Assessment of Risk Factors Correlated with Age-Adjusted Mortality by Region for Prostate Cancer in Japan: An Epidemiological Study
Received: 03-Sep-2021 / Accepted Date: 17-Sep-2021 / Published Date: 24-Sep-2021 DOI: 10.4172/2161-1165.1000412
Abstract
Introduction: Previous studies have suggested that smoking and drinking alcohol are prostate cancer risks. However, how regional unevenness of radiation oncologists/urologists in Japan or other risk factors including social background correlate with age-adjusted mortality of prostate cancer remain unknown.
Methods: In addition to known factors like smoking, we examined whether various other factors, such as the regional unevenness of radiotherapists/urologists, correlated with age-adjusted mortality rate (AAMR) for prostate cancer by prefecture. Statistical data were obtained from Annual Report of Hospital-Based Cancer Registries, Comprehensive Survey of Living Conditions and etc. We used the 10-year averages of AAMR from 2009 to 2018 in each prefecture to determine mortality rates.
Results: There was no correlation between the number of radiation oncologists/urologists per 100,000 and the 10-year averages of AAMR by prefecture in Japan. On the other hand, smoking rates and drinking per capita were correlated, respectively (P=0.0002 and 0.0008).
Conclusion: The current study of statistics by prefecture in Japan also suggests that factors such as smoking and drinking alcohol are correlated with the risk of prostate cancer mortality. On the other hand, regional unevenness of radiation oncologists did not correlate. These results suggest that there is validity in this study design of analyzing factors of risk of prostate cancer mortality. And the study design was suggested to be useful for comparing various patient background factors.
Keywords: Prostate cancer; Risk factors; Alcohol consumption; Smoking rate; Poverty
Introduction
With the rapid aging of the population, the number of prostate cancer patients in Japan is increasing rapidly [1,2]. The projected number of prostate cancer cases in 2019 was 78,500, making it the number one cancer case for men. And the projected number of deaths was 12,600 and the Age-adjusted Mortality Rate (AAMR) was 2.2 [3]. Therefore, the question of how to detect and treat prostate cancer at an early stage has become an issue.
The incidence of prostate cancer has been reported to vary widely among ethnic, racial, and regional groups [4]. And the incidence of prostate cancer among Japanese immigrants to the United States was shown to be between that of Japanese and Americans [5]. Thus, it is suggested that not only genetic factors but also environmental factors increase the risk of morbidity. Environmental factors reported include lifestyle, metabolic syndrome, prostate inflammation, infection, prostate enlargement and chemical exposure [6]. Because there can be a variety of risk factors, it is difficult to determine that you had prostate cancer because of one factor. For example, with regard to lifestyle, smoking more often has been reported to increase the risk of prostate cancer in middle age [7-9]. The same is reported to be true for alcohol consumption [10]. In addition to these typical cancer risk factors, studies have examined whether prostate cancer is correlated with lifestyle factors such as high-fat diet, fatty fish consumption, milk consumption, fruit and vegetable consumption, physical activity, obesity, Japanese diet, Green tea and coffee consumption [11-26]. Prognostic factors may be considered in terms of lifestyle as well as social background. For instance, coffee consumption and poverty can be inversely correlated. And also poverty and suicide rates can be correlated [27,28]. In more detail, coffee consumption can be correlated with social background factors such as suicide rates, amount of savings per family and unmarried rates. From the perspective of the biopsychosocial model, these social background factors need to be fully considered in epidemiology as well [29].
Health care is provided equitably to all prefectures in Japan. All Japanese people have access to health care without any disparity in wealth [30]. In addition, Japan is characterized by the fact that the country is subdivided into 47 prefectures. Each prefecture has its own characteristics, or trends, in the people of the county, and there are various statistics on these characteristics. This is called “Kenminsei” or characteristics of the people of a prefecture. This Keminsei causes differences in various prognostic factors in prostate cancer such as alcohol consumption, smoking rates, and even poverty in each region [31].
In the current study, we set up a study design to examine the contribution of risk factors and its associated social background factors including the regional unevenness of radiotherapists/urologists to the annual adjusted mortality rate of prostate cancer by region.
Materials and Methods
Smoking and alcohol consumption have been suggested as mortality risks for prostate cancer in cohort studies [7-10]. In addition to these factors, we examined whether various other factors including social background correlated with AAMR for prostate cancer by prefecture. Statistical data were from the National Cancer Registry of Cancer Treatment Centers and Other Hospitals, the National Cancer Information Service, the National Cancer Institute Cancer Information Service, the Basic Living Survey, Vital Statistics, National Tax Administration statistics, the National Health and Nutrition Examination Survey, the Society of Radiotherapy, the Urological Society [32-38]. For mortality by prefecture, we used the mean of AAMR from 2009 to 2018.
Factors for regional unevenness in the number of radiation oncologists per 100,000 and the number of urologists per 100,000 were calculated from the rosters of specialists published by the respective societies and the male population of the prefecture.
We included all prefectures in our analysis. However, only Kumamoto prefecture was excluded in the analysis of smoking rate because data on smoking rates were missing for this prefecture.
Statistics
The correlations were tested by using the Pearson's productive correlation coefficient. All the statistical analyses were performed using EZR software, version 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 2.13.0; The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander (version 1.6-3), designed to include statistical functions that are frequently used in biostatistics. All the statistical analyses were two-sided, and a p value <0.05 was considered statistically significant.
Results
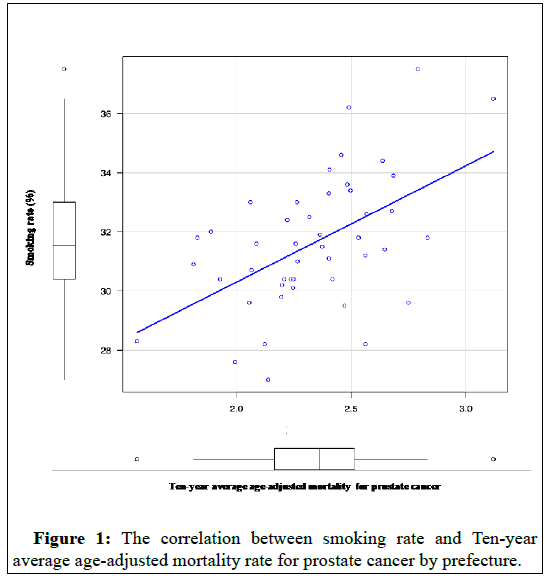
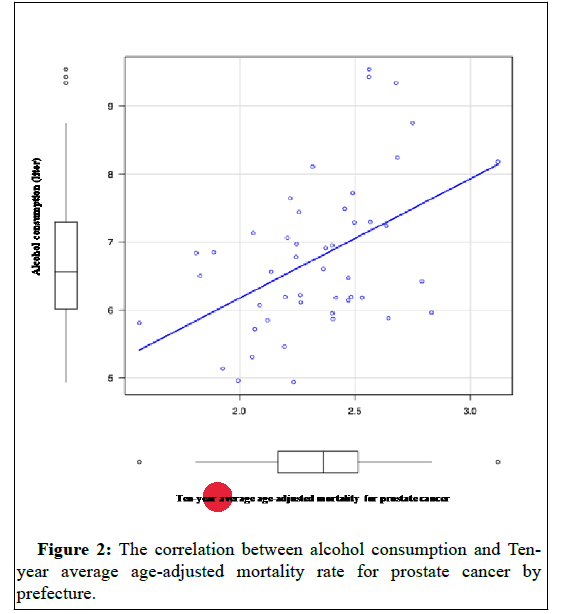
Smoking rates (p-value=0.0002) Figure 1 and alcohol consumption per capita (liters) (p-value=0.0008) Figure 2 were each correlated in 2017 for those aged 20 years and older.
In addition, suicide rate (p-value=0.015), household savings (pvalue= 0.0007, negatively correlated), unmarried rate over 50 years of age (p-value=0.0003), bread consumption (p-value=0.001, negatively correlated), consumption of sunfish (p-value=0.03), consumption of natto (p-value=0.009), coffee consumption (p-value=0. 03, negative correlation), and obesity rates (P-value=0.006) were correlated (Table 1).
| Risk factors | Correlation coefficient | 95% CI | P-value |
|---|---|---|---|
| Smoking rate | 0.529 | 0.282-0.71 | 0.0002 |
| Alcohol consumption | 0.47 | 0.212-0.667 | 0.0009 |
| Suicide rate | 0.353 | 0.0732-0.581 | 0.02 |
| Household savings | -0.475 | -0.453 | 0.0007 |
| Unmarried rate over 50 years of age | 0.506 | 0.256-0.692 | 0.0003 |
| Bread consumption | -0.466 | -0.457 | 0.001 |
| Mackerel pike consumption | 0.314 | 0.0298-0.552 | 0.03 |
| Natto consumption | 0.378 | 0.102-0.6 | 0.009 |
| Coffee consumption | -0.336 | -0.5145 | 0.02 |
| Obesity rate | 0.392 | 0.118-0.611 | 0.006 |
Table 1: Relationship between ten-year average age-adjusted mortality rate for prostate cancer and various risk factors.
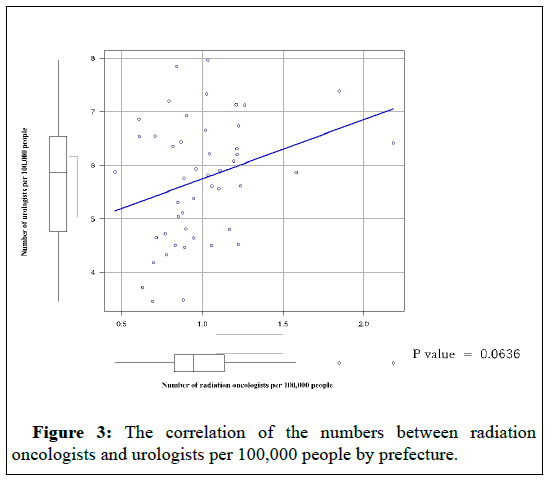
In contrast, there was no correlation between the number of radiation oncologists per 100,000 people, the number of urologists per 100,000 people, rate of PSA testing at health screenings in each prefecture in 2017, and the number of patients with stage IV prostate cancer according to cancer registry information. Other risk and preventive factors as referred to in the Japanese guideline did not correlate with seafood consumption, beef, chicken and pork consumption, vegetable consumption, milk consumption, green tea consumption, number of patients with hypertension, number of patients with diabetes, and sports population [6].
The correlation of the numbers between radiation oncologists and urologists per 100,000 people by prefecture is shown in Figure 3. There was a weak correlation between the number of radiation oncologists and the number of urologists, although not statistically different (P=0.06).
Discussion
A study design to assess risk factors based on country-or regionspecific morbidity and mortality and country-specific factors was recently conducted in the coronavirus disease 2019 (COVID-19) and BCG vaccination. However, this study has not been peer-reviewed, has confounding factors, and has not been scientifically evaluated [39]. On the other hand, this paper surprisingly led to randomized control trials and brought the mechanism of immunity in BCG into the limelight [40].
This study uses low-quality evidence observed at the population level to make broad inferences about the effectiveness of BCG at the individual level.
This epidemiological study has not been able to directly prove a relationship between BCG exposure rates and Covid-19 morbidity and mortality. This study was useful enough as a question to formulate a hypothesis. But this study should be treated as a hypothesis and not as evidence.
We used the same study design in our current study. The statistics by Japanese prefectures also suggest that lifestyle and social background factors other than alcohol and smoking are associated with risk of prostate cancer mortality.
This study design was used for the first time in prostate cancer. It may be useful in the epidemiology of prostate cancer to generate hypotheses, rather than as evidence, in the epidemiology of prostate cancer.
The current study suggests that, contrary to our expectations, the urban uneven distribution of physicians does not have a significant impact on patient outcomes. This suggests that the number of metastatic prostate cancer incidence does not correlate with the number of urologists and radiation oncologists.
The treatment of prostate cancer is well established for local treatment, and outcomes are excellent compared with other types of cancer. Rather, the problem is how to detect local prostate cancer at an early stage, rather than detecting it in its metastatic state.
Randomized control trials have already shown that as the screening exposure rate for PSA increases, the mortality rate decreases [41-43]. On the other hand, 10% of prostate cancers in clinical practice are found with bone metastases in Japan [44]. This suggests that screening rates in Japan are still at an inadequate level. There are also some factors that contribute to the disadvantage of PSA screening [45].
Although the goal of PSA screening for all men over 60 years of age could be targeted, the patient's disadvantage needs to be taken into account. Therefore, the current study suggests the importance of intervening proactively with PSA screening according to the risks presented in the current study, i.e., depending on the patient's background.
This has already been advocated for tailor-made screening; the EAU guidelines categorize risk according to age, family history, race, and previous PSA values [46].
As mentioned above, previous screening guidelines and previous papers have not included risk assessment of patients' backgrounds.
Conclusion
However, clinicians could easily predict that low-income, obese, and unmarried men with drinking and smoking habits do not come to the clinic until they have symptoms of pain, which tends to delay detection. On the other hand, it is difficult to prove this prediction. This is because it is difficult to prove it without confounding factors. Therefore, we hypothesize that the current study will lead to the following: will there be a difference in mortality between prefectures that provide free, annual, and aggressive PSA screening for lowincome obese unmarried men in their 50s who smoke or drink alcohol and prefectures that provide conventional, passive and optional PSA screening?
Whether tailor-made PSA screening by patient background risk factors can reduce mortality from prostate cancer should be studied prospectively.
Acknowledgements
Not applicable.
Funding
None.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
TN analyzed and interpreted the data. TN designed research. TN conducted data analysis. AH conducted review and editing. TN was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
References
- Yoshimi I, Mizuno S (2003) Cancer Statistics Mortality trends of prostate cancer in Japan: 1960-2000. Jpn J Clin Oncol 33: 367.
- Matsuda T, Matsuda A (2011) Time Trends in Prostate Cancer Mortality Between 1950 and 2008 in Japan, the USA and Europe Based on the WHO Mortality Database. Jpn J Clin Oncol 41: 1389.
- Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, et al. (2015) Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 45: 884-891.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
- Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, et al. (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63: 963-966.
- The Japanese Urological Association (2016) Prostate Cancer Practice Guidelines 2016.
- Plaskon LA, Penson DF, Vaughan TL, Stanford JL (2003) Cigarette Smoking and Risk of Prostate Cancer in Middle-Aged Men. Canc Epidemiol Biomark Prev 12: 604-609.
- Rohrmann S, Linseisen J, Allen N, Bueno-de-Mesquita HB, Johnsen NF, et al. (2013) Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 108: 708-714.
- Darlington GA, Kreiger N, Lightfoot N, Purdham J, Sass-Kortsak A (2007) Prostate cancer risk and diet, recreational physical activity and cigarette smoking. Chronic Dis Can 27: 145-153.
- Sawada N, Inoue M, Iwasaki M, Sasazuki S, Yamaji T, et al. (2014) Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: The Japan Public Health Center-based prospective study. Int J Cancer 134: 971-978.
- Fleshner N, Bagnell PS, Klotz L. (2004) Dietary fat and prostate cancer. J Urol 171: S19-S24.
- Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A (2001) Fatty fish consumption and risk of prostate cancer. Lancet 357: 1764-1766.
- Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A, et al. (2012) Dietary fatty acid intake and prostate cancer survival in Örebro County, Sweden. Am J Epidemiol 176: 240-252.
- Giovannucci E, Rimm EB, Wolk A, Ascherio A, Stampfer MJ, et al. (1998) Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res 58: 442-447.
- Key TJ, Allen N, Appleby P, Overvad K, Tjønneland A, et al. (2004) Fruits and vegetables and prostate cancer:no association among 1104 cases in a prospective study of 130544 men in the European Prospective Investigation into Cancer and Nutrition(EPIC). Int J Cancer 109: 119-124.
- Liu Y, Hu F, Li D, Wang F, Zhu L, et al. (2011) Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol 60: 1029-1044.
- Allott EH, Masko EM, Freedland SJ (2013) Obesity and prostate cancer:weighing the evidence. Eur Urol 63: 800-809.
- Masuda H, Kagawa M, Kawakami S, Numao N, Matsuoka Y, et al. (2013) Body mass index influences prostate cancer risk at biopsy in Japanese men. Int J Urol 20: 701-707.
- Mori M, Masumori N, Fukuta F, Nagata Y, Sonoda T, et al. (2011) Weight gain and family history of prostate or breast cancers as risk factors for prostate cancer: results of a case-control study in Japan. Asian Pac J Cancer Prev 12: 743-747.
- Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, et al. (2004) A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci 95: 238-242.
- Miyanaga N, Akaza H, Hinotsu S, Fujioka T, Naito S, et al. (2012) Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate- specific antigen. Cancer Sci 103: 125-130.
- Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S (2008) Green tea consumption and prostate cancer risk in Japanese men: A prospective study. Am J Epidemiol 167: 71-77.
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, et al. (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res 66: 1234-1240.
- Bosire C, Stampfer MJ, Subar AF, Wilson KM, Park Y, et al. (2013) Coffee consumption and the risk of overall and fatal prostate cancer in the NIH-AARP Diet and Health Study. Canc Cau Contr 24: 1527-1534.
- Wilson KM, Bälter K, Möller E, Adami HO, Andrén O, et al. (2013) Coffee and risk of prostate cancer incidence and mortality in the Cancer of the Prostate in Sweden Study. Canc Cau Contr 24: 1575-1581.
- Park C, Myung S, Kim T, Seo HG, Jeon Y, et al. (2010) Coffee consumption and risk of prostate cancer:a meta-analysis of epidemiological studies. BJU Int 106: 762-969.
- Bantjes J, Iemmi V, Coast E, Channer K, Leone T, et al. (2016) Poverty and suicide research in low- and middle-income countries: systematic mapping of literature published in English and a proposed research agenda. Glob Ment Health 3: e32.
- Elhadad MA, Karavasiloglou N, Wulaningsih W, Tsilidis KT, Tzoulaki I, et al. (2020) Metabolites, Nutrients, and Lifestyle Factors in Relation to Coffee Consumption: An Environment-Wide Association Study. Nutrients 12: 1470.
- Kusnanto H, Agustian D, Hilmanto D (2018) Biopsychosocial model of illnesses in primary care: A hermeneutic literature review. J Family Med Prim Care 7: 497-500.
- Ministry of Health, Labour and Welfare (2020) An Outline of the Japanese Medical System.
- Sofue T (1961) Anthropology in Japan: Historical Review and Modern Trends. Bienn Rev. Anthropol 2: 173-214.
- Cancer Registry and Statistics (2020) Cancer Information Service, National Cancer Center, Japan.
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, et al. (2020) Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. BJM 28: 1-9.
- Curtis N, Sparrow A, Ghebreyesus TA, Netea MG (2020) Considering BCG vaccination to reduce the impact of COVID-19. The Lancet 395: 1545-1546.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, et al. (2014) The European Randomized Study of Screening for Prostate Cancer (ERSPC)at 13 years of follow-up. Lancet 384: 2027-2035.
- Hugosson J, Carlsson S, Aus G, Bergdah S, Khatami A, et al. (2010) Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 11: 725-732.
- Oberaigner W, Siebert U, Horninger W, Klocker H, Bektic J, et al. (2012) Prostate-specific antigen testing in Tyrol, Austria:prostate cancer mortality reduction was supported by an update with mortality data up to 2008. Int J Public Health 57: 57-62.
- Fujimoto H, Nakanishi H, Miki T, Kubota Y, Takahashi S, et al. (2011) Oncological outcomes of the prostate cancer patients registered in 2004: Report from the Cancer Registration Committee of the JUA. Int J Urol 18: 876-881.
- Hamashima C, Nakayama T, Sagawa M, Saito H, Sobue T (2009) The Japanese guideline for prostate cancer screening. Jpn J Clin Oncol 39: 339-351.
Citation: Nagano T, Hoshi A (2021) Assessment of Risk Factors Correlated with Age-Adjusted Mortality by Region for Prostate Cancer in Japan: An Epidemiological Study. ECR 11:412. DOI: 10.4172/2161-1165.1000412
Copyright: © 2021 Nagano T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2486
- [From(publication date): 0-2021 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 1769
- PDF downloads: 717