Research Article Open Access
Aromatic Hydrocarbon Degrading Fungi Inhabiting the Phyllosphere ofOrnamental Plants on Roadsides of Urban Areas in Sri Lanka
| Undugoda LJS, Kannangara S* and Sirisena DM | |
| Department of Botany, Faculty of Science, University of Kelaniya, Kelaniya, Sri Lanka | |
| *Corresponding Author : | Kannangara S Department of Botany, Faculty of science University of Kelaniya, Kelaniya, Sri Lanka E-mail: sagarikadpk@kln.ac.lk |
| Received November 23, 2015; Accepted January 22, 2016; Published January 31, 2016 | |
| Citation: Undugoda LJS, Kannangara S, Sirisena DM (2016) Aromatic Hydrocarbon Degrading Fungi Inhabiting the Phyllosphere of Ornamental Plants on Roadsides of Urban Areas in Sri Lanka. J Bioremed Biodeg 7:328. doi: 10.4172/2155-6199.1000328 | |
| Copyright: © 2016 Undugoda LJS, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The phyllosphere of Ixora chinensis, Ervatamia divaricata, Hibiscus rosa-sinensis and Amaranthus cruentus, which are highly abundant on roadsides of five polluted areas in Sri Lanka (Colombo Fort, Maradana, Orugodawattha, Panchikawattha and Sapugaskanda) were rich in aromatic hydrocarbon (AH) degrading phyllosphere fungi compared to the plants from the less polluted site. HPLC results revealed significantly higher concentrations of phenanthrene, naphthalene, toluene and xylene concentrations in the phyllosphere of these plants collected from the five polluted sites, promoting the colonization of AH degrading fungal population. In fact, the phyllosphere of Ixora chinensis collected from Colombo Fort had the highest AH degrading fungal population. Also the phyllosphere of these plants had the highest phenanthrene (96.1 ng/g), naphthalene (160 ng/g), toluene (85.66 ng/g) and xylene (54.39 ng/g) concentrations. Thirty eight such fungal strains were isolated from the leaves of four ornamental plants collected from the above five polluted sites. Plate assay results showed, out of them twenty four phyllosphere fungal strains had at least one AH compound utilization ability. However, according to the HPLC and colorimetric assays, only nineteen fungal strains had the AH degradation ability. Then the best AH degrading phyllosphere fungi were identified into species levels (Penicillium oxalicum, Aspergilllus aculeatus, Aspergillus oryzea and Colletrotrichum siamense) using molecular techniques followed by PCR amplification, amplicons sequencing and BLASTN search. Penicillium oxalicum was the best naphthalene and phenanthrene degrader with 80% and 96% degradation abilities respectively. Significantly higher toluene degradation was demonstrated by Aspergilllus aculeatus. Colletrotrichum siamense showed the significantly highest xylene degradation ability (68.9%). These fungi were the dominant species in the highly polluted sites, Maradana and Colombo Fort.
| Keywords |
| Phyllosphere fungi; Air pollution; Biodegradation; Ornamental plants; Aromatic hydrocarbon |
| Introduction |
| Air pollution is one of the environmental problems of great concern, in the modern world. Aromatic hydrocarbons (AHs) are a major hazardous air pollutant in the ambient air, which have high carcinogenicity and geno-toxicity to the all-living beings [1]. The main sources of AHs in the air are vehicular emissions and industrial processes. AHs in the ambient air deposit onto the phyllosphere of plants mainly by the dry deposition, because of their hydrophobic character and high molecular weight. |
| The phyllosphere represents the largest biosphere-atmosphere inter-phase on the earth and is dominated by leaves. Like other biological surfaces, the phyllosphere harbors a large number of diverse microorganisms, including bacteria, yeasts, oomycetes, fungi, and algae [2]. |
| The predominant microorganisms in the phyllosphere are bacteria and fungi. They can degrade AHs by three mechanisms: The first is biodegradation of the AH resulting in carbon and energy for the microorganisms. The second is co-metabolism where two or more AHs are degraded, but only one contributes towards the carbon and energy requirements of the cell. The third is an intracellular process to make AHs more water soluble, as a detoxification method necessary for cell excretion [3]. |
| PAH degrading bacteria were about 1-10% of the total heterotrophic phyllosphere populations of ornamental plants highly abundant in roadside of Thailand and consisted of diverse bacterial species such as Acinetobacter, Pseudomonas, Pseudoxanthomonas, Mycobacterium, and uncultured bacteria [4]. |
| Pseudomonas, Microbacterium, Rhizobium, and Deinococcus species were the highly available phenanthrene degrading bacteria in the phyllosphere of Ixora chinensis collected from the roadsides of Bankok [5]. The results of Ref. [6] revealed naphthalene degrading bacteria isolated from the marine sample collected from Mumbai as well as petroleum soil sample from Trimbak road Satpur, Nashik consisted of bacterial species as Micrococcus spp, Bacillus spp, Staphylococcus spp, Pseudomonas spp. Pseudomanas putida which was isolated from effluent treatment plant (Frielas, Portugal) had higher toluene and xylene degradation ability [7,8]. |
| Fungi have a high ability of AH degradation over other organisms due to the presence of enzymes, such as lignin peroxidase, manganese peroxidase, and laccase, which can interact with several types of AHs and degrade those. They also have other enzyme systems, such as cytochrome P450 monooxygenase and epoxide hydrolase that oxidize Ahs [9]. Fungi are also tolerant to high concentrations of recalcitrant compounds and are able to flourish in extreme conditions, such as at high temperatures and low pH. In addition, the fact that fungi form large, branching mycelia makes it possible for them to grow and distribute through a solid matrix to degrade AHs within contaminated areas by secreting extracellular enzymes [10]. The fungal strains Curvularia sp. F18, Lentinus sp. S5, and Phanerochaete sp. T20 which were highly abundant in the petroleum oil contaminated soil samples of Thailand showed higher fluorene, phenanthrene, fluoranthene, and pyrene degradation ability [11]. But there is a shortage of published research articles related to the fungal strains which can degrade both PAHs and MAHs (monoaromatic hydrocarbons). Fungal degradation of these both types of AHs is important to survive under harsh condition which is a mixture of many pollutants. So that phylloremediation is one of the mostly used bioremediation methods that make use of AH degrading bacteria, fungi and algae which mostly inhabit leaves in polluted sites, to clean AH pollutants [12]. |
| Whereas the AH mineralization and degradation pathways of bacteria have been well studied, knowledge of similar activities in fungi is limited to various white rot fungi and a few species of soil fungi. According to the available literature the phyllosphere of ornamental plants, usually found on the roadsides of urbanized areas, polluted with AHs are rich in AH degrading fungal strains and the fast AH degrading phyllosphere fungal strains can be used as efficient bioremediators because of their high ability to tolerate AH pollutants while degrading those AHs into non toxic forms. In Sri Lanka there is a severe shortage of published records in this aspect. Therefore, the present study was conducted with an attempt to isolate and identify the phyllosphere fungal strains inhabiting some of the most abundant ornamental plant leaf surfaces in highly polluted areas in Sri Lanka, and investigate their ability to degrade four AHs; phenanthrene, naphthalene, toluene and xylene as these fungi have the potential to be used for in-situ bioremediation at environmental sites where contamination is caused by several types of AHs, a more common scenario. |
| Methods and Materials |
| Leaf sample collection |
| Leaf samples were collected from the ornamental plants, Ixora chinensis, Ervatamia divaricata, Hibiscus rosa-sinensis and Amaranthus cruentus which are highly abundant in roadsides of five urbanized areas, Colombo Fort, Orugodawattha, Maradana, Panchikawattha and Sapugaskanda in Sri Lanka. Leaf samples were also collected from the control site, Meemure, a remote rural area in Sri Lanka which is exposed to very low levels of pollutants compared with sites in urban areas. |
| Isolation of phyllosphere fungi |
| Four grams of freshly picked leaves were immersed in 100 ml of potassium phosphate buffer (pH 7.0) and shaken at 180 rpm for 1 hr. A dilution series of leaf wash (10-3-10-9) was prepared with peptone water and then 100 μl of each dilution was spread on Czepeck-Dox medium. This was done in 50 replications. Fungal colony counts were taken after a five day incubation period at room temperature. Then, each colony was streaked on Bacto-Bushell-Haas (BBH) medium (pH 7.0 at 25°C) which contains MgSO4 0.2 g/L, CaCl2 0.02 g/L, KHPO4 1.0 g/L, K2HPO4 1.0 g/L, NH4NO3 1.0 g/L and FeCl3 0.05 g/L. This medium was then supplemented with 100 ppm of AHs (toluene, xylene, naphthalene and phenanthene) separately and checked for their ability to grow in the presence of AHs. Then the number of AH degrading fungal colonies in leaf wash was determined. Finally, the AH degrading fungal population, in terms of colony forming units (CFU)/g was calculated using the following formula. |
| A: Average number of fungal colonies; B: Final dilution; C: Volume of water used for suspension of sample; D: Weight of leaf sample. |
| Finally, the frequency of occurrence of AH degrading fungi was calculated in order to categorize them in to the categories of dominant (81-100%), common (61-80%), frequent (41-60%) and occasional (0- 40%) as indicated by Ref. [13], using the following formula and also to obtain an idea about their distribution on the leaf phyllosphere. |
| Screening of AH utilization ability of the phyllosphere fungal strains |
| In order to observe the AH utilization ability of each fungal strain, a mycelial disk (5 mm in diameter) was taken from the pure cultures of each fungal strain and placed on the middle of petri dishes with Bacto Bushnell Haas (BBH) agar medium supplemented with AH compounds at 100 ppm concentration separately. Then, colony diameter was measured after seven day incubation period to test the AH utilization ability of isolated phyllosphere fungal strains. |
| Screening of AH degradation ability of the phyllosphere fungal strains |
| The AH degradation potential of isolated fungi was qualitatively tested using a modified colorimetric method [13]. Two agar plugs (1 cm2 each) of a pure growth of each isolate, were inoculated into Bacto Bushnell – Haas broth (50 ml/250 Erlenmeyer flask) with sterile AH (100 ppm), redox indicator, methylene blue (2% v/v) and Tween 80 (0.1% v/v). The control flask had no organisms. Incubation was at room temperature (28-30°C) with constant shaking at 180 rev/min for 7 days with fungi. After incubation, the culture was filtered to separate biomass and other residues followed by centrifugation at 8000 rpm for 15 minutes. Then the absorbance of the supernatant was measured at 609 nm (for methylene blue) by using the UV-Vis spectrophotometer and then calculated the% of degradation. |
| Analysis of AH degradation ability of the phyllosphere fungal strains |
| AH degradation ability of these fungal strains was quantitatively analysed using HPLC method. Two agar plugs (1 cm2 each) of a pure growth of each isolate, were inoculated into Bacto Bushnell – Haas broth (50 ml/250 Erlenmeyer flask) with sterile AH (100 ppm). After the seven day incubation period at room temperature, the residual PAHs (phenanthrene and naphthalene) and MAHs (toluene and xylene) in the broth were extracted using acetone and hexane solvents and methanol solvent respectively. Cleanup procedure was carried out using chromatographic column (250 mm long, 4.6 m diameter) which was packed totally porous spherical C-18 material (packed size, 5 μm). The extracts were analyzed by Agilent 1100 series HPLC (Agilent Technologies, Waldbronn, Germany) equipped with an Agilent 1200 diode array detector. 20 μl of each sample was injected into A ZORBAX ECLIPSE Plus C18 column (Agilent Technologies, USA) (4.6 × 100 mm × 3.5 μm particle size). Acetonitrile-water mixture (75:25) was used as a mobile phase for PAHs and methanol-water mixture (75:25) was used as a mobile phase for MAHs at a flow rate of 1.0 ml min-1. A standard curve of each AH compound was plotted using the following concentrations 1, 10, 20, 40, 60, 80 ppm and 100 ppm respectively to determine retention time and to study linearity of the detector. Then AH concentrations in the samples were calculated against standard curves. Finally, the percentage of AH degradation was calculated by the following formula: |
| AH degradation%=100 × [(Mi-Ms)/Mi] |
| Where, Ms is the concentration of AH in each treatment and Mi is the initial AH concentration present in the sample [14]. |
| HPLC analysis of the deposited AH pollutants on the phyllosphere of leaf samples |
| Ten grams of freshly picked leaves were immersed in 100 ml of hexane: acetone (1:1) and shaken at 180 rpm for 1 hr. Then, leaf wash was filtered and then evaporated using a rotary evaporator. Cleanup procedure was carried out using chromatographic column (250 mm long, 4.6 m diameter) which was packed totally porous spherical C-18 material (packed size, 5 μm). |
| The PAHs (phenanthrene and naphthalene) and MAHs (toluene and xylene) in the extract were analyzed using an Agilent 1100 series HPLC (Agilent Technologies, Waldbronn, Germany) equipped with an Agilent 1200 diode array detector. A ZORBAX ECLIPSE Plus C18 column (Agilent Technologies, USA) (4.6 × 100 mm × 3.5 μm particle size) maintained at room temperature was used for this purpose. Acetonitrile-water mixture (75:25) was used as a mobile phase for PAHs and methanol-water mixture (75:25) was used as a mobile phase for MAHs at a flow rate of 1.0 ml min-1. |
| Identification of the best AH degrading fungal strains |
| The fungi were identified up to genus level based on their morphological and microscopic characteristics (especially reproductive structures) following the identification keys [15]. |
| AH degrading phyllosphere fungal strains were identified up to species level by determination of the nucleotide sequence of ITS region of rDNA operon. ITS region of the AH degrading phyllosphere fungi was amplified by PCR with primer pair ITS 1 (5’-TCCGTAGGTGAACCTGCGG-3’) forward primer and ITS 4 (5’- TCCTCCGCTTATTGATATGC-3’) reverse primer [16]. The PCR reaction was performed in 40 μl volumes containing 5 μl of the genomic DNA sample, 10 × PCR buffer containing 750 mM Tris-HCl (pH 8.8 at 25°C), 200 mM (NH4)2 SO4, 0.1% Tween 20; 2.5 mM MgCl2; 0.16 mM dNTP Mix; 20 pmol of forward and reverse primers and 0.75 U Taq DNA polymerase (MBI, Fermentas, Lithuania). Amplification was carried out in a thermal cycler (Eppendorf Mastercycler 5330) with initial denaturation at 94°C for 5 minutes, followed by 40 cycles of denaturation at 94°C for 30 seconds, annealing at 52°C for primer pair ITS 1 (5’- TCCGTAGGTGAACCTGCGG-3’) and ITS 4 (5’- TCCTCCGCTTATTGATATGC-3’) for 1 minute, and extension at 72°C for 1 minute. The thermal cycles were terminated by a final extension for 5 minute at 72°C. Then PCR product (20 μl) was run in a 1.5% agarose gel and a gel piece with resulted band (900 bp) [16] was excised. |
| Direct sequence determination of the purified DNA sample was performed using sequencing primers with an Applied Biosystems automated sequencer (ABI3730XL) at Macrogen, Seoul, Korea. Almost complete ITS region sequences of strains designated as 15ASP, 9SF, 4SF and 13GTD were obtained and compared with ITS sequences available in GenBank using BLASTN searches [17]. |
| Statistical analysis |
| Data presented for colorimetric assay, plate assays and HPLC analysis were the means of ten replicates. The SPSS 16.0 software was used in determination of standard error (SE), least significant difference (LSD), ANOVA and regression analysis. |
| Results and Discussion |
| Presence of AH degrading fungi on leaves |
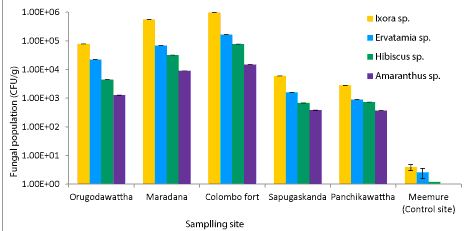
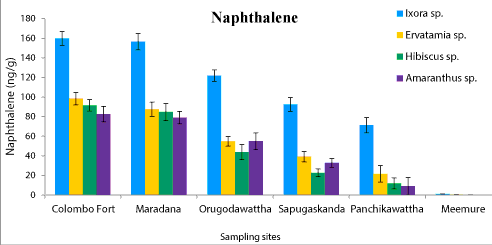
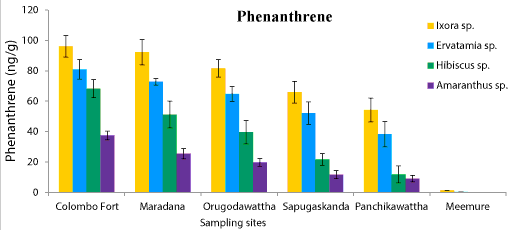
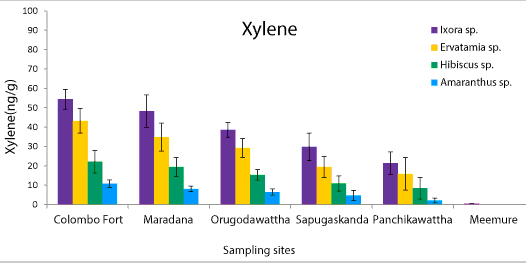
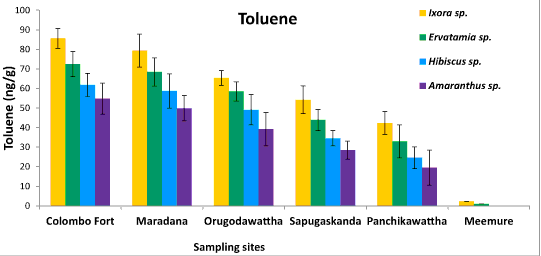
| Phyllosphere fungal population of four ornamental plants, Ixora chinensis, Ervatamia divaricata, Hibiscus rosa-sinensis and Amaranthus cruentus collected from five polluted sites ranged from 6.2 × 105 to 8.1 × 108 CFU/g. Among them, Ixora chinensis collected from Colombo Fort showed the highest phyllophere fungal population. AH degrading phyllosphere fungal population found in each leaf sample of four plant species from five polluted sites was 1.28 × 103-9.6 × 106 CFU/g (Figure 1). But AH degrading phyllosphere population of the less polluted site was very low compared to the selected five polluted sites. Moreover, the AH degrading phyllosphere fungal population of Ixora chinensis from all polluted sites, was higher than that of other three ornamental plant species, at p=0.05 levels of significance (Figure 1). Even in the less polluted site, Ixora chinensis had the highest AH degrading fungal population. Many reports show that plant tissues with high wax content like Ixora chinensis accumulate more AHs and it leads to promote the colonization of AH degrading fungi in the phyllosphere [5,18]. In fact, the HPLC analysis of the deposited AHs in the leaf samples of four ornamental plants collected from the five polluted sites revealed, the phyllosphere of Ixora chinensis showed the highest naphthalene (160 ng/g) (Figure 2), phenanthrene (96.1 ng/g) (Figure 3), toluene (85.66 ng/g) (Figure 4) and xylene (54.39 ng/g) (Figure 5) concentrations. Ref. [5] indicated higher AH concentration of the phyllosphere increases the AH degrading phyllosphere bacterial density. Similarly, in this study higher AH content of the phyllosphere of this plant leads to increase the AH degrading fungal population. |
| Samples from Colombo Fort, Orugodawattha and Maradana showed a comparatively higher fungal consortium, compared to those from Sapugaskanda and Panchikawattha. Simultaneously, all four AH concentrations of the leaf samples collected from Colombo Fort, Orugodawattha and Maradana was higher compared to those from Sapugaskanda and Panchikawattha. Furthermore, leaf samples from Colombo Fort showed the highest phyllosphere fungal density at p=0.05 significance level compared to that of the other four polluted sites (Figure 1). Simultaneously, the HPLC results indicated, leaf samples of Colombo Fort had the highest AH concentrations compared to the other sites (Figures 2-5). Naphthalene and phenanthrene concentrations of these leaf samples were comparatively higher than toluene and xylene. This may due to the daily increasing vehicle exhaust and industrial emissions. As consequence of high AH content in the phyllosphere, the AH degrading phyllosphere fungal population of leaf samples were increased. Furthermore, Maradana had the secondly high concentration of AHs in the leaf samples (Figure 2-5) and AH degrading fungal population in samples collected from that area, was only a little less than in samples from Colombo Fort (Figure 1). The leaf samples from Sapugaskanda and Orugodawattha had higher AH concentrations compare to Panchikawattha. The reason for this is, besides vehicular emissions, Sapugaskanda and Orugodawattha areas are exposed to waste product of oil refinery processes, which elevate the AH levels in the ambient air. As a result of this, the phyllosphere fungal consortium was higher in samples from these two sites, compared to that of the Panchikawattha area. However, Sapugaskanda is an area located out of the Colombo city, but the AH concentration of leaf samples from that site, was as high as that of samples from Orugodawattha. The reason for this is, the current location of the oil refinery, presence of an industrial zone and the high vehicular traffic in the area, all of which increase the AH levels in the ambient air [19]. Simultaneously, AH degrading phyllosphere fungal population of leaf samples from Sapugaskanda was as high as Orugodawattha. All in all, there is a significant correlation between AH degrading fungal consortium in the phyllosphere and the AH concentration of the phyllosphere. The results of [20], also revealed, the accumulation of AHs on plant leaves was found to correlate with the atmospheric AH concentrations. Consequently, AH degrading phyllosphere fungal population is a bioindicator for AH level in the ambient air of urban areas. |
| AH utilization ability of phyllosphere fungal strains |
| The AH utilizing fungal strains can either degrade AHs into nontoxic form while using it as a sole source of carbon or absorb AHs into the fungal cells without degradation. The phyllosphere of leaf samples was rich in different fungal strains, which belong to different genera. Approximately 38 fungal strains were present on the phyllosphere of leaf samples from the five polluted areas. However, colony diameter results of each fungal strain obtained from plate assay revealed, out of these 38 strains, 24 strains were able to utilize AHs in the BBH medium which may be degradation or only absorption (Table 1). These fungal strains should be AH degraders if they use as efficient biodegrades. Consequently, their AH degradation ability was qualitatively analyzed using colorimetric assay. |
| AH degrading ability of phyllosphere fungal strains |
| These isolates produced a color change in the BBH broth due to the reduction of the methylene blue indicator by the oxidation of aromatic hydrocarbon by these phyllosphere fungal strains. The total color change (blue to colorless) supports the fact that the isolates are potential hydrocarbon oxidizers [21] and this color change can measure as an absorbance value using UV-Vis spectrophotometer. |
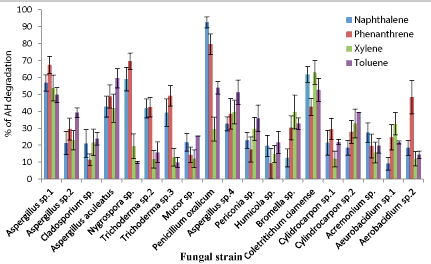
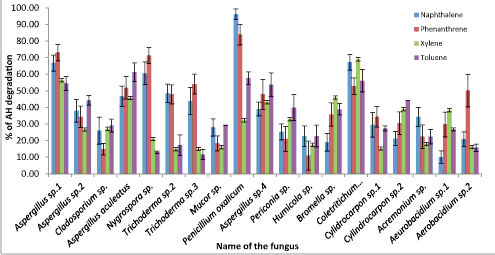
| The higher fungal AH degraders had low absorbance values after the incubation period compared to the control whereas the low fungal AH degraders showed high absorbance values compared to the control. The % of AH degradation results elicit, out of 24 AH utilizing phyllosphere fungal strains 19 fungal strains were able to oxidize AHs in the BBH broth while giving higher color changes (Figure 6). These 19 AH degrading fungal strains belong to 13 genera; Aspergillus, Cladosporium, Nigrosporium, Trichoderma, Mucor, Penicillium, Pericornia, Humicola, Broomella, Coletritichum, Cylindrocarpon, Acremonium and Aeurobacidium. Mainly two mechanisms are involved in these fungal AH biodegradation: one utilizes the cytochrome P-450 system [9] and the other uses the soluble extracellular enzymes of lignin catabolism, including lignin peroxidase, manganese peroxidase [22] and laccase [23]. Then, AH degradation ability of these selected 19 fungal strains was quantitatively analyzed using HPLC method. |
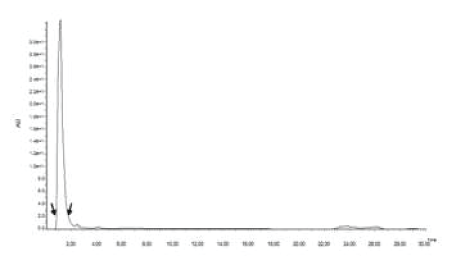
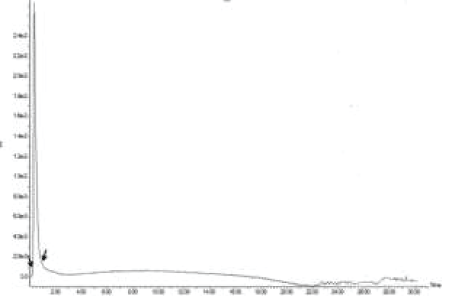
| Although, according to the finding of Ref. [21], A. niger and A. versicolor showed the highest PAH degradation ability, the HPLC results of this study revealed, Penicillium oxalicum had the highest phenanthrene and naphthalene degrading ability, 80% and 96% respectively (Figure 7) [24]. Indicated white-rot fungus Phanerochaete chrysosporium had the xylene and toluene degradation ability. But in this study, the best xylene degrader (68.9%) was Coletritichum siamense, which could also degrade phenanthrene (52%), naphthalene (67%) and toluene (56%) in the BBH medium in high rates. Aspergillus aculeatus was the best toluene degrader which was able to degrade 60% of toluene in the BBH medium (Figure 7). However, toluene degradation ability of all isolated fungal strains was comparatively low. Most of the naphthalene and phenanthrene degraders were also able to degrade monoaromatic hydrocarbons, xylene and toluene efficiently, but most of the monoaromatic hydrocarbon degraders have less ability in degrading polyaromatic hydrocarbons (Figure 7). However the HPLC chromatograms obtained for the AHs degradation of phyllosphere fungal strains revealed only one main peak for the remaining AHs and very small few peaks which can be neglected. In fact, chromatogram of P. oxalicum after and before naphthalene degradation revealed the absence of any significant peaks rather than naphthalene specific peak. This indicates the less accumulation of other toxic metabolites during the process of degradation (Figures 8 and 9). |
| Identification of the best AH degrading phyllosphere fungi |
| The best phyllosphere AH degraders were identified upto species level using molecular techniques. ITS region (ITS1 - 5.8 RNA - ITS2) of fungal strains designated as 9SF, 13GTD, 15ASP and 4SF was amplified using ITS1-F and ITS4-R primers. Gel electrophoresis of PCR products showed the expected 900 bp bands [16]. |
| Nucleotide sequencing of the ITS regions of the rRNA genes (ITS1 - 5.8S RNA - ITS2) was performed for the 9SF, 4SF, 13GTD, and 15ASP isolates followed by BLASTN searches to identify close relatives (high sequence identity) in the Genbank database [17]. Nucleotide sequence of 15ASP strain was most closely related to Aspergillus aculeatus in 100% nucleotide sequence similarity and 9SF had 100% nucleotide sequence similarity to Aspergillus oryzea. The other two fungal strains were most closely related to Penicillium oxalicum 13GTD (100% sequence similarity) and Coletrotrichum siamense 4SF (99% sequence similarity). The nucleotide sequences of isolates 4SF, 13GTD, 15ASP, and 9SF have been submitted to the Genbank database under accession numbers KP863925, KP863926, KP863927 and KP791804. |
| Frequency of occurrence of AH degrading phyllosphere fungi |
| Based on the frequency of occurrence, AH degrading phyllosphere fungal strains isolated from five polluted sites and one control site were categorized as dominant (81-100%), common (61-80%), frequent (41-60%) and occasional (0-40%) [25] (Table 2). The phyllosphere fungi Aspergillus aculeatus, Aspergillus oryzea, Penicillium oxalicum and Coletritichum siamense were found to be dominant in the highly polluted sites Colombo Fort and Maradana while they were common in the other two polluted sites, Orugodawattha, and Sapugaskanda. However, these best AH degraders were occasional in the control site. A majority of low AH degraders were occasional in all five polluted sites and absent in the phylloshere of the control site. These best degraders were dominant in the areas highly polluted with AHs. |
| The best AH degrading phyllosphere fungal strains were A. aculeatus, Penicillium oxalicum and Colletrotrichum siamense. Among them A. aculeatus was the best toluene degrader and C. siamense showed the highest xylene degrading ability. Moreover P. oxalicum showed the highest naphthalene and phenanthrene degrading ability as well as their xylene and toluene degrading ability was as high as the best MAH degraders. All these AH degraders dominate the phyllosphere of highly polluted sites. These characteristics lead to their potential use as efficient bioremediators in the environment cleaning process. Out of these three strains P. oxalicum is especially an efficient bioremediator, because of its multi AH degrading ability which increases the survival in the presence of various air pollutants. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15139
- [From(publication date):
January-2016 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 14113
- PDF downloads : 1026
