Assessment of Bovine Umbilical Cord-Derived Stem Cells in Conjunction with Glutamine for Mitigation of Hepatic Injury in the Context of Acute Pancreatitis
Received: 07-Mar-2024 / Manuscript No. DPO-24-129081 / Editor assigned: 11-Mar-2024 / PreQC No. DPO-24-129081 (PQ) / Reviewed: 26-Mar-2024 / QC No. DPO-24-129081 / Revised: 16-Apr-2025 / Manuscript No. DPO-24-129081 (R) / Published Date: 23-Apr-2025
Abstract
Acute Pancreatitis (AP) is a localized-to-systemic inflammatory disorder that triggers the release of various proinflammatory mediators. Unfortunately, effective treatments for this condition are currently lacking and a substantial portion of AP patients succumb to liver failure due to the intricate physiological and pathological interplay between the liver and pancreas. Umbilical Cord Mesenchymal Stem Cells (UCMSCs) exhibit notable attributes, including multipotent differentiation capacity, colony-forming potential and robust migratory responses to injury. Glutamine, an essential amino acid in cell culture and a source of immune cells, is used in early nutritional interventions to help with pancreatitis. In this study, our objective was to evaluate the therapeutic efficacy of a combinatorial approach involving UCMSCs and glutamine in the management of hepatic injury associated with pancreatitis.
To accomplish this objective, we established an acute pancreatitis murine model and administered UCMSCs via tail vein injections while concurrently providing oral glutamine supplementation. The homing of these cells was observed in rats through fluorescence microscopy. A comprehensive array of techniques was employed to assess treatment outcomes. Histopathological examination, as revealed by HE staining, demonstrated a significantly reduced extent of pancreatic tissue damage in both the UCMSC-treated and combined therapy groups compared to the AP control group. Serum amylase and lipase activities were markedly diminished in the UCMSC and combined therapy groups in comparison to the untreated AP group (P<0.05).
Furthermore, the expression levels of proinflammatory cytokines such as IL-6, IL-1 β, and TNF-α were significantly attenuated, while those of the anti-inflammatory IL-10 and SOD were notably elevated in the UCMSCtreated and combined therapy groups (P<0.05). The assessment of apoptosis, a pivotal aspect of tissue damage in pancreatitis, demonstrated a significantly higher apoptotic index in the untreated AP group (P<0.05), whereas it was significantly lower in the UCMSC-treated and combined therapy groups (P<0.05).
Our findings indicate that the combined therapeutic approach involving UCMSCs and glutamine substantially mitigates hepatocyte apoptosis induced by Acute Pancreatitis (AP). However, there was no statistically significant difference in efficacy between combination therapy and direct Mesenchymal Stem Cell (MSC) therapy in ameliorating liver injury in the context of pancreatitis (P>0.05). While combination glutamine therapy presents a promising and novel therapeutic avenue, its efficacy not diverges from that of direct cell-based regimens.
Keywords
Acute pancreatitis; Stem cells; Liver injury; Cell therapy
Introduction
Acute pancreatitis, an inflammatory disorder of pancreas, is caused by the activation of pancreatic enzymes in the Pancreatic Acinar Cell (PAC) due to various stimuli. The activation of pancreatic enzymes due to autodigestive processes stimulates an inflammatory response (Neutrophil and macrophage infiltration, as well as the production of the cytokines tumor necrosis factor and interleukins 1, 6 and 8) [1]. This can allow the overflow of pancreatic protease, which directly irritates and corrodes other organs in the abdominal cavity, leading to a massive inflammatory response and serious complications. The global incidence of AP is about 34 per 100,000 and is trending upward [2]. AP is classified as Mild AP (MAP), Moderately Severe AP (MSAP) and severe AP (SAP), approximately 20% of cases may finally progress to SAP with a mortality rate of 10%-20% [3].
The liver plays a bridging role in the process of multiple organ injury secondary to acute pancreatitis and is an important site of extra-pancreatic organ injury during all acute pancreatitis due to its specific location in the gastrointestinal circulation and its role in metabolic processes. Liver failure accounts for 83% of deaths [4]. With changes in diet, patients with acute pancreatitis are now becoming younger and more severe. One study reported that liver injury occurs in approximately 80% of AP patients and the severity of liver injury is positively correlated with the severity of pancreatitis and the duration of pancreatitis [5]. The exact mechanism of liver injury in AP is not fully understood. The pancreas begins in the caudal ventral and dorsal pancreatic buds of the protointestine, located in almost the same area as the beginning of liver development and early on the protointestine is wrapped in an intact membranous tethered structure, which develops into the mesentery as the embryo gradually matures [6]. In recent years it has been hypothesised that this membranous tethered structure is used in the development of the liver and it has been hypothesised that this structure is preserved during the development of the liver and pancreas and that the mature liver, bile and pancreas form physiological adhesions through these [7]. This has led to the hypothesis that a local microcirculatory system between the pancreas and liver may be formed through the vasculature and lymphatic vessels of the mesentery, which act as a bridge for the various cytokines that cause direct damage to the liver during the course of pancreatitis.
Since their first isolation and identification in 1976, Mesenchymal Stem Cells (MSCs) have been used as a reliable source of cells for regenerative medicine in the study of multiple diseases because of their modulatory effects on a variety of anti-inflammatory molecules. MSC therapy is currently being used in various gastrointestinal diseases such as graft-versus-host disease, inflammatory bowel disease and liver cirrhosis [8,9]. MSC therapy therefore has the potential to be a new approach to treating acute and chronic pancreatitis by suppressing inflammation. Glutamine is a crucial substrate for the immune system and various other cells, including those in the gastrointestinal tract. It plays a role in maintaining the integrity of the gut barrier, supporting the function of immune cells and providing energy for rapidly dividing cells, which can be particularly important in situations of injury or illness. However, there are no studies evaluating the feasibility of glutamine in combination with MSCs therapy.
Materials and Methods
Experimental materials
Forty ICR mice, 6-8 weeks old, weighing 28 g-35 g, purchased from Peking University Medical Department, animal licence number: SCXK (Beijing) 2021-0013. Total Superoxide Dismutase Test Kit, TNF alpha Mouse Uncoated ELISA Kit purchased from Nanjing Jiancheng Bioengineer Institute, Mouse IL-1 beta Uncoated ELISA, IL-6 Mouse Uncoated ELISA Kit, IL-10 Mouse Uncoated ELISA Kit purchased Thermo Fisher, a-amylase assay kit purchased Changchun Huili Biotech co, LTD. Trypsin, Fetal Bovine Serum (FBS), DMEM/F12 medium, paraformaldehyde fixative, Phosphate Buffer (PBS) were purchased from Servicebio; collagenase; DAPI, blocked goat serum, FITC labeled goat anti-rabbit secondary antibody from Beijing Boosen Biotechnology Co., Ltd; CD73, CD90, CD105, CD166, I resistance were purchased from Abcam Company, USA; Bcl-2, caspase-3, GAPDH antibodies and TUNEL kits was purchased from Servicebio. L-Arginine was purchased from GlpBio (USA). Glutamine was purchased from Sigma (USA).
Isolation and culture of UCMSCs
The umbilical cord tissue was sourced from healthy Holstein cow embryos at three months of age. These specimens were generously provided by the changing base of the Beijing Institute of Animal Husbandry and Veterinary Medicine, Chinese Academy of Agricultural Sciences. The extracellular matrix of the minced tissues was dissociated using 2 mg/mL collagenase type I for 45 min. To neutralise the enzymatic process, complete DMEM/F12 supplemented with 12% Fetal Bovine Serum (FBS) was employed. The resultant cell suspension was centrifuged at 1200 rpm for 10 minutes after being filtered through a sieve with a mesh size of 80 µm. The cell pellet was then reconstituted in a full DMEM/F12 mix with 10% FBS, seeded onto 6-well plates and cultivated at 37.5℃ in a humid incubator with 5% CO2. When the MSCs reached about 80% fusion by microscopy, the cells were digested by using 4 mg/mL trypsin II and harvested in 15 ml sterile centrifuge tubes and subjected to passaged culture.
Immunofluorescence staining
We performed immunofluorescence staining. After the concentration of third generation UCMSCs reached 40%-50%, cells were fixed in PBS containing 4% Paraformaldehyde (PFA) for 15 min at room temperature and washed for 3 × 5 min. Cells were then permeabilised with 0.1% Triton X-100 for 30 min at room temperature and washed for 3 × 5 min. Non-specific binding sites were blocked by incubating the cells with 10% sheep serum at room temperature for the non-specific binding site was blocked by incubating the cells with 10% sheep serum for 30 min at room temperature. After blocking, MSCs were incubated overnight at 4℃with the following primary antibody: FITC-bici CD73, CD90, CD105, CD29 and CD45 antibodies (1:200, Abcam, Cambridge, MA, USA). After incubation, MSCs were washed three times in PBS containing FITC-coupled goat anti-rabbit secondary antibody (1:100, Santa Cruz, CA, USA) for 2 h at 37℃ in the dark and washed for 3×5 min. MSCs were counterstained with 1 µg/mL DAPI for 15 min at room temperature in the dark. Finally, fluorescence was captured under a fluorescence microscope signal (Nikon TE-2000-E, Tokyo, Japan).
Multi-differentiation potential of UCMSCs
Osteogenic differentiation of UCMSCs: UCMSCs were seeded into 60 mm cell culture dish (2.0 × 105 cells/well). When third generation UCMSCs reached 30% confluence, the medium was placed with osteogenic medium. After 21 days’ cultivation, Alizarin Red staining was performed to detect calcium deposition.
Adipogenic differentiation of UCMSCs: As stated previous, third-generation UCMSCs reached 30% confluence were grown in medium for adipogenic differentiation. After 14 days, Oil Red O staining was used to assess the level of lipid buildup.
Chondrogenic differentiation of UCMSCs: About chondrogenic diffrentiation, third-generation UCMSCs reached 30% confluence were cultured in chondrogentic medium. After 21 days, the degree of chondrogentic expression was determined using Alcian Blue staining.
Clone formation assay of GMSCs
Third generation stem cells were isolated, centrifuged and then diluted into a single cell suspension with culture medium. After counting, 100 cells were inoculated into cell culture dishes. 7 days to observe cell morphology under an inverted microscope using Giemsa staining.
Animal modelling and grouping
Mouses were randomly divided into 4 groups (n=10). Control group, AP group, MSCs group (umbilical cord mesenchymal stem cells group) and AP+Glu+MSCs group (UCMSCs combined with glutamine treatment group). AP group, after anaesthesia, the mice were given two intraperitoneal injections of 20% L-Arg (200 mg/100 g•bw) at 1 h intervals in the control group and an equal volume of sterile saline in the control group. AP+MSCs group, after modelling, UC-MSCs were injected into the tail vein of rats at a rate of 2 × 105/100 g. AP+Glu+MSCs group, Mice were fed glutamine 0.4 g/(kg-d) after UCMSC injection (2 × 105/100 g) via tail vein after modelling. Both control and AP groups were given 300 microlitres of sterile saline tail vein.
Histopathological observations
The pancreatic and liver tissues of each group were fixed with formaldehyde, dehydrated, sectioned and stained with HE. And their pathomorphology was observed under light microscope. Three pathologists were blinded and scored the degree of oedema, haemorrhage, cellular necrosis and inflammatory cell infiltration in the pancreas according to the literature criteria and the average score of the 10 high magnification fields of view was used as the final score for each section. Twenty-four hours after modelling, no rats died in the control group (success rate: 100%), three died in the AP group (success rate: 70%), two died in the AP + MSCs group (success rate 80%) and one died in the AP+MSCs+Glu group (success rate: 90%). All surviving mice were anaesthetised by intraperitoneal injection of pentobarbital (50 mg/kg) at 24 h postoperatively, blood was collected from the eyeballs, executed and the serum and pancreatic tissues were retained for subsequent experimental testing. The serum and pancreatic and liver tissues were retained for subsequent experimental testing.
ELISA for serum amylase, lipase and inflammatory factors Inflammatory factors
After thawing the serum at room temperature, the expression levels of amylase, lipase, SOD and inflammatory factors (IL-6, TNF-α, IL-10, IL-β) were detected by ELISA according to the steps described in the kit instruction.
CM-Dil Labled UCMSCs
UCMSC were mixed with CM-Dil live cell dye (Thermo Fisher, USA) and incubated in the dark according to the instructions of CM-Dil live cell dye (Thermo Fisher, USA). After 30 min, the solution was processed by centrifugation and the precipitate was retained. The precipitate was resuspended in 9% saline and injected intravenously into a successfully established AP mouse model to observe its colonisation in pancreatic and liver tissues.
Detection of apoptosis using TUNEL assay
Fixed liver tissue was dehydrated, embedded, sectioned and dewaxed to water by automatic dehydrator. The tissue was immersed in xylene Ⅰ for 5-10 min, xylene Ⅱ for 5-10 min, anhydrous ethanol Ⅰ for 5 min, anhydrous ethanol Ⅱ for 5 min, 95% alcohol for 5 min, 85% alcohol for 5 min and water for 10 min, respectively. 5 min, anhydrous ethanol Ⅰ, anhydrous ethanol Ⅱ 5 min, 95% alcohol 5 min, 85% alcohol 5 min, 75% alcohol 5 min. 5 min, 85% alcohol, 75% alcohol 5 min, UP water 5 min, citric acid microwave repair 8 min, PBS wash 3 times 8 min, PBS washed three times, each time 5 min; dark preparation of fluorescent TUNEL incubation solution (A: B=1:30) was prepared in the dark and incubated at 37℃ for 1 h. The sample was washed with PBS three times, each time for 5 min. 5 min; add DAPI to stain the nucleus for 15 min, rinse with PBS, seal with glycerol gelatin and store at -20℃. The samples were stored at -20℃ (the reagents were prepared and used in the dark). The above specimens were processed according to the SOP of pathological examination. The above specimens were dehydrated, trimmed, embedded, sliced, stained, sealed and microscopically examined according to the SOP procedure. The above specimens were dehydrated, trimmed, embedded, cut, stained, sealed and examined by microscope according to the SOP procedures.
Determination of the expression levels of apoptotic proteins using western blotting
Liver tissue was removed and placed into 2 mL grinding tubes, then 3 mm steel beads and RIPA lysate (mass ratio sample: lysate=1:10) were added to each tube. Then add 3 mm steel balls and RIPA lysate (mass ratio of sample: lysate=1:10) into each tube. The tubes were placed in a high-speed low-temperature tissue grinder at -20℃ for 4 times, each time for 60 s. Remove the tubes and put them into a refrigerator at 4℃ for lysis; after 30 min, remove the tubes and centrifuge them at 4℃ for 10 min at 12,000 × g. Remove the supernatant and measure the protein concentration with the BCA Protein Quantification Kit. Take 50 μL of each group, add 5 × Loding buffer at the ratio of 4:1, mix well and then store at -80℃ for 15 min at 95℃ in a thermal cycler. The concentration of primary antibody: Bax 1:5 000, Bcl-2 1:2 000, Caspase-3 1:2 000, β-tubulin 1:2 000, incubate overnight at 4℃ with gentle shaking; wash the PVDF membrane with TBST three times, each time for 5 min; put the PVDF membrane into the secondary antibody (dilution: 1:5 000), incubate at room temperature for 2-3 h with gentle shaking; incubate the PVDF membrane with TBST for 3 times and incubate at room temperature for 2-3 h with TBST. Wash the PVDF membrane with TBST 3 times, each time for 10 min. lay the PVDF membrane on the exposure plate, mix the A and B reagents of ECL emitting solution and add them dropwise and react for 1 min; put the exposure plate with the membrane into the dark room and adjust the exposure time and exposure according to the strength of the signal. Finally, use image J to analyses the grey value.
Statistical analysis
Graph Pad Prism 9 software was used to perform one-way ANOVA and to plot the graphs. The significance threshold was set to p<0.05. All data are expressed as the mean ± SEM.
Identification of UCMSCs
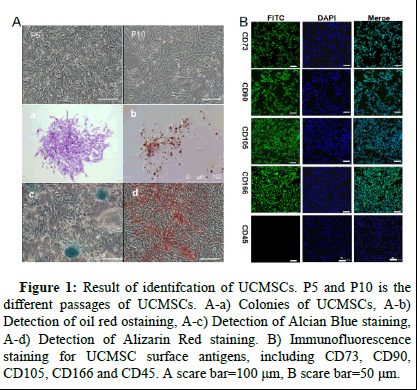
The morphology of UCMSCs in different passages (P5 and P10) showed typical long spindle-shaped cells (Figure 1). The ability of UCMSCs to generate colonies suggests that GMSCs have a good capacity for colony formation (Figure 1 a). Next, we determined the multispectral differentiation potential of UCMSCs in vitro. "In an in vitro experimental setting, UCMSCs exhibited distinct phenotypic responses contingent upon the specific culture conditions employed. The observed outcomes included the formation of rounded intracellular lipid droplets indicative of adipogenic differentiation (Figure 1 b), the aggregation of chondrocytic cells suggestive of chondrogenesis (Figure 1 c) and the development of osteoblastic features (Figure 1 d), respectively, under divergent culture stimuli.
Results
Immunofluorescence analyses revealed robust positive expression of mesenchymal stem cell (MSC)-specific marker genes, including CD73, CD90, CD105 and CD166 (Figure 1). The fluorescent signals emitted a green hue, demonstrating distinct cellular morphology characterized by well-defined edges, while DAPI staining enabled clear visualization of cell nuclei. Importantly, the primitive hematopoietic progenitor cell marker CD45 exhibited complete absence of fluorescence signals, consistent with the canonical surface marker profile of MSCs.
Figure 1: Result of identifcation of UCMSCs. P5 and P10 is the different passages of UCMSCs. A-a) Colonies of UCMSCs, A-b) Detection of oil red ostaining, A-c) Detection of Alcian Blue staining, A-d) Detection of Alizarin Red staining. B) Immunofluorescence staining for UCMSC surface antigens, including CD73, CD90, CD105, CD166 and CD45. A scare bar=100 μm, B scare bar=50 μm.
HE staining of pancreatic and liver tissue
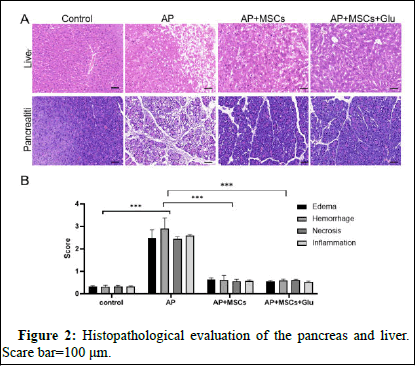
Control group: No inflammatory cell infiltration in pancreatic tissue, no obvious atrophy, degeneration and necrosis of follicles. AP group: Degeneration and necrosis of some follicular epithelial cells, necrotic cells are swollen and structurally blurred; cytoplasm is vacuolated, nuclei are shrunken or lysed and boundaries are unclear; interstitium is infiltrated with inflammatory cells (Figure 2). AP+Glu+UCMSC and AP+MSCs group: The peritoneum of pancreatic tissue was composed of thin layers of fibrous tissue, with clearer lobes; the structure of pancreatic islets and follicles was intact, the degree of pancreatic edema was smaller than that of TP group (Figure 2).
Figure 2: Histopathological evaluation of the pancreas and liver. Scare bar=100 μm.
In the control group, the liver lobules were structurally intact, the hepatocytes were uniform in size, there were no pathological changes such as degeneration and necrosis and there was no inflammatory cell infiltration; in the AP group, punctate necrotic rupture of the hepatocytes was seen, with obvious congestion and edema, accompanied by inflammatory cell infiltration; the vascular congestion and edema were significantly reduced in the AP+MSCs and AP+MSCs+Glu group and occasional punctate necrosis was seen, with a small amount of inflammatory cell infiltration and inflammation in the hepatic tissues was obviously ameliorated and no cases of piecemeal necrosis and cellular rupture were detected (Figure 2).
Serum amylase, lipase, SOD and inflammatory factor findings
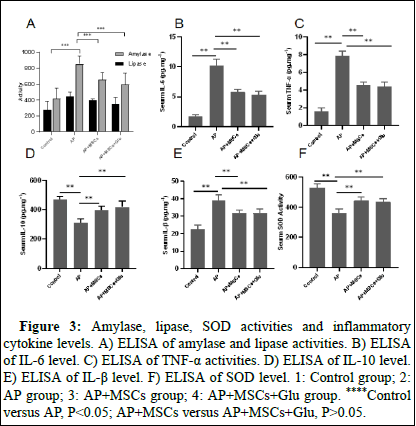
The serum amylase and lipase concentrations in the AP group were higher than those in the control group at 24 h after surgery (Figure 3, P<0.05) and the serum amylase and lipase levels in the AP+MSCs group and the AP+MSC+Glu group were significantly lower than those in the AP group (Figure 3, P<0.05). There was no statistically significant difference between the AP+MSCs group and the AP+MSCs+Glu group (Figure 3, P>0.05). SOD activities in AP group were lower than control group (Figure 3, P<0.05) and AP+MSCs group and the AP+MSCs+Glu group SOD activities were significantly higer than AP group (Figure 3, P<0.05). There was no statistically significant activities difference between the AP+MSCs group and the AP+MSCs+Glu group (Figure 3, P>0.05). Serum inflammatory factors showed that IL-6 and TNF-α were significantly higher and IL-10 and IL-β were significantly lower in the TP group than in the control group (Fig.3, P<0.05). Compared with the AP group, the serum concentrations of IL-6, IL-β and TNF-α in the AP+MSCs group and the AP+MSCs+Glu group were lower, whereas the concentrations of IL-10 were higher (Figure 3, P<0.05) and there was no statistically significant difference between the AP+MSCs group and the AP+MSCs+Glu group (Figure 3, P>0.05). The inflammatory response of rats after 24 h of modelling was more obvious.
Figure 3: Amylase, lipase, SOD activities and inflammatory cytokine levels. A) ELISA of amylase and lipase activities. B) ELISA of IL-6 level. C) ELISA of TNF-α activities. D) ELISA of IL-10 level. E) ELISA of IL-β level. F) ELISA of SOD level. 1: Control group; 2: AP group; 3: AP+MSCs group; 4: AP+MSCs+Glu group. ****Control versus AP, P<0.05; AP+MSCs versus AP+MSCs+Glu, P>0.05.
In vivo colonization of UCMSC
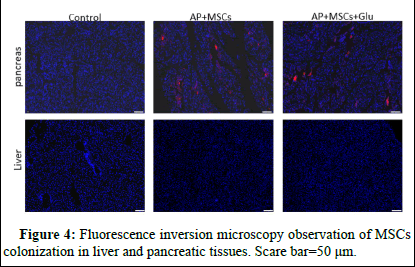
CM-Dil-labelled UCMSC was injected into AP mice through the tail vein and then the colonization in the damaged and control group pancreatic tissue and liver was assessed by fluorescence microscopy. The figure shows the presence of red fluorescence distribution in the pancreas of the AP+MSCs group and AP+MSCs+Glu group compared to the control group indicated that there was a handful significant colonization of pancreatic tissues by UCMSCs after injection. The presence of colonization of MSCs was not observed in liver tissue (Figure 4).
UCMSCs after injection. The presence of colonization of MSCs was not observed in liver tissue (Figure 4).
Figure 4: Fluorescence inversion microscopy observation of MSCs colonization in liver and pancreatic tissues. Scare bar=50 μm.
Liver apoptosis
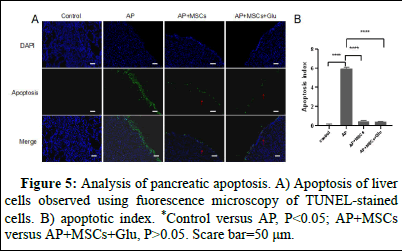
Hepatocytes in the AP group underwent apoptosis (Figure 5 ), whereas no apoptosis occurred in the control group and apoptosis in the AP group was more concentrated in the periphery of the liver tissue. The amount of apoptosis was significantly reduced in the AP+MSCs group and the AP+MSCs+Glu group. The apoptosis index of the AP group was significantly higher than that of the control group (p<0.05, Figure 5 B) and the apoptosis index of the AP+MSCs group and the AP+MSCs+Glu group was significantly lower than that of the AP group (p<0.05, Figure 5 B). MSCs+Glu groups had significantly lower apoptotic indices than the AP group (p<0.05, Figure 5 B). The apoptotic index of AP+MSCs group was slightly higher than that of AP+MSCs+Glu group, but there was no significant difference (p>0.05).
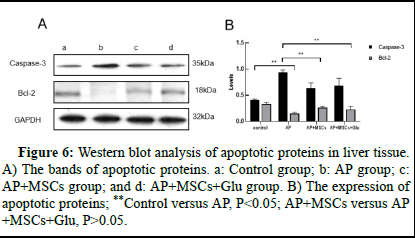
For Caspase-3 protein, the expression was higher in the AP group compared to the control group (p<0.05, Figure 6B) and lower in the AP+MSCs and AP+MSCs+Glu groups (p<0.05, Figure 6B), whereas the expression content of Bcl-2 protein increased in the AP+MSCs and AP+MSCs+Glu groups compared to the AP group (p<0.05, Figure 6B), there was no significant difference between AP+MSCs group and AP+MSCs+Glu group (p>0.05, Figure 6).
Figure 5: Analysis of pancreatic apoptosis. A) Apoptosis of liver cells observed using fuorescence microscopy of TUNEL-stained cells. B) apoptotic index. *Control versus AP, P<0.05; AP+MSCs versus AP+MSCs+Glu, P>0.05. Scare bar=50 μm.
Figure 6: Western blot analysis of apoptotic proteins in liver tissue. A) The bands of apoptotic proteins. a: Control group; b: AP group; c: AP+MSCs group; and d: AP+MSCs+Glu group. B) The expression of apoptotic proteins; **Control versus AP, P<0.05; AP+MSCs versus AP+MSCs+Glu, P>0.05.
Discussion
Acute pancreatitis leads to a cascade of events involving stimulation or production of digestive enzymes, damage to pancreatic tissue and an inflammatory response. The disease has a high morbidity and mortality rate and pancreatic alveolar cell injury leads to the release of a variety of enzymes resulting in a localized inflammatory response, which can be severe enough to lead to multi-organ failure. The liver damage that can result from pancreatitis and the degree of liver damage can exacerbate the severity of AP. Treatment options for pancreatitis are limited and focus primarily on controlling the inflammatory response to stop the means of subsequent complications.
In the condition of rapid development of regenerative medicine and tissue engineering technology, UCMSCs have attracted considerable attention for their unique immunomodulatory ability and low immune rejection and have been widely used in the fields of tissue repair, regeneration and restoration of tissue function and treatment of a variety of diseases. Using bone marrow stem cells from mice transplanted with severe pancreatitis induced by arginine, demonstrated that the bone marrow stem cells were effective in reducing mortality and serum amylase activity in pancreatitis, thereby protecting the pancreas from severe injury.
Wang et al. employed bone marrow stem cell transplantation to address pancreatitis-induced lung injury in rats. Their study revealed significant improvements in pulmonary edema and a concurrent reduction in TNF-α and SP mRNA expression in lung tissue. These results suggest the potential of MSC transplantation in alleviating pulmonary edema and inflammation in pancreatitis-associated lung injury. Furthermore, it is worth noting that umbilical cord-derived MSCs are a more accessible and safer alternative to bone marrow-derived stem cells. This assertion is based on their ease of procurement and enhanced safety profile. In another study, the therapeutic efficacy of umbilical cord-derived MSCs was investigated in the context of pancreatitis. Key findings included the marked inhibition of inflammatory cytokine secretion and the attenuation of pancreatic stellate cell activation. Additionally, these cells exhibited the ability to suppress pancreatitis-induced vesicular cell apoptosis, attributed to the downregulation of the PI3K signaling pathway within pancreatic tissues. Several studies have also demonstrated that ucmscs may be involved in immune regulation to suppress inflammatory factors and reduce tissue fibrosis.
Glutamine is considered a non-essential amino acid that provides immune nutrition during catabolic stresses such as trauma, sepsis and burns. Glutamine serves a variety of functions in the human body, and it is a fuel source for lymphocytes. Glutamine supplementation improves intestinal barrier function, lymphocyte function and reduces pro-inflammatory factors by inhibiting nuclear factor-kb and p38 mitogen-activated protein kinase in critically ill patients. There are no experiments using glutamine alone to treat the presence of AP. Garib et al. assessed the inflammatory and survival responses following parenteral glutamine infusion in a sodium taurocholate-induced AP model using high-dose glutamine; pulmonary HSP70 and hepatic HSP90 expression was lower than in the non-treated group; parenteral glutamine pretreatment was safe and improved early inflammatory mediator properties without affecting mortality.
In this study, we successfully isolated UCMSC from cattle, characterised the expression of their surface antigens and assessed the in vitro differentiation capacity. An AP mouse model was established to simulate the pathophysiological changes in pancreatic tissues after undergoing trauma and it was found that the AP mouse model successfully led to liver injury in mice. After the model was established, we injected UCMSCs through the tail vein of mice and gavaged glutamine. Cellular colonization was observed in pancreatic tissue, however, no colonisation in damaged liver tissue was observed. Jung et al. also found a small amount of cellular colonisation in the damaged pancreas after using human MSCs to treat acute pancreatitis in rats. Yin et al. found that when MSCs injected through the tail vein were used in the treatment of early pancreatitis, the cells mostly colonised the lungs, with only a small proportion reaching the pancreas and kidney tissue. This suggests that MSCs are more likely to achieve attenuation of liver injury in pancreatitis by participating in immune regulation. Compared with the AP group, pancreatic edema was significantly reduced in UCMSCs and combination treatment, the structure of pancreatic lobules was not completely disrupted, the structure of exocrine cells was normal in the injury area and the infiltration of inflammatory cells was significantly reduced. Hepatic lobules with intact tissue, significantly less cellular rupture and significantly less inflammatory cell infiltration and cellular necrosis. Levels of pro-inflammatory cytokines TNF-α, IL-6 and IL-β were significantly reduced, while levels of anti-inflammatory cytokines IL-10 and SOD were significantly increased. In addition, caspase-3 expression level was down-regulated and Bcl-2 expression was up-regulated in the liver tissues of the AP+MSC and AP+MSC+Glu groups compared with the AP group. The TUNEL staining results also suggested that UCMSCs and MSC+Glu could be involved in protecting and repairing tissues by inhibiting apoptosis of liver cells. This suggests that UCMSC and glutamine are involved in regulating the expression of pro-inflammatory and anti-inflammatory factors to participate in the recovery of AP mice.
In summary, our comparative analysis revealed a lack of statistically significant distinctions between MSC-based therapy and the combined therapy approach, both in terms of histopathological assessments of the liver and pancreas, as well as in the measurement of inflammatory factor levels and apoptotic indices. This implies that while both treatment modalities exhibited efficacy in mitigating liver injury in the context of pancreatitis, there exists no significant variance in their overall treatment effectiveness.
Conclusion
The available body of evidence on glutamine supplementation combined with cellular therapy remains limited, and the optimal regimen, offering the highest therapeutic efficacy, necessitates validation through more expansive and rigorous clinical trials.
Limitations of the Study
This trial is only validating the feasibility of the treatment regimen and more detailed and in-depth studies of the treatment mechanism and clinical design are needed.
Author Contributions
JZ was involved in providing and designing the experimental idea and writing the paper, ZY constructed the animal model, carried out the cell culture and were involved in the data image organisation. WG reviewed the manuscript.
Declaration of Interests
The authors declare that they have no competing interests.
Institutional review board statement
All protocols used in this study were approved by the Bureau of Animal Experimental Welfare, Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS2023-2). All experiments were conducted in accordance with the "Welfare of Experimental Animals" of the Institute of Animal Science, Chinese Academy of Agricultural Sciences and the "Guide for the Care and Use of Laboratory Animals" published by the National Institutes of Health of the USA.
Data Available Selection
All data generated or analyzed during the course of this study are included in this published article's Supplementary file-raw data. -Datasets generated and/or analyzed during the course of the current study are not publicly available due to laboratory-related requirements, but are available from the corresponding author upon reasonable request.
References
- Lee PJ, Papachristou GI (2019) New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol 16: 479-496.
[Crossref] [Google Scholar] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, et al. (2013) Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 62: 102-111.
[Crossref] [Google Scholar] [PubMed]
- Mei F, Yu J, Li M, Xiang M, Hong Y, et al. (2019) Magnesium isoglycyrrhizinate alleviates liver injury in obese rats with acute necrotizing pancreatitis. Pathol Res Pract 215: 106-114.
[Crossref] [Google Scholar] [PubMed]
- Triester SL, Kowdley KV (2002) Prognostic factors in acute pancreatitis. J Clin Gastroenterol 34: 167.
[Crossref] [Google Scholar] [PubMed]
- El Sebae GK, Malatos JM, Cone MKE, Rhee S, Angelo JR, et al. (2018) Single-cell murine genetic fate mapping reveals bipotential hepatoblasts and novel multi-organ endoderm progenitors. Development 145: dev168658.
[Crossref] [Google Scholar] [PubMed]
- Coffey JC, Walsh D, Byrnes KG, Hohenberger W, Heald RJ (2020) Mesentery a ‘New’organ. Emerg Top Life Sci 4: 191-206.
[Crossref] [Google Scholar] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
[Crossref] [Google Scholar] [PubMed]
- Kubo K, Ohnishi S, Hosono H, Fukai M, Kameya A, et al. (2015) Human amnion-derived mesenchymal stem cell transplantation ameliorates liver fibrosis in rats. Transplant Direct 1: 1-9.
[Crossref] [Google Scholar] [PubMed]
- Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726-736.
[Crossref] [Google Scholar] [PubMed]
Citation: Guan W, Zong J, Liu Y, Guan Y, Yan Y, et al. (2025) Assessment of Bovine Umbilical Cord-Derived Stem Cells in Conjunction with Glutamine for Mitigation of Hepatic Injury in the Context of Acute Pancreatitis. Diagnos Pathol Open 10: 251.
Copyright: © 2025 Guan W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 174
- [From(publication date): 0-0 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 108
- PDF downloads: 66