Research Article Open Access
Biodegradation of Fresh and Used Engine Oils by Pseudomonas aeruginosa LP5
| Oluwafemi S Obayori*, Lateef B Salam and Oluwatoba S Ogunwumi | |
| Department of Microbiology, Lagos State University, Ojo, Lagos, Nigeria | |
| Corresponding Author : | Oluwafemi S Obayori Faculty of Science, Department of Microbiology Lagos State University, Ojo, Lagos, Nigeria Tel: (234)802-319-2652 E-mail: femiobayori@yahoo.com |
| Received November 02, 2013; Accepted January 18, 2014; Published January 23, 2014 | |
| Citation: Obayori OS, Salam LB, Ogunwumi OS (2014) Biodegradation of Fresh and Used Engine Oils by Pseudomonas aeruginosa LP5. J Bioremed Biodeg 5:213. doi:10.4172/2155-6199.1000213 | |
| Copyright: © 2014 Obayori OS, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Pseudomonas aeruginosa strain LP5, a hydrocarbon degrader isolated from petroleum contaminated soil based on its ability to grow on pyrene, was used to degrade two different grades of fresh and used engine oil (SAE 40W and SAE 20W 50) in liquid cultures. The organism degraded more than 90 percent of all oil types within 21 days with values of 95%, 93%, 96% and 92% for fresh SAE 40W, used SAE 40W, fresh SAE 20W 50 and used SAE 20W 50 respectively. Similarly, growth rates were slightly higher in the fresh oils with values of 0.17, 0.13, 0.14 and 0.13 d-1 recorded respectively for fresh SAE 40W, used SAE 40W, fresh SAE 20W 50 and used SAE 20W 50. Significantly higher initial rates of degradation of 177.42 mg l-1d-1 and 207.14 mgl-1d-1 were recorded for fresh SAE 40 and SAE 20W 50 in the first 21 days compared to the values for the used oil counterparts, which were 73.23 mgl-1d-1 for SAE 40 and 74.37 mgl-1d-1 for SAE 20W 50. In the used oils, lower peaks that were not present at Day 0 reappeared on Day-21. All peaks greater than C20 also disappeared. In all cases, it was the medium fraction ranges of C14, C15, and C17 that remained with discernible peaks on Day 21, albeit at very low heights of less than 10% of Day 0 values. Thus whereas degradation rates differ on oil types and fresh oil appear more amenable to early degradation, the organism Pseudomonas aeruginosa LP5 showed remarkable potential for use in degradation of both fresh and used engine oils.
| Keywords |
| Biodegradation; Engine oil; Hydrocarbons; Pseudomonas |
| Introduction |
| Engine oil is one of the several refined products or cuts of crude oil. It is composed of long-chain saturated hydrocarbons (base oil) additives [1]. It is used to lubricate the parts of an automobiles engine, in order to keep everything running smoothly [2]. The most important characteristic of the lubricating oil for automotive use is its viscosity. Although it averagely constitutes about two percent of crude oil, engine oil is the most widely used cut of petroleum [3]. |
| Generally, engine oil can enter into the environment through leak of oil tankers, cleaning of tanks by merchants, warship carrying engine oil, and operations by motor mechanics. The Nigerian environment is characterized by nonchalant, indiscriminate and highly unregulated disposal of petroleum products including engine oil [4,5]. Mechanic workshops often dispose of used oil into open grounds where it eventually finds its way into drainages, canals and underground water. It is equally noteworthy that even small releases of petroleum hydrocarbons into aquifers could lead to concentrations of dissolved hydrocarbons far in excess of regulatory limits [6]. |
| Fresh and used oils differ markedly in their chemical compositions. Fresh motor oil contains a higher percentage of fresh and lighter hydrocarbons. The base oil contains C16-C36 hydrocarbons, and more than 75% c-alkanes. The rings number of cyclic-alkanes (c-alkanea) in the base oil is from 1 to 3 and any ring contains 5 or 6 members. Most of the c-alkanes in the base oil have long alkyl side chains [7]. It usually contains very small amounts of polycyclic aromatic hydrocarbons (PAHs) [8]. On the other hand, used oil may contain minute quantities of additive, metals such as lead, zinc, barium and magnesium resulting from engine wear and higher percentages of alkyl benzenes, naphthalene, methyl naphthalene and higher polycyclic aromatic hydrocarbons (PAHs) probably as a result of pyrosynthesis [9,10]. Prolonged exposure and high oil concentration may cause the development of liver or kidney disease, possible damage to the bone marrow and an increased risk of cancer [11,12]. They are capable of causing different undesirable changes in the anatomical features of man [13]. Some of these include, green house effect, contamination of water bodies leading to impairment of aquatic life, impairment of plants metabolism through plant root toxicity, and adverse effect on soil organisms such as bacteria, fungi, and other multicellular organisms [14]. Moreover, research findings have shown statistically significant impact of such reckless disposal on plants, including height reduction, chlorophyll loss and protein level reduction [15,16]. |
| Bioremediation remains one of the most effective ways to reclaim soils and aquifers polluted with petroleum hydrocarbons. Such efforts would depend on the availability of petrogenic organisms with capacity to degrade the broad array of components in the contaminant. Reports abound of degraders of engine oil spanning strains of genera such as Achromobacter, Bacillus, Enterobacter, Escherichia, Gordonia, Mycobacterium, Pseudomonas, Rhodococcus, Serratia and Staphylococcus among others [3,7,17-22]. Also, there had been some reports specifically detailing degradation of used engine oil by bacteria [1,20]. |
| Oftentimes, individual strains are only capable of degrading a few components of the oil pollutant and complete biodegradation require the activity of consortium [3,23]. However, there is increasing research on the isolation of individual organisms that can not only degrade the major components of engine oils but also demonstrate versatility for other more recalcitrant hydrocarbons, as these pollutants are mostly found together in the same environmental compartments. Here we report the degradation of fresh and used engine oil by Pseudomonas aeruginosa LP5, an organism previously reported as a pyrene degrader. The study was carried out in furtherance of its assessment for use in bio-augmentation. |
| Materials and Methods |
| Motor oil |
| Two different grades of fresh engine oil commonly used in Nigeria were namely SAE 40W and SAE 20W 50 from Conoil were used in this study. Used counterparts of the grades were obtained from a motor mechanic who used the oil grades to service specific vehicles on regular basis. The used oils were collected in well washed and airdried plastic bottles. The fresh oils were coded with respective grade number preceded with F or U denoting respectively Fresh and Used thus: FSAE40, USAE40, FSAE 20W-50 and USAE 20W-50. |
| Microorganism and inoculum development |
| The isolation and characterization of the isolate used in this study had been described elsewhere [24]. The organism, Pseudomonas aeruginosa LP5, maintained in glycerol: nutrient broth (1:1) at -20°C. Resuscitation was carried out by streaking onto Luria-Bertani agar with very low percentage of PAH (0.005%). Colonies were harvested with sterile inoculating loop, pooled and transferred to screw-capped bottles containing 5 ml of physiological saline (0.9% NaCl). Enough cells were transferred to achieve an OD600 of approximately 1.5. |
| Medium and culture conditions |
| The mineral salt medium (MSM) described by Kastner et al. [25] was used. The pH of the medium for bacteria was adjusted to 7.2 and further fortified with trace elements solution (1 mlL-1) previously described by Bauchop and Elsden [26]. The trace elements solution was sterilized separately, and later added aseptically to the medium. Unless otherwise stated, all incubations were carried out at room temperature (27 ± 2.0°C). |
| Growth of isolates on engine oil |
| Replicate flasks containing 50 ml of MSM with 0.5 ml of engine oil were prepared. The flasks was inoculated to achieve an initial concentration (OD600) of 0.1 and incubated at room temperature for a period of 21 days. Flasks inoculated with heat-inactivated cells served as controls. Increase in organism concentration was determined at OD600 using a UV/Vis spectrophotometer (Jenway 6305). Residual oil concentrations were also determined at the same interval. Specific growth rates were calculated from the linear plots of the natural log of the optical density (ln OD) over the initial optical density (OD0) against time [27]. |
| Extraction of residual oil |
| Residual oil was extracted via liquid-liquid extraction by adding 10 ml of hexane to broth culture in flask and shaking thoroughly as described by Adebusoye et al. [28]. After removing the aqueous phase with separating funnel, the residual oil concentration was determined by gas chromatography. Similarly, control flasks were also extracted. Results were expressed as percentages of respective controls. |
| Analytical method |
| Hexane extracts (1.0 μl) were analyzed with Hewlett Packard 5890 Series II gas chromatograph equipped with flame ionization detector (FID) and 30 m long HP-5 column (internal diameter, 0.25 mm; film thickness, 0.25 μm). The carrier gas was nitrogen. The temperature of injector and detector maintained at 250ºC and 350ºC respectively. The column was programmed at an initial temperature of 70ºC; this was held for 2 min, then ramped at 10ºC/min to 320º C and held for 10 minutes. |
| Results |
| The Growth profile of Isolate on crude oil |
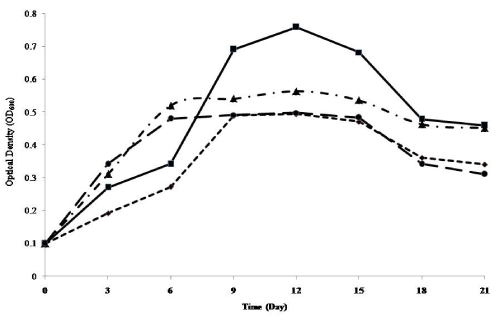
| The growth profiles of P. aeruginosa LP5 are shown in Figure 1. The organism grew best on FSAE 40. From an initial OD of 0.100 on Day 0, the population increased exponentially to 0.759 on day 12 while it declined steeply to 0.478 on Day 18. On USAE 40, growth was initially slow, with OD of 0.272 on Day 6 then a steep growth to 0.489 and it maintained a plateau until it started declining on Day 15. In all cases, lag periods of less than 2 days were indicated by increase from day 0 to day 3. |
| The growth on FSAE 20W-50 also followed a plateau pattern with OD of 0.520 on Day 6, highest value of 0.540 on Day 12 and a gentle decline to 0.451 on Day 21. Also on USAE 20W-50 P. aeruginosa LP5 showed population growth with OD rising from 0.342 on day 3 to 0.493 on day 12 and decline from 0.483 on day 15 to 0.311 on day 21. |
| Table 1 shows the growth and degradation kinetics of LP5. Growth rates ranged between 0.13 and 0.17 d-1, with the highest value recorded for FSAE40. The doubling time ranged between 4.08 and 5.33 d, with the used oils exhibiting the higher doubling time. On all the oil types, higher degradation rates were observed in the first 12 days than the last 9 days. The values for FSAE 40, USAE 40, FSAE 20W-50 and USAE 20W-50 were 177.42, 75.23, 207.14 and 74.37 (mg l-1d-1) respectively in the first 12 days. Equally 78% of FSAE 40 was degraded within this period as against 66% of USAE 40, while the values for FSAE 20W- 50 and USAE 20W-50 were 79% and 62 % respectively. However, in general the percentage degraded ranged between 92% and 96%. |
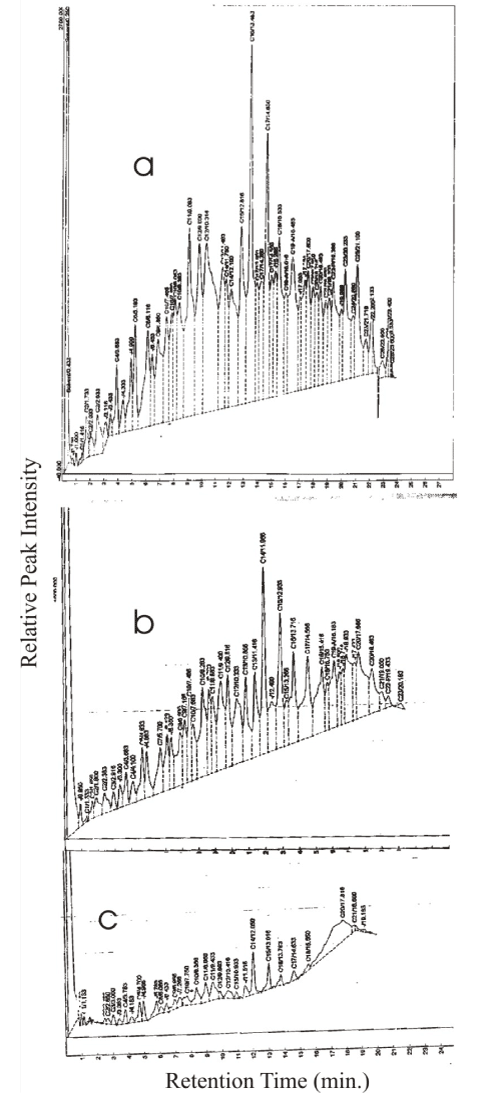
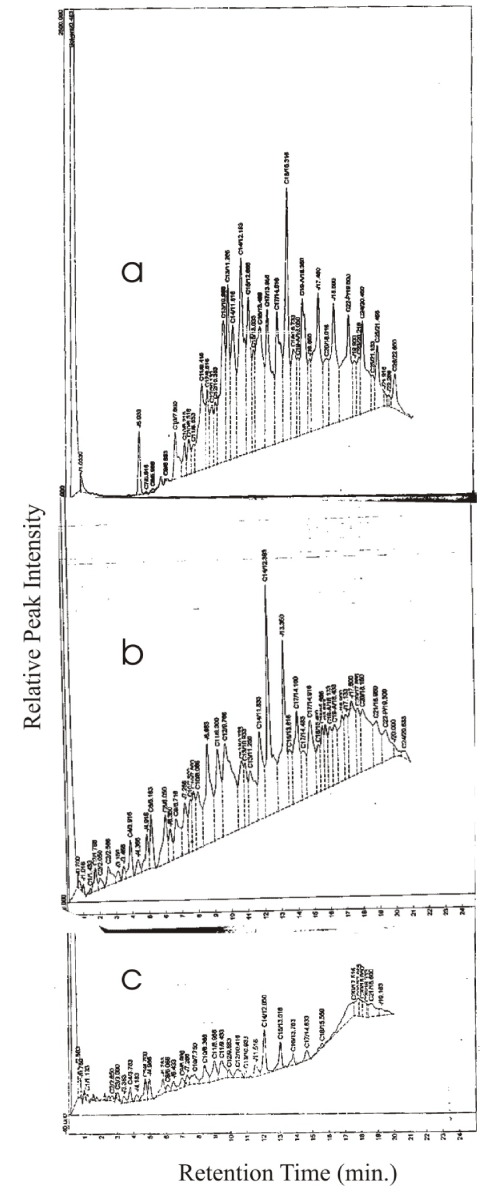
| The changes in GC fingerprints of fresh SAE 40W during degradation by P. aeruginosa LP5 are displayed in Figure 2. Whereas the oil had full range of hydrocarbon fractions CI to C26, with peaks normally distributed around C16, which was highest, on Day zero (Figure 2a), there was drastic reduction of most of the peaks by Day 12, with total disappearance of C25, C26 and C27. In addition, there was a marked reduction of C16 and C17, which were originally the most abundant. By Day 21 (Figure 2b), all the peaks had been reduced to an average of 5%. Furthermore, peaks higher than C20 had disappeared. |
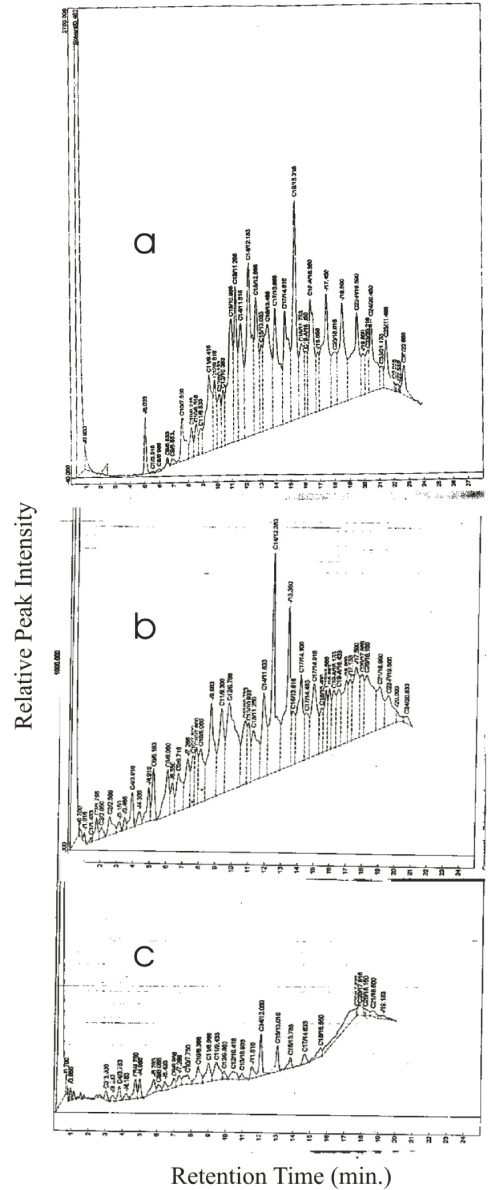
| The used SEA 40W on the other hand showed no initial traces of C5 and lower carbon hydrocarbons and the most prominent peak was C18 fraction and the disappearance of fractions higher than C24 (Figure 3). As for the fresh oil, all the peaks had reduced to less than 10% of their original height at Day 21 and > C20 peaks had disappeared. |
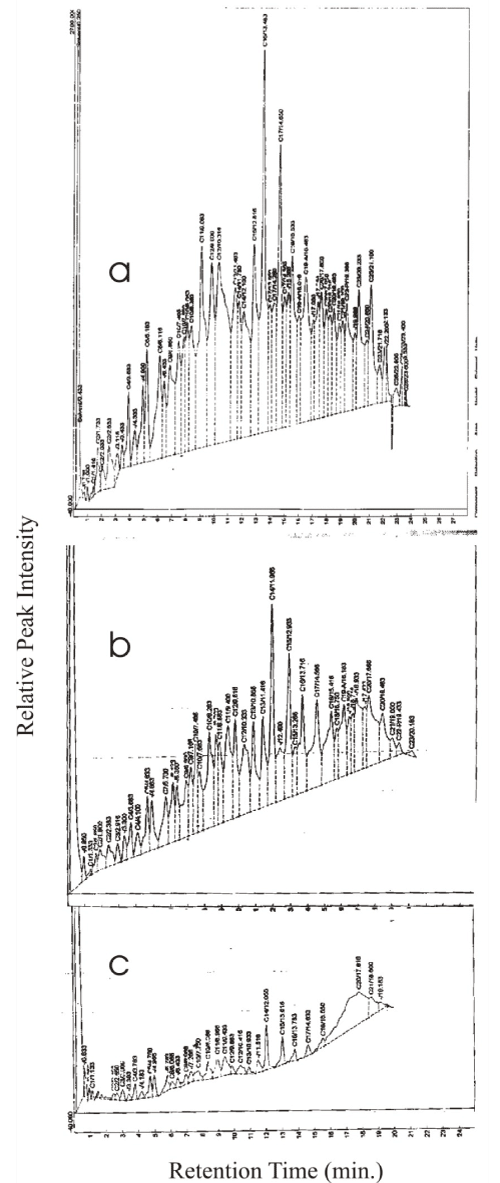
| The GC fingerprint of fresh SAE 20W-50 (Figure 4) showed that prior to degradation (Figure 4a), the peaks ranged from C1 to C28 hydrocarbons. The highest peak was C16 at 2,163.08. This particular peak drastically reduced to 308.55 at Day 12 and on Day 21 (Figure 4b), it was 23.70. All the other peaks equally reduced by more than 50% on day 12 but C14 and C15 remained prominent while fractions above C23 had disappeared. By Day 21, however, virtually all the peaks had reduced to less than 3 % with the exception of C14, C15, C19, C20 and C21. |
| As for SAE 40W, on SAE 20W-50 the lower molecular range C1 to C5 were virtually absent in used oil (Figure 5a). C6 was prominent while C26 was the highest carbon fraction. Distribution was skewed towards the higher fractions. The highest peak was C18 at 1,292.9. The value reduced to 179.2 on Day 12 and by Day 21, it was 9.4. On the other hand the lower molecular weight carbons that were not initially present (C1,C2, C3,C4,C5) reappeared on Day 12. The fractions C14 and C16 became the most prominent peaks while fractions above C24 had disappeared (Figure 5b). By Day 21 averagely, less than 10% of starting peaks remained. However, C14, C15, C17, and C21 retained more than 10 % (Figure 5c). |
| The percentages of various n-aliphathic components of fresh and used engine oils degraded by P. aeruginosa LP5 after 21 days of incubation are shown in Table 2. The result showed that tetracosane (C24) was completely degraded in all cases, which also applies for nonadecane (C19), icosane (C20), heinecosane (C21) and docosane (C22) in used SAE40. Tetradecane (C14) was degraded to between 74.7 to 86 percent, with degradation being lowest in SAE 40. Except pentadecane (C15) the general trend was degradation levels higher than 90 percent. |
| Discussion |
| The biodegradation of hydrocarbons in polluted environments is mainly through the activities of bacteria and fungi. Biodegradation is one of the means by which hydrocarbons contaminants can be removed from the environment [24]. |
| Typically, individual organisms degrade only a limited range of hydrocarbons. Pseudomonas species represents one of the most versatile groups of organisms involved in the degradation of hydrocarbons [29]. The organism used in this study was isolated on the basis of its ability to grow on pyrene [24] and has been studied with remarkable outcomes [30,31]. Reports of degraders of fresh engine oil by Pseudomonas species are rife in the literature [3,18,21,22]. Previously we reported the ability of a Pseudomonas sp. LP1 to grow and produce biosurfactant on fresh engine oil [31]. |
| Pseudomonas aeruginosa strain LP5 grew well on engine oil (used and fresh) and in all cases stationary phase was attained within 12 days with considerable disappearance of oil and biomass accumulation as revealed by turbidity and increase in population of the organism as indicated by increase in optical density. The microbial profiles can be divided into 3: the adaptation period, maximum oil degradation period and decaying period where the rate of degradation slows down, which may be due to exhaustion of available nutrients or production of toxic metabolites [32]. |
| The growth rates of LP5 on fresh engine oils SAE 40W and SAE 20W-50, 0.17 and 0.14 d-1 respectively were higher than the value for LP1 (0.122 d-1). However, it is noteworthy that this growth rates were far lower than values previously obtained for Pseudomonas species isolated by Amund and Adebiyi [17], which ranged from 0.97 to 1.33 d-1 and the values for Pseudomonas sp. and Rhodococcus sp. strains isolated from hydrocarbon contaminated sites in Lagos, Nigeria by Ogunbayo et al. [33]. However, higher growth rate may not necessarily mean higher degradation rate, especially considering that different strains may be adapted to converting different percentages of carbon utillised to biomass and carbon dioxide. |
| Whereas the Pseudomonas species reported by Jayashree et al. [22] degraded 90.2% of fresh oil in 30 days and Adelowo et al. [18] reported 73.3% and 80.0% for Pseudomonas sp. and Achromobacter sp. respectively, LP5 degraded higher percentages. The values degraded by LP5 in the first 12 days on SAE 40W and SAE 20W-50 ( 78 and 79 % respectively) compared well with the values recorded by Harikrishna et al. [34] for a Bacillus subtilis strain which degraded 71% in 10 days. These facts further underscore the versatility of LP5. |
| The initial slow rate of degradation observed on used engine oil compared with the fresh oils may be due to presence of more recalcitrant fractions of hydrocarbons and toxicity exerted by impurities such as metals and combustion products, which were present in the used oil, necessitating longer period of adaptability. However, the fact that degradation within 21 days was higher than 90 % in all cases, including used oil, demonstrated the organism did not required long period of acclimation. Furthermore, it equally showed that the organism was not susceptible to inhibition by the highly volatile lower fractions and additives present in fresh oil and the high molecular weight aliphatics and poly-aromatics in used oil. There are other reports of equally versatile degraders of used engine oil. For instance, Thenmozhi et al. [35] showed that a Pseudomonas aeruginosa strain isolated from hydrocarbon-polluted site was able to utilize 81% of used engine oil within 4 weeks. So also, Basuki et al. [20] reported the removal of 35 components out of 47 components of used oil by Acinetobacter junii TBC 1.2. |
| The reduction in peaks between Day 0 and Day 12 concomitant with rapid growth shows that the organism metabolized actively within this period. Certain hydrocarbons such as C14, C17, C18, and C21 reduced drastically by day 12 but increased on day 21, this may be due to the breakdown of larger HC molecules into smaller units. The total disappearance of C19, C22, C23, C24, C25, C26 and C27 on Day 12 suggests that these may be saturated linear alkanes, which easily lend themselves to complete degradation or fragmentation into shorter fractions. The fingerprints of crude petroleum is generally constituted by a continuum, built up by a number of fused peaks belonging to the compounds present in relatively low concentrations superimposed. However, some single peaks are shot up distinctively denoting compounds with relatively higher concentrations. The n-alkanes, belong to the group that are easily recognized because they are generally more intense and equispaced in the chromatogram [36]. This explains why even after near complete disappearance of major peaks particularly of C12, C20 and C21, other smaller peaks with the same carbon numbers persist. These peaks represent branched aliphatics and aromatics, are known to be generally more resistant to degradation than linear aliphatics. |
| The near absence of all lower fraction < C10 except C6 in the used SAE 40 on Day 0 may be attributed to abiotic removal in the crank engine [37]. It is equally interesting that whereas in the fresh oil the most prominent peak was C14, C18 fraction was more prominent in used oil. This may have resulted from cracking of higher fractions greater than C34 - C50 due to high crankcase temperature. |
| The drastic reduction of C17 and C16 fractions of fresh SAE 20w 50 in the first 12 days and reduction of other peaks by more than 50 % signify the ability of LP5 to utilize these fractions. However, the prominence of C14 and C15 may be the result of recalcitrance of these fractions, or breakdown of higher fractions leading to loss of carbon. Nevertheless, the continuous prominence of C14 over other peaks even on day 12 weighs the argument more in favour of its recalcitrance, possibly an aromatic, as hydrocarbon degrading Pseudomonas strains are generally acknowledged to be good degraders of C10 to C16 range aliphatics [38] |
| The marked similarities in the GC fingerprints of used SAE 40 and SAE 20w 50 can be explained by the same thermal effect in the crankcase [37]. Reappearance of fractions lower the C6 may be attributed to carbon loss by higher fractions during degradation. The medium range hydrocarbons, which are the major constituents of engine oil have been shown to be easily degraded by bacteria [31]. |
| The organism used in this study degraded all fractions of hydrocarbon in excess of C22 up to 100 percent in 21 days as shown in the chromatograms displayed in Figures 2 to 5 and in Table 2. This is similar to previous trends recorded in hydrocarbon degradation by other authors [36]. However, certain differences are discernible. For instance, whereas we recorded less than hundred percent degradation for medium range linear alkanes (C12 to C20) Gagandeep and Malik [38] recorded within just 10 days 100% degradation of dodecane, pentadecane, hexadecane, heptadecane and heneicosane by a bacterial strain GD18 when growing on 2T engine oil. But interestingly, just as the tetradecane (C14) exhibited the lowest biodegradability in our study (Table 2) compared with other aliphatics, the level of degradation of C14 recorded by this authors fell below 100 percent. |
| Conclusion |
| This study shows that Pseudomonas aeruginosa LP5, an organisms isolated from contaminated Nigeria soil based on its ability to degrade pyrene, has degradative potentials for fresh and used engine oil. It further confirms the potential of this isolate for use in bioremediation of hydrocarbon-polluted sites, particularly in environments polluted with mixtures of wide range of hydrocarbon components. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16883
- [From(publication date):
January-2014 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 11951
- PDF downloads : 4932
