distilled ethanol. DNA was dissolved in a solution containing 10 mM Tris-Cl (pH 8.0) and 1 mM EDTA. DNase free RNase (5.0 μl of 10 mg/ml) was added and incubated at 37°C overnight to remove RNA. The quantified DNA was then kept at -70°C while working stock was placed at 4°C. The quality as well as quantity of DNA was estimated by agarose gel electrophoresis [23].
A small volume of DNA solution was loaded on 1% agarose gel along with a known amount of Hae III digest of Phi X 174 DNA molecular marker. The amount of DNA was then estimated by comparing the relative intensities of the DNA bands of samples and molecular marker. The comparison was done visually under UV light or by capturing the image in a gel documentation system using software, Gene Tools (Syngene Corporation, UK).
PCR analysis
PCR analysis was done to amplify the coding sequence of Glu-B1x using allele-specific primers as previously reported. Primers were prepared on the basis of published sequences used for amplifying subunit 20 of Triticun durum . It used the complete coding region, including the signal peptide and designed from nucleotide sequences of respective genes [19,24-29].
(A) UTV F: 5’ATGGCTAAGCGCCTGGTCCT 3’
(B) UTV R: 5’TGCCTGGTCGACAATGCGTCGCTG3’
PCR reactions were performed in a final reaction volume of 25 μl using 100 ng genomic DNA, 1U Taq DNA polymerase, 250 ng each of two primers and 200 μM of each dNTP. Amplification conditions were as follows: initial denaturation at 94°C for 2 min, followed by 35 cycles at 94°C for 1 min, 58°C for 2 min, and 72°C for 2 min and 30 s and a final incubation at 72°C for 7 min. Amplified products were analyzed on 2.5% (w/v) agarose gels and run for 5 h.
Results
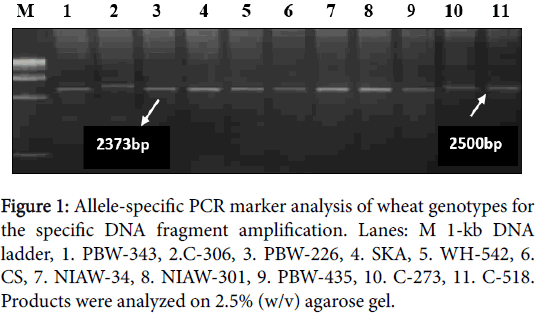
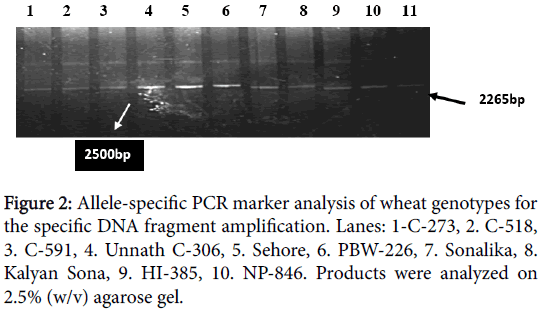
With primer combination UTVF/UTVR, the templates carrying subunit 7 amplified fragment of 2373 bp which is same as expected from the nucleotide sequence information specific to this gene and the templates carrying subunit 20 amplified ~2500 bp fragment. DNA from cultivars with either subunit 7 alone or in combinations e.g., 7+8, 7+9 directed amplification of the Bx7 marker fragment, whereas varieties with subunit 20 amplified the Bx20 marker fragment. The same primer also gave specific fragment of expected size 2265 bp with subunit 17+18 but varieties with other subunits like 13+19 did not amplify any band (Table 1, Figures 1 and 2).
Figure 1: Allele-specific PCR marker analysis of wheat genotypes for the specific DNA fragment amplification. Lanes: M 1-kb DNA ladder, 1. PBW-343, 2.C-306, 3. PBW-226, 4. SKA, 5. WH-542, 6. CS, 7. NIAW-34, 8. NIAW-301, 9. PBW-435, 10. C-273, 11. C-518. Products were analyzed on 2.5% (w/v) agarose gel.
Discussion
The wheat cultivars possessing different allelic composition at GluB-1 locus can be distinguished using allele-specific primer [25,26,29]. These primers are not only specific for genes but also for identical alleles of a given genes hence very useful for identifying genotypes. One of the major drawbacks of using SDS-PAGE for obtaining protein profile is that some functionally different HMW glutenin subunits, such as Bx7 vs Bx7*, By8 vs By8* or By20* and By20 which have similar relative electrophoretic mobility but functionally distinct cannot be clearly identified [30,31].
In triticale, alleles supposed to encode subunits By20 and By18 do not correspond to the wheat alleles with similar symbols. For those new HMW glutenin subunits, new names were attributed: By20* instead of By20 and By18* instead of By18. Allele-specific markers help in the discrimination between the alleles encoding By20 and By20* and By18* and By18 which are having different migration time on SDS-PAGE [21]. Although a large number of DNA markers are available for quality traits of wheat (Table 2) including a number of markers for Glu-B1 alleles none associated with 1Bx20 of Triticum aestivum. Shewry et al. [29] used the allele-specific primer developed by Anderson and Greene [25] for amplifying 1Bx20 in Triticum durum and reported 96% sequence homology between two alleles 1Bx7 and 1Bx20 of Triticum durum (pasta wheat).
| S No | Gene | Allele(s) | Phenotype | References |
|---|---|---|---|---|
| 1 | Dx, Dy | Various | Dough strength | Marchylo et al. [32-34] D’Ovidio et al. [19] De Bustos et al. [35] |
| 2 | Dx5 | Glu-D1 | Dough strength | Smith et al. [36] D’Ovidio and Anderson [18] Ahmad [26] Ma et al. [37] Rodovanovic and Cloutier [27] |
| 3 | Dy10/Dy12 | Glu-D1 | Dough strength | Ahmad [26] |
| 4 | Ax, Ay | Various | Dough strength | Lafiandra et al. [38] |
| 5 | Ax null | Glu-A1 | Dough strength | D’Ovidio et al. [24] Lafiandra et al. [38] |
| 6 | Ax1, Ax2* | Glu-A1 | Dough strength | Ma et al. [37] Rodovanovic and Cloutier [27] |
| 7 | Bx7/Bx17 | Glu-B1 | Dough strength | Ahmad [26] |
| 8 | Bx7*, Bx7OE | Glu-B1 | Dough strength | Rodovanovic and Cloutier [27] Butow et al. [30] |
| 9 | Bx | Glu-B1 | Dough strength | Butow et al. [31] |
| 10 | By18*, By20*, By8, By8* | Glu-B1 | Dough strength | Lei et al. [28] |
Table 2: Molecular markers reported for HMW subunit genes of wheat.
Based on this it is assumed that this primer would possibly amplify the 1Bx20 gene of Triticum aestivum . PCR analysis was performed using allele-specific primers with the aim of amplifying complete coding region of the x20 (Glu-B1) genes. Also, the subunit 20 of C-306 which is bread wheat (T. aestivum) and other varieties amplified product larger than the products of the subunit 7 as well as that amplified by subunit 20 of durum wheat [29]. This gene was larger than subunit 7 and could be easily distinguished from amplicon of subunit 20 of durum wheat. It produced an expected 2373 bp fragment for the allele Bx7 and approximately 2500 bp fragment for Bx20. The variations in size, observed in two different allelic subunits were mainly due to variation in the length of the central repetitive domain, typical of these proteins. Deletions or duplications, probably caused by unequal crossing over, have given rise to size polymorphism. This marker is co-dominant and amplified different fragment sizes for different alleles suggesting specificity for various Glu-B1 alleles. Therefore, the same marker is helpful to discriminate the wheat varieties with different Glu-B1 alleles.
Conclusion
The allele-specific marker employed in the present study will be useful in varietal differentiation of bread wheat at early developmental stages on the basis of variation in Glu-B1 allelic composition. Marker for subunit 1Bx20 of Triticum aestivum will assist introgression of this subunit in wheat cultivars with high yield, disease resistance and other desirable parameters. It will also facilitate combination of subunit 20 with other subunits at Glu-A1 and Glu-D1 locus in breeding lines for selection of varieties with medium gluten strength for augmentation in chapatti making quality.
Acknowledgements
The authors would like to thank Dr SFD Souza, former Head, NABTD; IARI, New Delhi; IIWBR (DWR), Karnal and NBPGR, New Delhi for supplying the seeds of wheat genotypes. Ruchi Rai would also wish to thank DST for WOSA fellowship. Shilpi Singh would like to thank UGC for DS Kothari fellowship.
References
- Gandhi VP, Zhou ZY, Mullen J (2004) India's wheat economy: Will demand be a constraint or supply?. Economic and Political Weekly, pp: 4737-4746.
- Shurpalekar SR, Prabhavathi C (1976) Brabender farinograph, research extensometer and hilliff chapati press as tools for standardization and objective assessment of chapati dough. Cereal Chemistry 53: 457-469.
- Swaranjeet K, Maninder K, Bains GS (1982) Chapaties with leavening and supplements: Changes in texture, residual sugars, and phytic phosphorus. Cereal Chemistry 59: 367-372.
- Rao PH, Leelavathi K, Shurpalekar SR (1989) Effect of damaged starch on the chapati-making quality of whole wheat-flour. Cereal Chemistry 66: 329-333.
- Kolster P, Vereijken JM (1993) Evaluating HMW glutenin subunits to improve bread-making quality of wheat. Cereal Foods World (USA).
- Ram BP, Nigam SN (1982) Puffing and textural characteristics of chapati in relation to varietal differences in gluten composition. Journal of Food Science 47: 231-233.
- Kolster P, Vereijken JM (1993) Evaluating HMW glutenin subunits to improve breadmaking quality of wheat. Cereal Foods World 38: 76-82.
- Qarooni J (1996) Wheat characteristics for flat breads: Hard or soft, white or red?. Cereal Foods World (USA).
- Singh RB, Kulshereshtha VP (1996) Wheat In: Fifty years of Crop Science research in India. Indian Council of Agricultural Research, New Delhi, pp: 219-249.
- Rehman SU, Paterson A, Piggott JR (2007) Chapatti quality from British wheat cultivar flours. LWT-Food Science and Technology 40: 775-784.
- Anjum FM, Lookhart GL, Walker CE (2000) Highâ€molecularâ€weight glutenin subunit composition of Pakistani hard white spring wheats grown at three locations for 2 years and its relationship with endâ€use quality characteristics. Journal of the Science of Food and Agriculture 80: 219-225.
- Srivastava AK, Prasada Rao UJS, Haridas Rao P (2003) Studies on protein and its highâ€molecularâ€weight subunit composition in relation to chapatiâ€making quality of Indian wheat cultivars. Journal of the Science of Food and Agriculture 83: 225-231.
- Sreeramulu G, Banerjee R, Bharadwaj A, Vaishnav PP (2004) Understanding the functionality of wheat high molecular weight glutenin subunits (HMW-GS) in chapati making quality. Special Publication-Royal Society of Chemistry, pp: 162-168.
- Hemalatha MS, Manu BT, Bhagwat SG, Leelavathi K, Rao UJP (2007) Protein characteristics and peroxidase activities of different Indian wheat varieties and their relationship to chapati-making quality. European Food Research and Technology 225: 463-471.
- Békés F, Kemény S, Morell M (2006) An integrated approach to predicting end-product quality of wheat. European Journal of Agronomy 25: 155-162.
- Gianibelli MC, Larroque OR, MacRitchie F, Wrigley CW (2001) Biochemical, genetic, and molecular characterization of wheat glutenin and its component subunits. Cereal Chemistry 78: 635-646.
- Rasheed A, Xia X, Ogbonnaya F, Mahmood T, Zhang Z, et al. (2014) Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biology 14: 128.
- D'ovidio R, Anderson OD (1994) PCR analysis to distinguish between alleles of a member of a multigene family correlated with wheat bread-making quality. Theoretical and Applied Genetics 88: 759-763.
- D'ovidio R, Porceddu E, Lafiandra D (1994) PCR analysis of genes encoding allelic variants of high-molecular-weight glutenin subunits at the Glu-D1 locus. Theoretical and Applied Genetics 88: 175-180.
- Goldsbrough AP, Bulleid NJ, Freedman RB, Flavell RB (1989) Conformational differences between two wheat (Triticum aestivum)‘high-molecular-weight’glutenin subunits are due to a short region containing six amino acid differences. Biochemical Journal 263: 837-842.
- Salmanowicz BP, Dylewicz M (2007) Identification and characterization of high-molecular-weight glutenin genes in Polish triticale cultivars by PCR-based DNA markers. Journal of Applied Genetics 48: 347-357.
- Nalini E, Bhagwat SG, Jawali N (2004) A simple method for isolation of DNA from plants suitable for long term storage and DNA marker analysis. BARC Newsletter 249: 208-214.
- Prasad S, Reddy KS, Jawali N (1999) Abundance of random amplified hybridising microsatellites in mungbean(Vigna radiata L. Wilczek). Asia-Pacific Journal of Molecular Biology and Biotechnology 7: 173-177.
- D'ovidio R, Masci S, Porceddu E (1995) Development of a set of oligonucleotide primers specific for genes at the Glu-1 complex loci of wheat. Theoretical and Applied Genetics 91: 189-194.
- Anderson OD, Greene FC (1989) The characterization and comparative analysis of high-molecular-weight glutenin genes from genomes A and B of a hexaploid bread wheat. Theoretical and Applied Genetics 77: 689-700.
- Ahmad M (2000) Molecular marker-assisted selection of HMW glutenin alleles related to wheat bread quality by PCR-generated DNA markers. Theoretical and Applied Genetics 101: 892-896.
- Radovanovic N, Cloutier S (2003) Gene-assisted selection for high molecular weight glutenin subunits in wheat doubled haploid breeding programs. Molecular Breeding 12: 51-59.
- Lei ZS, Gale KR, He ZH, Gianibelli C, Larroque O, et al. (2006) Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat. Journal of Cereal Science 43: 94-101.
- Shewry P, Gilbert S, Savage A, Tatham A, Wan YF, et al. (2003) Sequence and properties of HMW subunit 1Bx20 from pasta wheat (Triticum durum) which is associated with poor end use properties. Theoretical and Applied Genetics 106: 744-750.
- Butow BJ, Gale KR, Ikea J, Juhasz A, Bedö Z, et al. (2004) Dissemination of the highly expressed Bx7 glutenin subunit (Glu-B1al allele) in wheat as revealed by novel PCR markers and RP-HPLC. Theoretical and Applied Genetics 109: 1525-1535.
- Butow BJ, Ma W, Gale KR, Cornish GB, Rampling L, et al. (2003) Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high-molecular-weight glutenin allele has a major impact on wheat flour dough strength. Theoretical and Applied Genetics 107: 1524-1532.
- Marchylo BA, Kruger JE, Hatcher DW (1989) Quantitative reversed-phase high-performance liquid chromatographic analysis of wheat storage proteins as a potential quality prediction tool. Journal of Cereal Science 9: 113-130.
- Marchylo BA, Handel KA, Mellish VJ (1989) Fast horizontal sodium dodecyl sulfate gradient polyacrylamide gel electrophoresis for rapid wheat cultivar identification and analysis of high molecular weight glutenin subunits. Cereal Chemistry (USA).
- De Bustos A, Rubio P, Soler C, Garcia P, Jouve N (2001) Marker assisted selection to improve HMW-glutenins in wheat. In Wheat in a Global Environment, pp: 171-176.
- Â Smith RL, Schweder ME, Barnett RD (1994) Identification of glutenin alleles in wheat and triticale using PCR-generated DNA markers. Crop Science 34: 1373-1378.
- Smith RL, Schweder ME, Barnett RD (1994) Identification of glutenin alleles in wheat and triticale using PCR-generated DNA markers. Crop Science 34: 1373-1378.
- Ma W, Zhang W, Gale KR (2003) Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 134: 51-60.
- Lafiandra D, Sanguineti MC, Maccaferri M, Deambrogio E (2007) Molecular markers and QTL analysis for grain quality improvement in wheat. In Genomics-Assisted Crop Improvement, pp: 25-50.
Citation: Rai RV, Singh S, Das BK, Bhagwat SG (2018) Application of Allele-Specific (AS-PCR) Marker for Identification of High-Molecular-Weight Glutenin Subunits (HMW-GS) at the GluB-1 Locus in Bread Wheat (Triticum aestivum L.). Adv Crop Sci Tech 6:387. DOI: 10.4172/2329-8863.1000387
Copyright: © 2018 Rai RV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4923
- [From(publication date): 0-2018 - Nov 12, 2025]
- Breakdown by view type
- HTML page views: 3928
- PDF downloads: 995