Biodegradation of Polyaromatic Hydrocarbons by Acclimatized Mixed Culture Using Shake Flask and Roller Bioreactors
Received: 06-Nov-2017 / Accepted Date: 14-Dec-2017 / Published Date: 02-Jan-2018 DOI: 10.4172/2155-6199.1000424
Abstract
The ability of acclimatized mixed culture from sewage waste sludge was tested to biodegrade (PAHs): naphthalene and phenanthrene each with a concentration of 300 mg/L. Sewage sludge was selected as an inexpensive source of mixed culture of microorganisms, usually available in large quantities in wastewater treatment plants. Two types of reactors were employed in the investigation: shake flask and roller bioreactors. Complete biodegradation of naphthalene and phenanthrene was achieved in the shake flask bioreactor after 13 and 14 days of treatment, respectively. The corresponding durations in the roller bioreactor were 11 and 12 days. The obtained results show that the said culture is capable of consuming PAHs as energy and carbon source and have a promising application in bioremediation of PAH contaminated environments. The biodegradation of naphthalene was enhanced when using the roller bioreactor compared to its biodegradation in the shake flask bioreactor. The microorganisms’ specific growth rate was raised from 0.014 to 0.022 h-1 due to the enhanced mixing in the roller bioreactor. No enhancement was observed for phenanthrene biodegradation when using the roller bioreactor: the microorganisms’ specific growth rate was equal to 0.016 h-1 for the shake flask bioreactor compared to 0.012 h-1 for the roller bioreactor. Logistic models were employed for the description of the microorganisms’ growth and the PAHs degradation in both shake flask and roller bioreactor. Additionally, second order inhibition model was used to describe the possible inhibitions. The results obtained from the models well-matched the biodegradation experimental data with R2 of more than 97%.
Keywords: Naphthalene; Phenanthrene; PAHs; Roller bioreactor; Shake flask reactor
Introduction
PAHs are a diverse group of over 100 organic compounds containing two or more benzene rings joined in linear, angular or cluster orientations. The structural characteristics of PAHs such as number of rings, angularity, their low solubility and high hydrophobicity make them recalcitrant and persistent in the environment. They are thermodynamically stable and possess high melting and boiling points together with low water solubilities and vapor pressures [1].
PAHs pose a serious threat to the environment, especially on sites related to the processing of oil and gas.
Exposure to PAHs has a significant health risk and many of these compounds are listed as majored pollutants by both the US EPA and the European Union due to their toxic, mutagenic, or carcinogenic properties. PAH exposure can occur via inhalation, ingestion, or dermal contact. The degree of toxicity is related to the molecular weight of the PAH, higher molecular weight compounds often exhibiting greater toxicity, although the route of exposure and presence of other toxic substances will influence the toxicological effects [2,3]
Conventional chemical or physical technologies have been used for PAHs abatement. The disadvantages of conventional techniques are operational cost and difficulties as well as the resulting pollutants [4].
Biodegradation presents a promising approach for the removal of PAHs. It involves the use of naturally occurring microorganisms, e.g., bacteria of the genera Burkholderia, Sphingomonas, Acinetobacter, Pseudomonas, Mycobacterium and Rhodococcus . These have successfully mineralized low and high molecular weight PAHs [5,6].
Romero et al. [7] isolated Pseudomonas aeruginosa from a heavily polluted effluent stream from a petroleum refinery. This species was found to be growing actively over high concentrations of phenanthrene; totally removing the pollutant in duration of 30 days.
Although pure cultures of several bacteria species can readily consume PAHs as a carbon source, a mixed culture may enhance degradation [8].
Combined metabolism of mixed culture of microorganisms may result in enhanced PAH degradation because intermediate biotransformation products from one microorganism may be utilized for metabolism by others [9,10].
Sewage waste sludge is an example of a mixed bacterial culture. It results from wastewater treatment plants and contains fungi, yeast, protozoa, in addition to numerous bacterial species.
The key factor influencing the biodegradation rate is the bioavailability of PAHs to the microorganisms. Stirred tank bioreactors are advantageous regarding solid-liquid mass transfer, since the rate of dissolution can be enhanced by the application of a suitable mixing protocol. However, an inherent aspect of these bioreactors is the clinging of solid hydrophobic particles to their walls and agitation mechanisms, preventing the efficient biodegradation of solid PAHs. The use of a roller bioreactor partially overrides such a problem [11,12].
In this study sewage waste sludge was investigated as a mixed bacterial culture for the biodegradation of naphthalene and phenanthrene which are commonly used as model compounds for PAH biodegradation with two system types: shake flask and roller slurry bioreactors. All the experimental data were fitted to logistic and second order inhibition models, in order to better characterize the biodegradation process.
Material and Methods
Materials
In the present study, the used naphthalene and phenanthrene, are of analytical grade (CDH India). The mineral salt medium (MSM) is of analytical grade also (CDH and Merck, India). The nutrient broth was purchased from HIMEDIA India.
Nutrients
For microorganisms’ growth, McKinney’s modified medium mineral salt medium (MSM) was employed. Tables 1 and 2 show composition and trace elements in one liter of MSM, respectively. Preparation of the medium involved the mixing of inorganic chemicals with distilled water to make a buffered solution of pH of 6.5-6.7.
| Substance | Mass or Volume |
|---|---|
| Trace element, ml | 1 |
| Fe(NH4)2SO4, mg | 10 |
| MgSO4, mg | 30 |
| CaCl2, mg | 30 |
| NaCl, mg | 30 |
| (NH4)2SO4, mg | 237 |
| K2HPO4, mg | 375 |
| KH2PO4, mg | 420 |
Table 1: Modified McKinney’s medium in 1 liter of distilled water.
| Substance | Mass (mg) |
|---|---|
| CuCl2 | 10 |
| NiCl2 | 20 |
| Na2MoO4 | 30 |
| MnCl2 | 30 |
| ZnSO4.7H2O | 100 |
| CoCl3 | 200 |
| H3BO3 | 300 |
Table 2: Trace elements composition in 1 liter of distilled water.
Nutrient broth was used in isolation and acclimatization experiments, it consisted of yeast extract 1.5 g/L, beef extract 1.5 g/L, sodium chloride 5 g/L, and animal tissues 5 g/L with final pH=7.4 at 25°C.
Microorganisms culture
The used sewage waste sludge was collected from the drying beds of a local wastewater treatment plant (Al-Rustamiyah).
The physical, chemical and biological characteristics of the sewage sludge are shown in Table 3. The prevailing bacteria in the microbial consortium was identified to be Pseudomonas aeruginosa. The measurements were carried out using API instrument (Biomerieux, France) in the Biotechnology Center, Al-Nahrain University, Baghdad, Iraq.
| Physical characteristic (dry dead biomass) | |
|---|---|
| Particle diameter, mm | 0.775 |
| Surface area (m2/g) | 94.53 |
| Actual density (kg/m3) | 1741.6 |
| Bulk density (kg/m3) | 609.9 |
| Particle porosity | 0.584 |
| T.S (mg/l) | 153950 |
| V.S(mg/l) | 78126 |
| Chemical characteristic (dry dead biomass) | |
| pH | 5.5-6.3 |
| CEC(meq/100g) | 51.2 |
| Lead, mg/l | 0. 02 |
| Chromium, mg/l | 0.01 |
| Cadmium, mg/l | 0.02 |
| Biological characteristic (live biomass) | |
| Bacteria | |
| Aeromonasspecies (CFU/ml) | 222000 |
| E-coli (CFU/ ml) | 430000 |
| Pseudomonas aerginrsa(CFU/ ml) | 703500 |
| Klebsiella species (CFU/ ml) | 210000 |
| Clostridium (CFU/ ml) | 370000 |
| Staphylococcus sp. (CFU/ ml) | 210000 |
| Streptococcus sp. (CFU/ ml) | 490000 |
| Salmonellasp (CFU/ ml) | 190000 |
| Shiglladysente (CFU/ ml) | 410000 |
| Fungi | |
| Penicillium sp. (CFU/ ml) | 180000 |
| Yeast | |
| Candida albicans (CFU/ ml) | 460000 |
| Protozoa | |
| Entamoeba species (CFU/ ml) | 16000 |
| Giardia lambihia (CFU/ ml) | 90000 |
Table 3: Physical, chemical and biological characteristic of sewage sludge.
To isolate the mixed culture from the obtained sludge, initially 10 ml of sludge was mixed with 250 ml of the aforementioned broth. The resulting solution was subsequently put in a rotary shaker, 150 rpm, for about one week at 37°C. Then 10 ml of the week-long shaken solution was mixed with another 250 ml of nutrient broth and put again in the rotary shaker for about a week at 37°C. The above procedure was repeated four times to ensure proper isolation of microbial mixed culture which took an overall duration of six weeks.
For acclimatization of the mixed culture to the relevant PAHs, 10 ml of isolated culture was added and mixed with 250 ml of nutrient broth. To this solution, 2 g of glucose and 0.1 g of either naphthalene or phenanthrene were added. The resultant solution was subsequently put in the rotary shaker for one week at 37°C. This protocol was repeated with a gradual increase and decrease of the PAH and glucose concentrations, respectively. It took a period of more than eight weeks to obtain a final well acclimatized mixed culture grown in naphthalene or phenanthrene.
Apparatus and experimental procedures
Naphthalene or phenanthrenes with concentrations of 300 mg/L were used with either the shake flask or the roller bioreactor. For the shake flask experiments, 400 ml of MSM was introduced into a 1000 ml Erlenmeyer flask, autoclaved and mixed with 10 ml (2.5%) of acclimatized mixed culture. Then a fixed amount of naphthalene or phenanthrene was added to maintain the required concentration. The mixture was then put in the rotary shaker at 150 rpm and 37°C. To ensure aeration and VOCs minimization, all flasks were sealed with cotton stoppers. Collection and analysis of samples were conducted at regular intervals to determine PAH and microorganism concentrations.
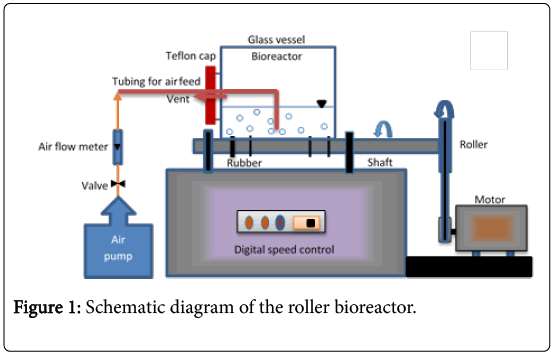
The roller bioreactor used in this study (shown in Figure 1) was fabricated in the local market. It consists of 5 L narrow mouthed glass bottle (0.15 m inside diameter and 0.3 m length), rotated on a roller arrangement at a speed of 50 rpm. The roller arrangement is a variable speed devise (0-100 rpm) containing two roller bars and run by a heavy-duty motor. Simultaneous rotation of the two bars is achieved by pulleys and a drive-belt. This bioreactor has a working volume of one liter and was operated at room temperature (~25°C). The reactor’s glass bottle was supplied with a Teflon cap with two openings; one opening equipped with stainless steel tubing, allowing continuous injection of air into the bioreactor, and the second opening was used as a vent. Air flow at a rate of 3.5 L/min was supplied to the bioreactor by an air pump (Rs-610 Zhongshan Risheng Electrical Products Co., China) to transfer the necessary oxygen for the biodegradation process.
One liter of autoclaved MSM was placed in the roller bioreactor’s glass bottle, mixed with 25 ml (2.5%) of acclimatized mixed culture. Then 300 mg of naphthalene or phenanthrene was added. The Teflon cap was fixed at the bottle mouth and the air allowed passing through the stainless steel tube to supply O2 for the biodegradation process.
The bottle was placed horizontally on the roller bioreactor and allowed to rotate at 50 rpm. Samples were taken at regular intervals for analysis by stopping the rotation of the bottle and withdrawing a 5 ml sample. Rotation was then resumed. All experiments were carried out in duplicates, and the values shown in the figures relate to mean values with a standard deviation lower than 15%.
PAHs concentration measurements
Concentration of naphthalene or phenanthrene during biodegradation experiments were determined by taking a 5 mL wellshaken sample every day, to which 10 ml of ethanol was added in order to dissolve the PAH and suppress bacteria. Subsequently, the sample was shaken for one minute (vortex mixer), centrifuged to separate the biomass, and filtered to eliminate any particles including biomass (0.22 μm nylon filter). The sample was then injected into High Performance Liquid Chromatography (HPLC), PerkinElmer series 200, USA. The stationary phase is C18 column (25 cm × 4.6 mm, 5 μm particle size) Discovery, from Supelco. A mixture of acetonitrile and water (65:35) constituted the mobile phase. Peaks were detected with a UV detector at 275 nm for naphthalene and 252 nm for phenanthrene. The HPLC reading was multiplied by 3 to obtain the PAH’s concentration before dilution.
Biomass concentration measurements
A 5 ml well-mixed sample was taken daily to measure the biomass concentration during biodegradation experiments. Measurement of the optical density (OD) was done using UV-V spectrophotometer (Model T80 from PG Instrument Ltd, England). In order to exclude the effect of bubbles and naphthalene particles on the OD value, the sample was filtered through coarse paper (Whatman Grade 41) into a cuvette. Supernatant absorbance was then measured at 600 nm, and the obtained-values were converted to grams of cell dry weight per liter using a calibration curve.
The calibration curve was developed by determining the biomass concentration using a dry-weight method. 200 ml of bacteria broth (1000 mg/ L glucose as growth substrate) was grown to a maximum biomass concentration (3 days at 30°C in shaker incubator). 100 ml of this broth was then transferred to centrifuge tubes and centrifuged at 10000 rpm for 15 minutes to allow the biomass to precipitate at the bottom of the tubes. A few drops of distilled water were introduced into the tubes to rinse their walls, followed by mixing using a vortex mixer. The procedures of centrifuging, decanting and rinsing were repeated three times. Finally, this biomass suspension was transferred to pre-weighed aluminum boats and dried at 105°C for 24 hours. The original broth was also subjected to a series of dilutions and analyzed for OD. These dilutions provided a range of known concentrations with measured ODs and were used to plot the dry-weight calibration curve.
The relation: (Biomass concentration (g/L)=0.9 × OD) consequently from the previous procedure.
Results and Discussion
Biodegradation of naphthalene and phenanthrene in shake flask bioreactor
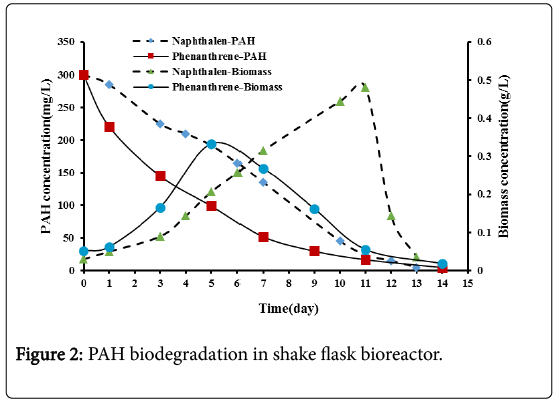
The effect of using acclimatized mix culture of microorganisms for the biodegradation of PAHs using shake flask bioreactor were investigated in these experiments. Figure 2 shows that the PAHs concentrations decreased continuously with time until depletion, indicating that the acclimatized mixed culture from sewage waste sludge could effectively degrade these compounds. Naphthalene was degraded completely after 13 days while phenanthrene was degraded completely after 14 days. The proximity of the biodegradation of these two compounds in spite of their significantly different chemical structures (two benzene rings in naphthalene vs. three fused rings in phenanthrene) and water solubilities (32 mg/L for naphthalene vs. 1.6 mg/L for phenanthrene at 25°C) is not eworthly. It may be due to the fact that the microorganisms were efficiently acclimatized on PAHs although phenanthrene was thought to be the more difficult compound to biodegrade.
Romero et al. [7] isolated Pseudomonas aeruginosa from a stream heavily polluted by a petroleum refinery. Complete removal of high dosages of phenanthrene (200 mg/L) in a period of 30 days was found.
Nasrollahzadeh et al. [10] studied the biodegradation of phenanthrene using mixed consortia of microorganisms from the effluents of a local industrial zone. The biodegradation data of phenanthrene indicate about 100%, 100% and 85% degradation at concentrations of 20, 50 and 100 mg/L, respectively within 6 days.
Also, Janbandhu and Fulekar [8] reported that the biodegradation data of phenanthrene indicated to about 100%, 56.9% and 25.8% degradation at concentrations of 100, 250 and 500 mg/L, respectively within 14 days by using adapted microbial consortium from an old petrochemical refinery field.
The results obtained in the present research, in which high dosage of phenanthrene (300 mg/L) was completely degraded after 14 days, are in line with previous results. This indicates that the microbial consortium from sewage waste sludge have a promising application in bioremediation of PAH contaminated environments.
Figure 2 shows the variation of biomass concentration with time. Biomass concentration increases with time which indicates that the microbes utilized the PAHs as energy and carbon source. An adaptation lag phase of one day for both naphthalene and phenanthrene was observed through these experiments. During this phase, cell mass may increase a little, without an increase in cell number density [13]. After the lag phase a period of an active.
After the exponential growth phase, a death phase was observed. A sharp decline in microbial growth for naphthalene and a gradual decline for phenanthrene were observed. The sharp decline in microbial growth indicates severe microorganisms’ starvation due to naphthalene depletion. While the gradual decline in phenanthrene may be due to the slow dissolution of phenanthrene into the liquid phase up to exhaustion. This observation was also mentioned by Nasrollahzadeh et al. [10] in their research.
Cell growth kinetics in a batch reactor can be represented by Malthus law (1):
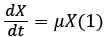
Where X is the biomass concentration (g/L) at time t, μ is the specific growth rate (h−1) and t is the incubation time (h).
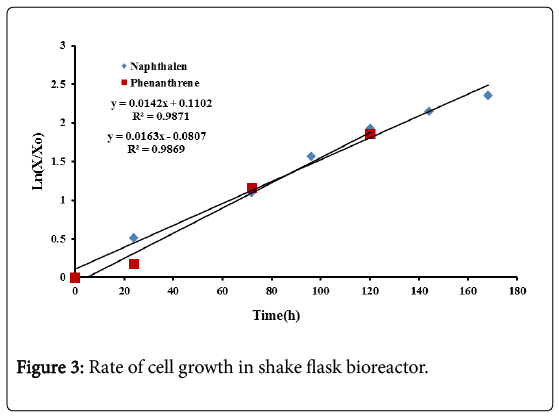
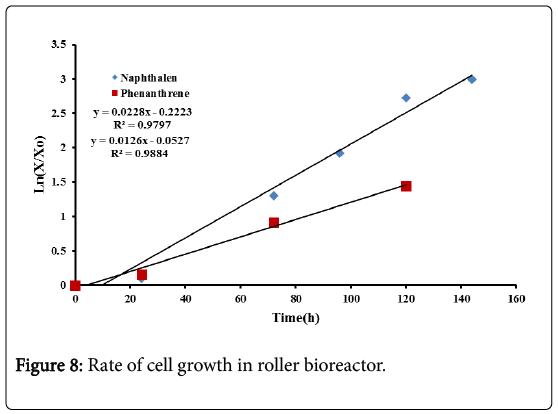
The specific growth rate for naphthalene and phenanthrene were determined from the slope of Ln(X/X0) against time, during the exponential growth phase as shown in Figure 3. X0 represents the initial biomass concentration.
The results show that the specific growth for naphthalene is 0.014 h−1 while that for phenanthrene is 0.016 h−1. The specific growth seems to be equal for the two compounds in spite of their different chemical composition and solubility.
Nasrollahzadeh et al. [10] reported that a specific growth rate of 0.022, 0.023 and 0.023 h-1 were obtained for phenanthrene concentrations of 20, 50 and 100 mg/l, respectively.
Romero et al. [7] reported that the specific growth rates for Pseudomonas aeruginosa isolated from a contaminated stream were equal to 0.041 and 0.037 h-1 for phenanthrene concentrations of 100 and 200 mg/L, respectively. Also, Lei et al. [14] found that the specific growth rate of Pseudomonas mendocina was equal to 0.033 h-1 for 100 mg/L phenanthrene.
In the present research the specific growth of phenanthrene is equal to 0.016 h-1 only, which is less than the corresponding values mentioned previously by other workers. This may be due to the relatively high concentration of phenanthrene (300 mg/L) which inhibits the microorganisms’ growth.
Various models have been proposed to represent microbial growth. An example of such models is the Logistic equation (2), which predicts the lag phase, the exponential growth rate and the stationary cell population (Xm) [11,12,15].
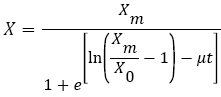 (2)
(2)
where X is the biomass concentration (g/L) at time t (h), X0 and Xm are the initial and stationary biomass concentrations (g/L), and μ is the specific growth rate (h-1).
Also, the second order inhibition model (3) was used to describe possible inhibitions as stated by Najafpour [16]. This equation has the potential to predict the inhibition which results from intermediates competing with PAH consumption:
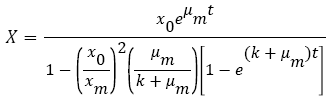 (3)
(3)
K is the decline or promotion constant (h-1).
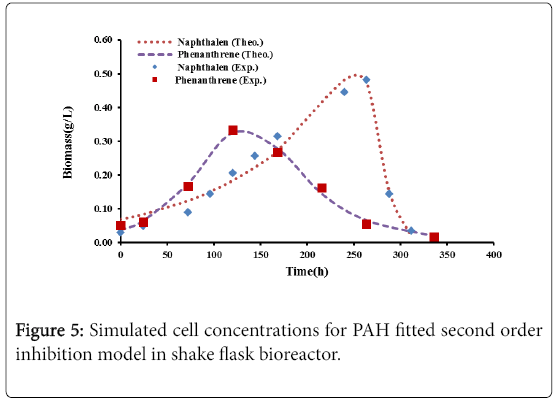
Table 4 gives the models parameters with their values. It can be seen that the parameters defining the growth models for naphthalene and phenanthrene have R2 of more than 0.98%.
| Biomass (logistic model) parameters | ||||
|---|---|---|---|---|
| naphthalene | phenanthrene | |||
| shake flask | roller | shake flask | roller | |
| Xm (g/L) | 0.525 | 0.47 | 0.903 | 0.592 |
| Xo (g/L) | 0.029 | 0.002 | 0.044 | 0.049 |
| µm (h-1) | 0.019 | 0.047 | 0.02 | 0.016 |
| R2 | 0.998 | 0.99 | 0.997 | 0.996 |
| Biomass (second order inhibition model) parameters | ||||
| naphthalene | phenanthrene | |||
| shake flask | roller | shake flask | roller | |
| Xm (g/L) | 0.25 | 0.1 | 0.35 | 0.43 |
| Xo (g/L) | 0.07 | 0.01 | 0.038 | 0.045 |
| µm (h-1) | 0.01 | 0.02 | 0.023 | 0.016 |
| K (h-1) | 0.1 | 0.02 | 0.017 | 0.022 |
| R2 | 0.986 | 0.99 | 0.995 | 0.949 |
| Degradation parameters | ||||
| naphthalene | phenanthrene | |||
| shake flask | roller | shake flask | roller | |
| Dm (%) | 100 | 92 | 94 | 91 |
| Do (%) | 6.311 | 12.712 | 12.836 | 10.961 |
| µD (h-1) | 0.014 | 0.048 | 0.025 | 0.034 |
| R2 | 0.992 | 0.97 | 0.96 | 0.971 |
Table 4: Growth and PAHs biodegradation kinetic parameters for shaker and roller bioreactor systems.
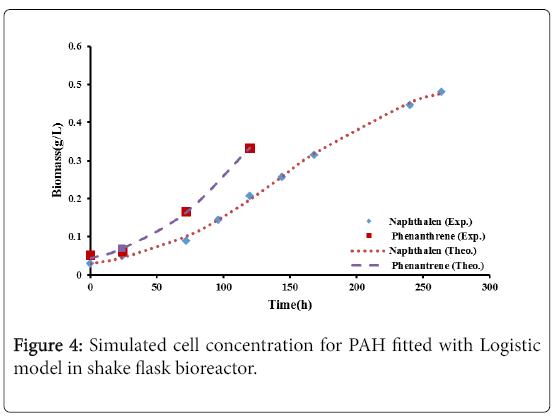
Figures 4 and 5 show the comparison between the experimental data with logistic and second order inhibition models. The theoretical growth curves fit quite well the experimental data.
The results for the biodegradation of naphthalene and phenanthrene in the shake flask bioreactor were fitted to the Logistic equation (4) [11,12].
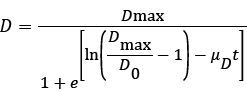 (4)
(4)
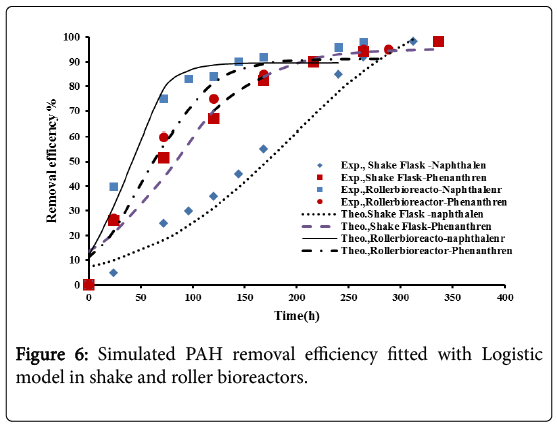
D is the naphthalene or phenanthrene removal efficiency (%) at time t, D0 and Dmax are the initial and maximum PAHs removal efficiencies (%), and μD is the specific degradation rate (h-1).
The model parameters with their values are shown in Table 4. Figure 6 shows a successful fitting between the theoretical and the experimental results.
Biodegradation of naphthalene and phenanthrene in roller bioreactor
The bioavailability of PAHs to the microorganisms is the key factor influencing the biodegradation rate. In this sense a roller bioreactor was used to improve the performance of the biodegradation process by supplying efficient mixing for better PAHs particle dissolution to the liquid phase.
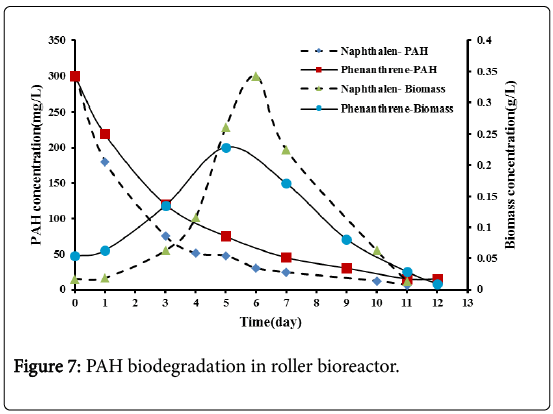
From Figure 7, it can be seen that a rapid biodegradation rate exists for both naphthalene and phenanthrene at an early stage of the experiment and then a slower biodegradation rate was observed. Naphthalene degraded completely after 11 days while phenanthrene degraded completely after 12 days with a slightly slower rate.
It was obvious that the biodegradation rate of naphthalene was enhanced when using the roller bioreactor due to its increased solubility which making it more bioavailable to the microorganisms [17]. No enhancement in the degradation rate of phenanthrene was observed when using the roller bioreactor. Phenanthrene solubility was only 1.6 mg/L; to enhance this low solubility efficient mixing is not enough. Different surfactants may be required as reported by many authors [11,12,18-21]. Moscoso et al. [11,12] reported that phenanthrene degradation values of 90% was achieved in flask scale and 100% in bioreactor scale after 3 days of operation when using Tween 80 surfactant (1% w/v).
From Figure 7, it can be seen that the biomass concentration increases with time indicating that the microbes consumed the naphthalene and the phenanthrene as energy and carbon source. A lag phase of 1 day for both naphthalene and phenanthrene was observed, as in the shake flask bioreactor. After the lag phase a period of exponential growth phase was observed. The maximum biomass concentration reached in the present experiments can be observed to be 0.34 g/L after 6 days for naphthalene and 0.23 g/L after 5 days for phenanthrene. The low solubility of phenanthrene may cause a limited cell growth.
Following the exponential growth, a death phase was observed indicating the depletion of PAHs.
From Figure 8 the specific growth rate for naphthalene was 0.022 h−1. In the shake flask bioreactor this specific growth rate was 0.014 h−1, indicating that the roller bioreactor is more efficient in the biodegradation treatment of naphthalene. For phenanthrene the specific growth rate was 0.012 h−1 compared to 0.016 h−1 for the shake flask bioreactor. This confirms that no enhancement in the biodegradation treatment of phenanthrene was obtained in the roller bioreactor. As pointed out previously, for very low PAHs solubilities, enhancement may be achieved by adding different surfactants.
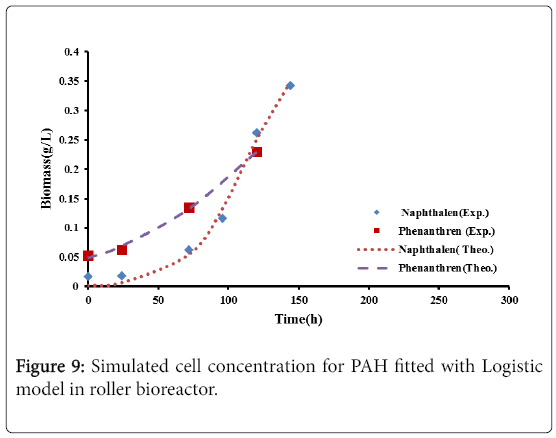
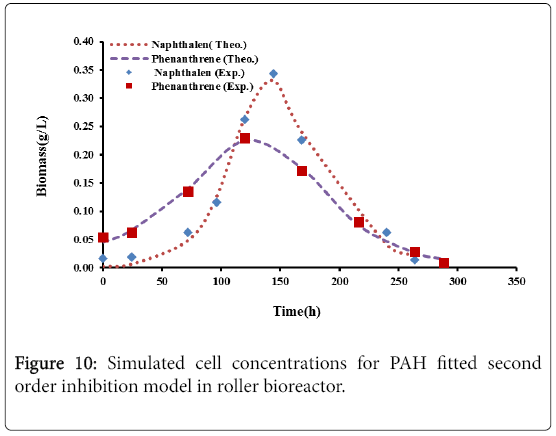
Figures 9 and 10, show the comparison between the experimental data with the results of logistic model and second order inhibition model for both naphthalene and phenanthrene.
It was observed that the experimental data were well represented by the proposed models. The parameters defining the logistic models and second order inhibition model for the roller bioreactor are presented in Table 3, with R2 of more than 97%. The results for the biodegradation of naphthalene and phenanthrene in the roller bioreactor were fitted to the Logistic equation (4). A fair fitting was obtained as shown in Figure 6. The model parameters with their values are shown in Table 4, with R2 of 97%.
Conclusion
The biodegradation of naphthalene and phenanthrene by an acclimatized mixed culture from sewage waste sludge was investigated in two types of reactors: shake flask and roller bioreactors. The results show that complete biodegradation of naphthalene and phenanthrene was achieved after 13 and 14 days, respectively in the shake flask bioreactor. While in the roller bioreactor complete biodegradation was achieved after 11 and 12 days, respectively. The specific growth rate was observed to be 0.014 and 0.016 h-1 for naphthalene and phenanthrene, respectively, in the shake flask bioreactor while the corresponding values were 0.022 and 0.012 h-1 in the roller bioreactor.
The results indicate that the roller bioreactor enhanced the biodegradation of naphthalene due to its efficient mixing which enhanced the dissolution of naphthalene particles to the aqueous phase making it more bioavailable to the microorganisms. The roller bioreactor didn’t show any enhancement to the biodegradation of phenanthrene, this may be due to the very low solubility of this polyaromatic compound in water and the need to use a surfactant to enhance its solubility.
Logistic model and the second order inhibition model were used to describe the microorganisms’ growth and the PAHs degradation. A good fitting was obtained between the models and the experimental results.
Acknowledgements
Financial support from the Ministry of Higher Education and Scientific Research is gratefully acknowledged.
References
- Cao B, Karthiga N, Kai-Chee L (2009) Biodegradation of aromatic compounds: current status and opportunities for biomolecular approaches. Applied Microbiology and Biotechnology 85: 207-228.
- Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. Journal of Hazardous Materials 169: 1-15.
- Mrozik A, Labuzek S (2003) Bacterial degradation and bioremediation of polycyclic aromatic hydrocarbons. Polish Journal of Environmental Studies 1: 15-25.
- Mamma D, Emmanuel K, Nikolaos P, Dimitris G, Paul C, et al. (2004) Biodegradation of phenol by acclimatized Pseudomonas putida cells using glucose as an added growth substrate. Journal of Environmental Science and Health, Part A 39: 2093-2104.
- Johnsen A, Lukas Y, Hauke H (2005) Principles of microbial PAH-degradation in soil. Environmental Pollution 133: 71-84.
- Yu SH, KeL,Wong YS, Tam NF (2005) Degradation of polycyclic aromatic hydrocarbons by a bacterial consortium enriched from mangrove sediments. Environment International 31: 149-154.
- Romero M, Cazau M, Giorgieri S, Arambarri A (1998) Phenanthrene degradation by microorganisms isolated from a contaminated stream. Environmental Pollution 101: 355-359.
- Janbandhu A, Fulekar M (2011) Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. Journal of Hazardous Materials 187: 333-340.
- Bouchez M, Blanchet D, Vandecasteele J (1995) Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Applied Microbiology and Biotechnology 43: 156-64.
- Nasrollahzadeh H, Najafpour G, Pazouki M, Younesi H, Zinatizadeh A, et al. (2010) Biodegradation of phenanthrene in an anaerobic batch reactor: Growth kinetics. Chemical Industry and Chemical Engineering Quarterly 16: 157-165.
- Moscoso F, Teijiz I, Deive J, Sanromán M (2012) Efficient PAHs biodegradation by a bacterial consortium at flask and bioreactor scale. Bioresource Technology 119: 270-276.
- Moscoso F, Deive F, Longo M, Sanromán M (2012) Technoeconomic assessment of phenanthrene degradation by PseudomonasstutzeriCECT 930 in a batch bioreactor. Bioresource technology104: 81-89.
- Yu R (2006) Bioremediation of Polycyclic Aromatic Hydrocarbon (PAH)-Contaminated soils in a roller baffled bioreactor. PhD Thesis, University of Saskatchewan, Saskatoon, Saskatchewan.
- Lei T, Pei M, Jian-Jiang Z (2002) Kinetics and key enzyme activities of phenanthrene degradation by Pseudomonas mendocina. Process Biochemistry 37: 1431-1437.
- Zeinab B, Ghasem N (2001) Growth kinetic models for phenol biodegradation in a batch culture of Pseudomonas putida. Environmental Technology 32: 1835-1841.
- Najafpour G (2007) Biochemical engineering and biotechnology. Elsevier Science, Amsterdam p: 51.
- Riess R, Mehdi N, John H, Gordon H (2005) Improved mass transfer and biodegradation rates of naphthalene particles using a novel bead mill bioreactor. Journal of Chemical Technology and Biotechnology 80: 662-668.
- Pantsyrnaya T, Delaunay S, Goergen J, Guédon E, Paris C, et al. (2012) Biodegradation of phenanthrene by pseudomonas putida and a bacterial consortium in the presence and in the absence of a surfactant. Indian Journal of Microbiology 52: 420-426.
- Schippers C, Gessner K, Müller T, Scheper T (2000) Microbial degradation of phenanthrene by addition of a sophorolipid mixture. Journal of Biotechnology 83: 189-198.
- Megharaj M, Ramakrishnan B, Venkateswarlu K, Sethunathan N, Naidu R (2011) Bioremediation approaches for organic pollutants: a critical perspective. Environ Int 37: 1362-1375.
- Ortega-Calvo J, Tejeda-Agredano M, Jimenez-Sanchez C, Congiu, E, Sungthong R, et al. (2013) Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation? J Hazard Mater 261: 733-745.
Citation: Ridha MJM, Mustafa YA, Razak AZ (2018) Biodegradation of Polyaromatic Hydrocarbons by Acclimatized Mixed Culture Using Shake Flask and Roller Bioreactors. J Bioremediat Biodegrad 9: 424. DOI: 10.4172/2155-6199.1000424
Copyright: © 2018 Ridha MJM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5926
- [From(publication date): 0-2018 - Aug 04, 2025]
- Breakdown by view type
- HTML page views: 4969
- PDF downloads: 957