Are PCV2-Cattle Infections at an Early Evolutionary Virus Adaption Stage?
Received: 14-Dec-2018 / Accepted Date: 21-Jan-2019 / Published Date: 28-Jan-2019 DOI: 10.4172/2332-0877.1000389
Abstract
Porcine circovirus type 2 (PCV2) has a major economic impact on pig production. Vaccination against the virus appears to keep PCV2 disease (PCVD) manifestations in check. Nevertheless, PCV2 has not been eliminated. Several reports indicate that cattle also seem to be susceptible to PCV2 infection. In this study, we detected increased PCV2 cross-species transmission and suggest a strategy for identifying hidden PCV2 infection in cattle. We compared a postweaning multisystemic wasting syndrome (PMWS) distressed-farm with a subclinically-infected farm and investigated how differences in virus concentration would affect PCV2 transmission to a susceptible host such as cattle. The pig population of farm 1 had recurrent PMWS cases among weaners. The 34 cattle in proximity to the PMWS-affected pigs included 14 cattle (41%) that carried a PCV2 load of on average 2.5 x 105 virus genomes per ml blood. Among these was a 7 years old cow with chronic mastitis that was infected with the highest PCV2 load of 1.3 x 106 virus genomes per ml blood. These numbers contrasted with farm 2 that housed PCV2-subclinically infected pigs in proximity to 31 cattle of which six (19%) were infected with an average of 1.7 x 105 virus genome per ml blood. No PCV2-specific humoral response was measured in these cattle. Additionally, we encountered the problem of how to measure PCV2 latent/persistent infections. In pigs, after a one-shot vaccination, we have previously observed a direct anti-PCV2 IgG response instead of the expected IgM response. This indicated that the pig immune system was already primed with PCV2. We therefore hypothesized that cattle may also be PCV2- primed. Although the cattle’s humoral immune system hardly responded to vaccination, we detected PCV2-specific IgG antibody in one cow after the first vaccination shot. This, taken together with the PCV2 transmission rate in hybrid farms indicated sporadic infections in the cattle population. Thus, it can be concluded that PCV2 infections were not well established in cattle in comparison to the situation in swine.
Keywords: Porcine circovirus type 2 diseases; Pig; Cattle; Vaccination; Cross-species transmission
Introduction
Porcine circovirus type 2 (PCV2) is the main etiological agent for porcine circovirus diseases (PCVD) including post weaning multisystemic wasting syndrome (PMWS) [1,2,3]. The virus’s associations with recurrent pandemics in pigs are seen as a real threat to pig production. Paradoxically, PCV2 is endemic in the pig population and can be found both in healthy as well as in diseased pigs [3,4]. The virus latent/ persistent presence [3,5] is a constant threat for the host immune system. PCV2 uses a measurable amount of host metabolism reflected by a reduction in pig’s daily weight gain [3,6]. Detection of anti-PCV2 IgG antibodies is not a reliable indicator of individual infection status [7]. Furthermore, in disease the anti-PCV2 IgG antibodies are often absent [8]. This, and the lack of a meaningful T-cell response against the PCV2 capsid protein [9] now makes more sense in the light of our proposed pathogenicity mechanism [3]. We have shown that pig foetuses already carry the virus in the thymus even before immune competence, which is central to the virus complex pathogenicity [5].
A study indicated the presence of anti-PCV antibodies in a broad range of sera including mouse and human [10]. However, more specific assays demonstrated that PCV2-specific antibody were not that common after all [11,12]. More than a decade after the initial report [13], another report surfaced that described a high similarity between PCV2 and virus DNA from cattle [14]. Other authors confirmed the presence of PCV2 DNA in hosts unrelated to pig [15] and even indicated that the virus could potentially cause similar syndromes in cattle like the ones seen in pigs [16]. Thus, it was important to question the susceptibility of cattle to PCV2 infections [17]. These research scientists’ experiments indicated that cattle are generally susceptible to PCV2 infections [17]. However, in these experiments it was not possible to reliably identify the infected cattle and show that PCV2 singly led to disease in cattle [17]. This is reminiscent of the findings in swine that show in addition to double PCV2 genotype group infections [18] several other factors might separately or combined be important for disease development [3,19,20].
We think the difficulties in interpretation of the pathogenicity mechanism also originated from the nature of the latent virus infection early in pig’s ontogeny [5] with sporadic, recurrent viremia [3,5]. For the diagnosis of disease, the viral load in the lymphatic system and viremia are an indication of disease progress in the animal [3,21,22]. Furthermore, we believe that the virus capsid load in the thymus is central as a diagnostic parameter to define disease [3]. The levels of virus concentrations seem to be dependent on the genetics of the host [3,23,24,25], the viral genotype group member/s [18] and/or another cofactor/s [1,2,20].
It is thought that PCV2 was transferred from wild boar to the domestic pig less than one hundred years ago [26]. It then took more than half a century and several pig generations before the first case of PMWS diseased in 1991 [27]. The current situation in cattle seems to be early in PCV2 infection evolution and hence interesting questions are raised about host infections by these viruses. We were primarily interested whether a higher virus pressure (as found in the environment of PMWS-affected pigs) versus that on a subclinically infected farm might be determined in a susceptible host like cattle.
Material and Methods
Ethics statement
The animal experiments and protocols were approved by the Animal Welfare Committee of the Canton Zurich (authorization no. 177/2011). Handling of and experiments with cattle were carried out in accordance with EU standards and the Swiss Animal Welfare law (Tierschutzgesetz SR455).
Two farms with both pig and cattle cohort
We selected two farms that contained a cohort of pigs in close proximity to a cohort of cattle: on farm 1, 30 dams (producing about 720 weaners per year) were kept together with 34 cattle in the same housing complex separated by a wall. The pig population on the farm was diagnosed with PMWS and no other pathogens were detected [28]. Weaned pigs from 30 sows persistently developed PMWS over a period of several months. Morbidity of up to 30% and a lethality of about 75% was estimated among the weaned pigs. On farm 2, 100 dams (producing about 2600 weaners per year) were housed separated about 30 m from the cattle housing. Routine diagnosis of the blood from pigs and weaners indicated PCV2 infections. However, no clinical signs of disease (no porcine circovirus disease history) and an absence of major respiratory and intestinal pathogens that may affect piglets were observed. The study ended shortly before two pigs developed PMWS [28]. On both farms, the workers changed clothes when moving from one shed to the other.
Cattle vaccination against PCV2
Eight cattle, aged 2-6 years, were used for the vaccination trial. Four cattle were vaccinated twice with an interval of three weeks with 2 ml of Circovac® (2 ml ≥ 2.1 log10 PCV2 antigen unit, 0.2 mg thiomersal and 500 mg paraffin as adjuvant) from Merial Inc. (Lyon, France) and the other four cattle were injected using the same vaccination regime with adjuvant only as controls. Vaccination or adjuvant was subcutaneously injected. Blood samples were taken before the first vaccination, 3 weeks after the first vaccination and 3 weeks after the second vaccination.
PCV2 detection in whole blood by PCR
Cattle blood was collected from the jugular vein and directly transferred into EDTA coated tubes S-Monovette (Sarstedt, Sevelen, Switzerland). We used an in-house sybr-green based real-time PCR as described in Klausmann et al. [3]. We quantified ≥ 40 virus genome copies per μl blood as a positive PCV2 infection.
Anti-PCV2-IgG and-IgM ELISAs
We used the competitive ELISA, SERELISA® PCV2 Ab Mono Blocking Systems of Symbiotic Corporation Europe SAS (Lyon, France) and the assay of INGEZIM CIRCOVIRUS IgG/IgM® of Ingenasa (Madrid, Spain) to detect PCV2-specific IgGs and PCV2- specific IgMs in cattle sera, respectively. The assays were used according to manual instructions and Kurmann et al.[6].
Statistics
Data were analysed using Stata® (StataCorp., 2011; Stata Statistical Software: Release 12.1; College Station, Texas, USA). Normal distribution was tested using the Wilk-Shapiro test (Stata term <swilk var>). Distributions were visualized using <glader var> or <graph box var>. A two-sided unpaired t-test (<ttest vary, by (varx2)>) was used for normally distributed data, the Kolmogorov-Smirnov test (<ksmirnov vary, by(varx)>) as well as the Kruskal-Wallis rank test (<kwallis vary, by(varx)>) was used for not normally distributed data, whereby varx=farm variable, vary=ELISA value or the prevalence by farm. Categorical data were analyzed using Chi2- or Fisher’s Exact-Test (<tab2 varx1 varx2, cchi2 chi2 exact expected>). A p-value ≤ 0.05 was considered significant.
Results
In our quest for proper real-time PCR controls, we noticed that many pigs had variable concentration of PCV2 DNA in whole blood. Consequently, we decided to establish a DNA standard for real-time PCR unrelated to sera or blood from swine and chose cattle blood as a control. To our surprise, we sporadically found PCV2 templates even in the blood from cattle by PCR. We sequenced several amplificates from cattle sera and blood. These sequences were of the PCV2b genotype, namely PCV2-CH.
We selected two farms, each with a cohort of pigs with different PCVD health statuses, in close proximity to a cohort of cattle. On both farms PCV2-infected cattle were found. We sampled cattle blood for the presence of PCV2 by real-time PCR: 20 (31%) out of 65 cattle on the two farms contained on average 2.26 x 105 PCV2 genomes per ml.
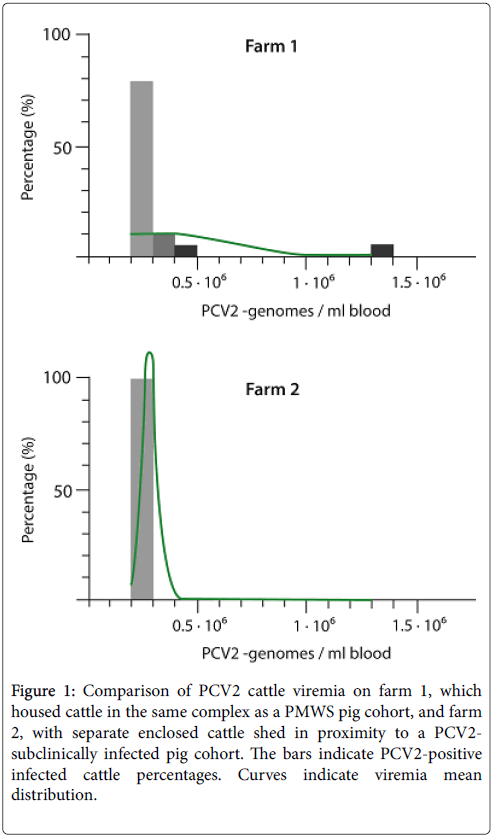
The pig population from farm 1 was previously diagnosed with PMWS, whereas farm 2 was subclinically infected with PCV2. On farm 1 (with PMWS manifestation), we found in close proximity to the pigs, 14 cattle (41%) out of 34 carrying virus loads with an average of 2.5 x 105 virus genomes per ml blood (Figure 1). This cohort contained a 7 years old cow, which was infected with the highest virus titter of 1.3 x 106 virus genomes per ml blood. This animal suffered from chronic mastitis.
Figure 1: Comparison of PCV2 cattle viremia on farm 1, which housed cattle in the same complex as a PMWS pig cohort, and farm 2, with separate enclosed cattle shed in proximity to a PCV2- subclinically infected pig cohort. The bars indicate PCV2-positive infected cattle percentages. Curves indicate viremia mean distribution.
Furthermore, on farm 2, 6 (19%) out of 31 cattle with an average of 1.7 x 105 virus genomes per ml blood were found in the neighbourhood of the subclinically infected pig cohort (Figure 1). We found by a one-sided Fischer analysis there was indeed a statistically significant difference between the PCV2 infection grades of the cattle cohorts in farm 1 compared to farm 2. The cattle carried significantly more viruses when exposed to the higher PCV2 concentrations of the PMWS pig cohort (Figure 1).
Even though 20 cattle were PCV2-viremic, not one animal of the 65 had a meaningful humoral immune response against PCV2 in the form of anti-PCV2 specific IgM or IgG antibodies measurable with the aid of the ELISA assays. This led us to surmise whether cattle might not generally be able to mount a humoral response to PCV2. We decided to vaccinate cattle, which were not PCV2-viremic, with the complete virus (Circovac®, Merial) or adjuvant control only. Three weeks after the first immunization relative low titer of anti-PCV2 IgG antibodies were measured (Table 1) when compared to PCV2-specific IgG titer of vaccinated pigs. Only one cow, that also had the highest response after the first immunization, developed a meaningful anti- PCV2 IgG antibody titer when boosted with a second immunization (Table 1).
| Vaccinated | |||
|---|---|---|---|
| B0 | B1 | B2 | |
| R1 | 0 | 147 | 5078 |
| R2 | 0 | 34 | 314 |
| R3 | 0 | 37 | 557 |
| R4 | 0 | 14 | 565 |
| Controls | |||
| R5-R8 | 0 | 0 | 0 |
Table 1: PCV2 specific vaccinated cattle versus adjuvant injected controls.
R1 to R8 were 8 cattle. B0 is the bleed before vaccination; B1 is the bleed three weeks after first vaccination; B2 is the bleed three weeks after boost. Numbers indicate mean PCV2 specific IgG concentrations in ELISA units.
Discussion
Many studies including those from our group have found that pig livestock is widely infected with PCV2 [2,5]. Moreover, scientific reports also indicate that cattle are susceptible to PCV2 infections and these viruses may even cause disease in cattle [16]. We therefore analysed the prevalence of PCV2 infections in cattle. This undertaking is not simple, as PCV2 infections are latent in pigs [3,5,29] and we must therefore assume a similar infection characteristic may also be found in other unrelated hosts. Thus, PCV2 might be present in the host without being detected in the blood as viremia. PCV2-specific antibody absence or specific antibody isotype presence may not necessary reflect the infection/disease status of cattle. This discrepancy in the pig has been reported by several groups including ourselves [3,7,8,29]. In cattle, this phenomenon has not been previously described, therefore we investigated the PCV2 status in two cohorts of cattle. We observed 31% of cattle were PCV2-viremic, but none showed an anti-PCV2 specific IgM or IgG humoral immune response. As this is a study in progress, we cannot definitively ensure that the assay used can detect a specific PCV2-IgM response from cattle. However, the absence of specific antibody against PCV2 in cattle is not easily explained by an unresponsiveness of the cattle immune response as we found that vaccination against PCV2 provoked an anti-PCV2 IgG antibody humoral response against the virus. Nevertheless, it appears when using the same vaccination protocols as used for pigs, that the response to PCV2 in cattle is less efficient compared to the pigs’ immune responses. This could reflect the natural historical exposure of pigs to PCV2 [5] but would also support the notion that PCV2 infection of cattle is in the early evolutionary phase of host adaption.
Normally, after a single vaccination, anti-PCV2 IgM-specific antibodies are indicative that the animals’ immune system had encountered the antigen for the first time. The direct appearance of anti-PCV2 IgG isotype antibody after virus-specific vaccination suggests that these cattle were previously infected with PCV2. It is interesting to note the potential of vaccination to reveal hidden latent virus infections.
Further evidence of early evolutionary PCV2 host infection adaption comes from the observation that close to PMWS-affected pig farms, other animals carry PCV2 more often than animals in proximity to the PCV2-subclinical infected pig cohort. As PMWSdisease ridden pigs shed higher concentrations of PCV2 [21,22] this would indicate that cross-species transmission of PCV2 is also virus concentration dependent and additionally that the cattle are not saturated with PCV2 infections. However, we only found a statistically significant difference between the PCV2 infection grades of the cattle cohorts from the two farms with a one-sided Fischer analysis. We think that bigger cattle cohorts in proximity to the pigs would remove any ambiguity regarding the conclusion.
We analysed blood of the three highest viremic cattle specific for PCV2 DNA by PCR and sequenced the amplificates. These sequences were not new, instead they belong to viruses of the PCV2b genotype, namely the PCV2b-CH, which dominated the Swiss epizooty [28] and seems to be present endemically throughout the pig population. As this virus sequence is not novel or different from the dominant genotype group member in pigs, this represents a real cross-species transmission of PCV2, expanding into another unrelated species, namely cattle.
Out of these cattle cohorts, a 7 years old cow was infected with the highest virus titter of 1.3 x 106 virus genomes per ml blood. The cow suffered from chronic mastitis. Although we previously presented evidence that PCV2-infected pigs have a deregulated T-cell response [3], further investigations are needed to examine if the presence of smaller concentrations of PCV2 virus titer may be sufficient to induce disease in different host such as cattle. In this respect, chronic mastitis in cattle might be an equivalent disease manifestation akin to PCVDs in pigs. However, follow up studies need to confirm or disprove this possibility.
Conclusion
PCV2b-CH is the dominant PCV2 infection in pigs, its transmission to cattle is reported here for the first time. The higher concentration of PCV2 from the pig PMWS farm supports cross-species transmission. It is possible either that the PCV2 capsid protein is not that immunogenic in cattle or both we may also be witnessing a real-time occurrence of cross-species transmission from pig to cattle, occurring even before the virus has adapted appropriately to the new host.
Acknowledgements
We thank Roseline Weilenmann at the Institute of Veterinary Pathology (Zurich) for her dedicated technical support. The laboratory work was partly performed using the logistics of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich. The project was also financially supported by the BNF.
References
- Allan GM, Ellis JA (2000) Porcine circoviruses: a review. J Vet Diagn Invest 12: 3-14.
- Segalés J (2012) Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res 64: 10-19.
- Klausmann S, Sydler T, Summerfield A, Lewis FI, Weilenmann R, et al. (2015) T-cell reprogramming through targeted CD4-coreceptor and T-cell receptor expression on maturing thymocytes by latent Circoviridae family member porcine circovirus type 2 cell infections in the thymus. Emerg Microbes Infect 4: e15.
- Segalés J, Calsamiglia M, Olvera A., Sibila M., Badiella L, et al. (2005) Quantification of porcine circovirus type 2 (PCV2) DNA in serum and tonsillar, nasal, tracheo-bronchial, urinary and faecal swabs of pigs with and without postweaning multisystemic wasting syndrome (PMWS). Vet Microbiol 111: 223-229.
- Sydler T, Brägger S, Handke M, Hartnack S, Lewis FI, et al. (2016) Latent porcine circovirus type 2-infected domestic pigs: A potential infection model for the effective development of vaccines against latent or chronic virus induced diseases. Vaccine 34: 1047-1053.
- Kurmann J, Sydler T, Brugnera E, Buergi E, Haessig M, et al. (2011) Vaccination of dams increases antibody titer and improves growth parameters in finisher pigs subclinically infected with porcine circovirus type 2. Clin Vaccine Immunol 18: 1644-1649.
- Meerts P, Misinzo G, Lefebvre D, Nielsen J, Botner A, et al. (2006) Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res 2: 6.
- Fort M, Olvera A, Sibila M, Segales J, Mateu E (2007) Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet Microbiol 125: 244-255.
- Stevenson LS, Gilpin DF, Douglas A, McNeilly F, McNair I, et al. (2007) T lymphocyte epitope mapping of porcine circovirus type 2. Viral. Immunol 20: 389-398.
- Tischer I, Bode L, Apodaca J, Timm H, Peters D, et al. (1995) Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch Virol 140: 1427-1439.
- Allan GM, McNeilly F, McNair I, Curran MD, Walker I, et al (2000) Absence of evidence for porcine circovirus type 2 in cattle and humans, and lack of seroconversion or lesions in experimentally infected sheep. Arch Virol 145: 853-857.
- Ellis JA, Konoby C, West KH, Allan GM, Krakowka S, et al. (2001) Lack of antibodies to porcine circovirus type 2 virus in beef and dairy cattle and horses in western Canada. Can Vet J 42: 461-464.
- Tischer I, Gelderblom H, Vettermann W, Koch MA (1982) A very small porcine virus with a circular single-stranded DNA. Nature 295: 64–66.
- Nayar GP, Hamel AL, Lin L, Sachvie C, Grudeski E, et al. (1999) Evidence for circovirus in cattle with respiratory disease and from aborted bovine fetuses. Can Vet J 40: 277-278.
- Li L, Shan T, Soji OB, Alam MM, Kunz TH, et al. (2011) Possible cross-species transmission of circoviruses and cycloviruses among farm animals. J Gen Virol 92: 768-772.
- Kappe EC, Halami MY, Schade B, Alex M, Hoffmann D, et al. (2010) Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl Munch Tierarztl Wochenschr 123: 31-41.
- Halami MY, Freick M, Shehata AA, Müller H, Vahlenkamp TW (2014) Susceptibility of calves to porcine circovirus-2 (PCV2). Vet. Microbiol 173: 125-131.
- Khaiseb S, Sydler T, Zimmermann D, Pospischil A, Sidler X, et al. (2011) Coreplication of the major genotype group members of porcine circovirus type 2 as a prerequisite to coevolution may explain the variable disease manifestations. J Virol 85: 11111-11120.
- Tomás A, Fernandes LT, Valero O, Segalés J (2008) A meta-analysis on experimental infections with porcine circovirus type 2 (PCV2). Vet Microbiol 132: 260-273.
- Baumgartner M, Brugnera E, Sydler T, Burgi E, Hassig M, et al. (2012) Risk factors causing postweaning multisystemic wasting syndrome (PMWS) onset in Swiss pig farms. Schweiz Arch Tierheilkd 154: 429-436.
- Brunborg IM, Moldal T, Jonassen CM (2004) Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J Virol Methods 122: 171-178.
- Opriessnig T, Meng XJ, Halbur PG (2007) Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet Diagn. Invest 19: 591-615.
- Lopez-Soria S, Segales J, Nofrarias M, Calsamiglia M, Ramirez H, et al. (2004) Genetic influence on the expression of PCV disease. Vet Rec 155: 504.
- Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, et al. (2006) Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet Pathol 43: 281-293.
- Sun X, Wertz N, Lager KM, Butler JE (2012) Antibody repertoire development in fetal and neonatal piglets. XV. Porcine circovirus type 2 infection differentially affects serum IgG levels and antibodies to ORF2 in piglets free from other environmental factors. Vaccine 31: 141-148.
- Firth C, Charleston MA, Duffy S, Shapiro B, Holmes EC (2009) Insights into the evolutionary history of an emerging livestock pathogen: porcine circovirus 2. J Virol 83: 12813-12821.
- Ellis J, Hassard L, Clark E, Harding J, Allan G, et al. (1998) Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J 39: 44-51.
- Wiederkehr DD, Sydler T, Buergi E, Haessig M, Zimmermann D, et al. (2009) A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet Microbiol 136: 27-35.
- Vybiral PR, Sydler T, Haessig M, Sidler X, Brugnera E (2017) Antibody Titers from Finisher Populations with Persistent/Latent PCV2 Infection Before, During and After the PCV2-SD Epizootic. Epidemiology: Open Access 7:5.
Citation: Sidler X, Sydler T, Hässig M, Brugnera E (2019) Are PCV2-Cattle Infections at an Early Evolutionary Virus Adaption Stage?. J Infect Dis Ther 7:389. DOI: 10.4172/2332-0877.1000389
Copyright: © 2019 Sidler X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4120
- [From(publication date): 0-2019 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 3194
- PDF downloads: 926