Biofuels Get in the Fast Lane: Developments in Plant Feedstock Production and Processing
Received: 09-Jul-2013 / Accepted Date: 24-Oct-2013 / Published Date: 26-Oct-2013 DOI: 10.4172/2329-8863.1000117
Abstract
In recent years, high volatility in oil prices and global climate change led to an increased interest in biofuel production to reduce dependency on foreign fossil fuel. Domestically produced plant feedstocks are environmentally friendly renewable substitutes for fossil-derived fuel and are expected to stabilize fuel prices. Plant-derived energy can offer rural development and other environmental, social and energy security benefits for local societies. Crops, grasses, trees, forest-residues and aquatic plants, all can be used as potential biofuel feedstocks. To meet the increased global and regional demand for bioenergy, evaluation and improvement of current and emergent plant feedstocks is urgently needed to reduce the cost of the resulting biofuels.
Keywords: Biofuels, Feedstock Production, Plant Feedstock, Bioenergy Production, Bioethanol
402358Emerging Bioenergy Production from Plants
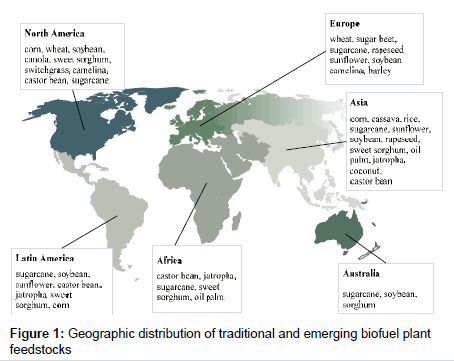
The most common biofuels are ethanol and biodiesel. Bioethanol represents the most widely used liquid biofuel in the world. Global ethanol production (~97% bioethanol) reached 28 billion gallons in 2010, with United States (corn) and Brazil (sugarcane) being the worlds’ leaders, producing 23 billion gallons and together accounting for 90% of total global production [1]. Other ethanol-producing countries include the European Union Member States (mostly Germany, Spain, France, Sweden, Italy and Poland), China, Canada, Australia and Thailand. By 2012, ethanol production absorbed over 50% of Brazil’s sugarcane crop and 37% of the coarse grain crop in the United States. Biodiesel markets are dominated by the European Union, United States, Argentina, Brazil and Southeast Asia (Malaysia, Indonesia, Singapore and China) (Figure 1). The European Union is the largest producer and consumer of biodiesel accounting for 80% of global production.
The top four biodiesel producing European Member States are Germany, France, Spain and the Benelux. Rapeseed is the major source of biodiesel in Europe while about 85% of United States biodiesel production comes primarily from soybean.
First-generation biofuels are made from sugars and oils found in the edible part of agricultural feedstocks such as starches from grains (corn, barley, grain sorghum), tubers (cassava and sweet potatoes), crops high in sugar content (sugarbeets, sugarcane) and oil-containing seeds (rapeseed, soybean, sunflower, coconut and palm oils). First-generation agricultural feedstocks have played an important role in establishing the basic infrastructure for biofuel production processes and producing a portion of transport fuels. Competition with food crops for land use and commodity prices, food security and greenhouse gas (GHG) emissions from land use will eventually lead to the progressive replacement of the traditional feedstocks with the second-generation biofuels derived from other non-food crops, lignocellulosic biomass and waste material. Lignocellulosic feedstocks include crops (corn stover, wheat straw, switchgrass and sweet sorghum) and fast-growing trees (poplars, willows and Eucalyptus). Conversion of lignocellulosic biomass into usable energy requires advanced technologies. Improving the efficiency and reducing the cost of cellulosic conversion technology is an important challenge and opportunity for the commercialization of this type of bioenergy source. Lignocellulosic biomass is one of most promising renewable sources for production of fuel ethanol in countries with ample supply such as Canada, United States and China. Similar to the second-generation biofuels, the third-generation biofuels originate from non-food feedstocks such as microalgae, which are more productive than land grown plants, do not compete with food crops for agricultural land and could produce more biodiesel than palm oil per year for the same size of land [2]. Third-generation biodiesel from microalga is still a project of the future and not of the present. This energy-dense biofuel source is expensive to produce in commercial scale (open or closed production systems) as it requires lots of land, water, light, sterile carbon dioxide (CO2), biomass harvesting and processing while oil production increases only under stress (starvation) conditions which negatively impact the reproduction of algae [3].
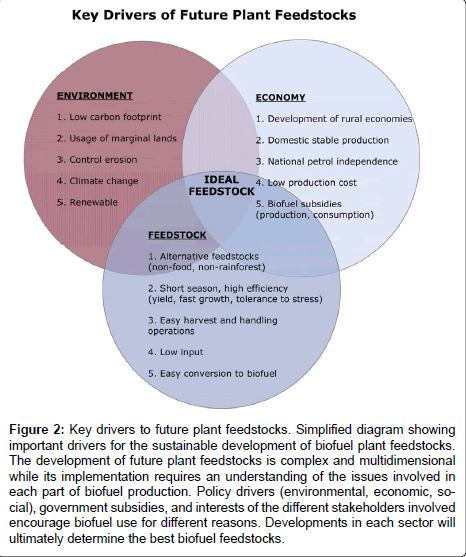
When evaluating potential feedstock for biofuel production the major considerations are low production cost, low carbon footprint, capability to grow on marginal lands, low input (low water and nitrogen use), tolerance to biotic and abiotic stresses, easy conversion to biofuel and a preference for non-food and non-rainforest plants. In addition, other factors including the harvesting, storage and transportation operations increase the cost of feedstock production (Figure 2). A recent study evaluated the efficiency of resource use, soil quality, net energy production and GHG emissions in several crops [4]. According to this study, oil palm (Indonesia, Malaysia), sugarcane (Brazil) and sweet sorghum (China) are the top ranking plants meeting all the aforementioned criteria. These plants were more efficient in terms of land use, water requirements, nitrogen inputs, pesticide applications and GHG emissions. Notably, maize and wheat performed poorly whereas sugar beet (northern Europe), cassava (Thailand), rapeseed (northern Europe), soybean (United States) were moderately efficient. The sustainability criteria set forth by biofuel policies of individual countries are expected to profoundly affect feedstock and biofuel markets over time and transform the agricultural landscape.
Figure 2: Key drivers to future plant feedstocks. Simplified diagram showing important drivers for the sustainable development of biofuel plant feedstocks. The development of future plant feedstocks is complex and multidimensional while its implementation requires an understanding of the issues involved in each part of biofuel production. Policy drivers (environmental, economic, social), government subsidies, and interests of the different stakeholders involved encourage biofuel use for different reasons. Developments in each sector will ultimately determine the best biofuel feedstocks.
Benefits and Disadvantages of Biodiesel
Biodiesel is an alternative fuel for diesel engines consisting of long chain (C16 to C18) alkyl esters. The use of plant oil as biofuel source dates back in 1900, when Rudolf Diesel first demonstrated that his prototype engine could run on peanut oil [5]. Today, the rising cost of fossil fuel and global climate change brought biodiesel back in the spotlight. Unlike the United States, the growing use of cars and trucks that run on diesel in Europe resulted in European Union initiatives for the production of biodiesel. Biodiesel is produced from biomass mainly by alkali-catalysed transesterification of plant oil-derived triacylglycerols (TAGs) and short chain alcohols [6]. During this process, three moles of biodiesel and one mole of glycerol are synthesized for every mole of fully converted TAG. Apart from the chemical catalysis, biodiesel production can be achieved using enzymatic (microbial lipases) transesterification of oils. This biocatalytic method of biodiesel production offers high selectivity, high purity product and fewer process steps compared to chemical catalysis but it needs improvements to reduce the high cost for industrial scale use and to speed up the reaction rate [7].
Biodiesel production from plant oils as an alternative to petroleum diesel has several advantages because it is considered as nontoxic and biodegradable liquid fuel. The miscibility with petrodiesel at different ratios is another advantage of biodiesel. The composition of biodiesel differs from petroleum diesel as it contains a reduced amount of carbon and higher hydrogen and oxygen. Because of this important difference, biodiesel gives near complete combustion with low particulate emissions. Biodiesel also yields reduced emission of carbon monoxide (CO). This is important because the abundance of carbon-based gases in the atmosphere contributes significantly to climatic changes and global warming. Plant feedstocks are not only efficient convertors of solar energy but play a significant role in removing CO2 from the atmosphere by photosynthesis across their lifecycle. Further, plant feedstock used for biofuel production returns CO2 to the atmosphere when the fuel is burned. In this respect, plant feedstock can be regarded as a sustainable source of fuel with beneficial atmospheric impact in terms of CO2 reduction. However, both the sustainability of biofuels which is associated with the production and management of feedstock and the contribution of biofuels to climatic change mitigation by reducing GHG emissions are highly debated matters. Another advantage of biodiesel is that it is essentially free of sulfur or aromatic compounds which add to the emission of particulate matter and pose a health hazard [8,9]. Regulations in many countries required the elimination of sulfur-containing compounds from conventional diesel in an effort to reduce exhaustion pollutants. The low-sulfur diesel solved concerns on environmental impact but at the same time created a lubricity problem for the engines since sulphur compounds of diesel fuel provided natural lubrication protecting the engine from excessive wear and prolonged its life. Inclusion of biodiesel in low-sulfur fuel improves lubricity [10] and eliminates the need for lubricity improving additives. Biodiesel has a relatively low cloud point and is resistant to oxidation [11]. An additional advantage of biodiesel over diesel is the high flash point which provides safety benefits from potential fire hazard. The disadvantages of biodiesel include increased oxides of nitrogen (NOx) exhaust emissions in some cases, high feedstock cost and lower volumetric energy content [12,13].
Important and Promising Plant Feedstocks for Biodiesel Production
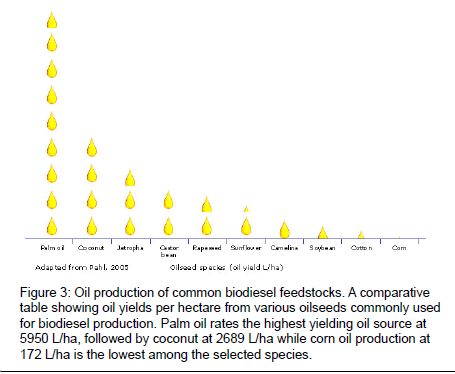
Given the competing demands for agricultural feedstocks, current biofuel policies support the cultivation of non-food feed stocks which are able to grow on marginal lands and produce biofuels within a sustainable agricultural system. Contrary to ethanol, biodiesel produced from different feedstocks varies in composition and quality characteristics. These differences may also be reflected in biodiesel prices. There is also great variation in oil yield based on plant species (Figure 3). Currently, non-edible oil plants for biodiesel production include Jatropha [14,15] Pongamia [14,16], tobacco [17-19], mahua [20,21], neem [22], castor bean [23], cotton [24] and camelina [25]. For most of these feedstocks, agronomic and crop production improvements are just beginning to be applied. An overview of selected biodiesel feedstocks is provided.
Figure 3: Oil production of common biodiesel feedstocks. A comparative table showing oil yields per hectare from various oilseeds commonly used for biodiesel production. Palm oil rates the highest yielding oil source at 5950 L/ha, followed by coconut at 2689 L/ha while corn oil production at 172 L/ha is the lowest among the selected species.
Rapeseed (Brassica napus) also known as canola in North America (edible, rapeseed variety with low erucic acid) is a member of the family Brassicaceae with the Mediterranean area being its probable origin. This coolseason annual crop is grown worldwide. The popularity of the crop may be due, in part, to its new varieties and today, rapeseed production in the world ranks second among oilseed crops after soybean. Between 1994 and 2008, the prices for rapeseed oil as biofuel feedstock have tripled due to high demand. Major advantages of rapeseed cultivation are the ease of growth and high oil yield per unit of land area. The plant performs best in well-drained soil and produces small seeds with 40 to 45% oil content. The seeds may contain up to 64% erucic acid (Δ13-22:1) which makes them inedible. Currently, rapeseed oil is the preferred oil for biodiesel in European Union and Eastern Europe where it makes up approximately 80% of plant oils used in European biofuels. It is also an extremely efficient feedstock for biodiesel in North America (the United States, Canada). Rapeseed is the most widely planted oil-bearing crop in China where it covers about 60% of the total oil crop cultivation land. The European Union is the world’s leader in rapeseed cultivation followed by Canada, China and India. Early breeding programs concentrated on improvements in agronomic characteristics such as high yield, seed size, stress tolerance (drought, frost), early flowering, disease resistance and modification of fatty acid content. Winter rapeseed (canola) planting is done between September and November in Europe, Ukraine, Russia, and parts of China resulting in a 20 to 30% larger yield compared to spring planting. Spring planting of rapeseed (canola) usually requires a minimum of 85 days from planting to seed maturation and is a common practice in some regions of China, India, Canada and United States.
Biodiesel made from rapeseed (canola) oil has a cloud point (the temperature at which wax crystals appear) of 1°C and pour point of -9°C which means improved cold weather performance [26]. The iodine value, which indicates the degree of unsaturation of oil and is a stability measurement, is greater for canola oil than the rapeseed oil (110 to 126 versus 97 to 108) because of differences in the levels of erucic acid, linoleic and linolenic acids. Although the pure rapeseed biodiesel is a cleaner-burning fuel than petroleum diesel partly due to the higher oxygen content (10% by weight), the crop requires nitrogen fertilizer to grow which in turn releases nitrous dioxide from the fertilizer to the atmosphere and is blamed for its indirect carbon footprint impact on the environment.
Castor bean (Ricinus communis) also known as ricin, is an important non-edible oilseed crop of the family Euphorbiaceae, native to North- East Africa from where it spread to the Mediterranean area and the Middle-East. This warm season crop is also found in India, North America, Caribbean and Asia. Castor bean is a C3 pathway, cross-pollinated diploid species with 2n=20 chromosomes. Castor bean is generally grown as a perennial crop in tropics, but it can be grown as an annual crop in temperate regions. Castor bean is an excellent biofuel crop with cycle duration about 140 to 180 days in temperate regions, a low demand for water, prolific growth, large production of oil, can withstand long periods of drought and ia able to grow well on degraded and marginal lands. The environmental benefit of growing castor bean is that castor bean plants may absorb about 34.6 t/ha of CO2 in two growing cycles per year. In general, castor bean cultivation is suitable for regions where the frost-free period is more than 110 days. The plants grow well on slightly alkaline or acid soils, while supply of nitrogen may increase yield. Water logging is a limiting factor for castor bean production because plants cannot tolerate prolonged low-oxygen soil conditions [27]. For the establishment of the plantation, the seeding rate is about 50,000 seeds/ha while the row spacing depends on the combine harvester and whether the cultivation is irrigated or rain fed. The cost of castor bean plantation can be almost half of the cost of planting rapeseed and one fourth of the cost of planting Jatropha [28]. Today, India, China, Brazil and Mozambique are the main producers of castor oil (FAOSTAT 2012 online resource: http://faostat.fao.org) with oil yields up to 1100 Kg/ha while oil yields in arid and semi-arid regions range from 350 to 900 kg/ ha, which in turn produce 350 to 900 kg/ha of biodiesel. The oil content of seeds with hulls removed varies from 24 to 55%. The low oil content of castor bean seeds is usually found in traditional varieties whereas with current hybrids farmers can expect almost double the amount of oil and adaptability to different climatic conditions. Castor beans should be planted in April to early May and a slow germination may take 10 to 21 days after planting. Varieties with precocious, median and delayed cycles are currently available. The plants may grow up to 3 m/year with 5 to 6 branches and terminal spikes containing male and female flowers in the same inflorescence. For increased seed production hybrids with only female flowers have been developed. Cross-pollination of the female flower with the protruding star-shaped stigma benefits from insects. The capsules are ready for harvesting when they are dry and the leaves have fallen from the plant. Caution is needed not to delay harvesting to avoid seed shattering at harvest. Usually, seed maturation takes about 120 days after planting and earliness in maturation is one desirable plant trait for regions where high temperatures and drought threaten the plants during the late growth stage. Identification of early maturation genotypes that escape drought provides a solution to this problem [29].
The development of new cultivars of castor plant aims towards semi-dwarf, unbranched plants suitable for mechanical harvesting with high seed oil yield [30], tolerance to salt [31], drought [32], pests and diseases. Also, although ricin toxin is not present in castor oil, a preference for plants low in ricin led scientists to investigate the naturally present variation in ricin levels in order to develop varieties free or low in ricin content such as the cultivar ‘Brigham’ which has a 10- fold reduction in ricin level [33]. Interestingly, the ricin toxin contains two subunits that are encoded by a multigene family [34,35]. Another breeding objective is to modify the fatty acid profiles of castor bean. Using the half-seed technique, a natural mutant of castor bean has been identified with high oleic and low ricinoleic acid concentration [36]. The valuable mutant revealed natural variation regarding ricin content and is a resource for breeding purposes. Chan et al. [37] in the most recent castor draft genome, found 28 potential genes encoding ricinlike proteins. These findings open the way for more targeted genome engineering approaches. Castor bean has low genetic diversity based on amplified fragment length polymorphisms (AFLP), simple sequence repeat (SSR) [38] and single nucleotide polymorphism (SNP) analysis in a worldwide collection of accessions [39]. Since 1970, several varieties of castorbean have been developed for oil production: ‘Hale’ (short, several racemes), ‘Brigham’ (low ricin content), ‘BRS Nordestina’ (hand harvest, semi-arid environments), ‘BRS Energia’ (hand or mechanized harvest), ‘GCH6’ and ‘GCH5’ (resistant to fusarium wilt), ‘Abaro’ and ‘Hiruy’ (hand harvest). In arid and semi-arid regions where this plant is mostly cultivated [29,40], local varieties are best suited for biodiesel production as they are adapted to the local environmental conditions, pest and diseases.
Castor oil is unique among other plant oils as it consists mainly of 12-hydroxy-9-octadecenoic acid, commonly known as ricinoleic acid, which is a monounsaturated 18-carbon fatty acid and represents over 89% of the fatty acid chains present in seed. Other significant components of the oil are the oleic and linoleic acids. Castor oil has the remarkable ability to withstand high and low temperatures [41] while its high content in hydroxylated fatty acid (ricinoleic acid) makes it more stable against oxidation and higher in oxygen content than other oils. The fuel-related properties of castor oil with the high density (0.956 to 0.963 g/ml at 20°C, above the maximum limit in EN 14214 European biodiesel specification), viscosity under 50°C and hygroscopicity suggest that castor biodiesel cannot be used in its pure form [42-46], but rather in blends with regular diesel or biodiesel made with other lipid feedstocks. Importantly, biodiesel made of castor oil has lower cost compared to other vegetable oil feedstocks because it is the only oil that is soluble in alcohol and does not require heat and the consequent energy requirement of other vegetable oils in transforming them into fuel [47]. Biodiesel from castor achieved an average of almost 90% less GHG emissions when compared to regular diesel. Other properties of the oil that make it useful for biodiesel purpose are the low sulphur content (0.04%), ash content of 0.02% and medium iodine value (82 to 90).
Jatropha (Jatropha curcas L.) is a large perennial tree of about 5 m height adapted to tropics and subtropics that belongs to the Euphorbiaceae family. Jatropha produces non-edible seeds with seed oil suitable for biodiesel production [48]. The plant is able to grow in temperate climates with average annual temperatures well above 20°C. Commercial scale production of Jatropha comes mainly from India where more than a million hectares of Jatropha plantations are dedicated to biodiesel production. Apart from India, Africa is becoming the second largest area of Jatropha cultivation with Mozambique, Ghana, Malawi, Tanzania and Zimbabwe developing large scale plantations. Southeast Asian countries including Indonesia, Malaysia and Phillipines are also keen to develop large scale Jatropha plantations. Pilot and small scale jatropha plantations have been initiated in China, Brazil and United States (mainly in Florida). Jatropha feedstock with the wide geographic range emerges as one of the prime choices for biodiesel production. The plant prefers well-drained soils with good aeration [49] and is well adapted to marginal soils with low nutrient content. Its cultivation in poor non-arable lands can prevent soil erosion. Although Jatropha ranks first among the biodiesel crops in water requirements, rainfed Jatropha is produced in many different countries with lower output of biofuel compared to irrigated plantations in India. It is found mainly at areas with lower altitude (0 to 500 m) but it can grow at higher altitudes and tolerate slight frost. Jatropha trees are usually propagated from cuttings (one-year-old terminal branches of 25 to 30 cm) or seeds [50]. Jatropha trees produced from seeds have a better root system compared to trees produced from cuttings and can tolerate frost. Fruits are harvested at seed maturity, which occurs about 40 days after flowering and then dried to less than 8% humidity in the sun. Jatropha oil yield can reach 3820 L/ha/year [51]. One factor that limits seed production is that only a fraction (10 to 20%) of the total flowers of the inflorescence are female, as a result each inflorescence yields approximately 10 fruits. The increase in the number of female flowers in each inflorescence is a target of ongoing research. In addition, one constrain in a large-scale production of Jatropha is the lack of mechanized harvest which limits its production in countries with cheap labor. Development of plant varieties suitable for mechanized harvest will result in the rapid expansion of the plant as biodiesel feedstock. Other desirable agronomic traits of Jatropha for biofuel production are the seed morphology, oil content [52,53], synchronization of fruit maturation, nanism, resistance to pests and diseases. However, the current understanding of the genetic basis of agronomic traits is poor [54]. Jatropha accessions available in India show only modest levels of genetic variation while wide variation has been found between the Asian and Mexican genotypes [55-57]. Breeding programs and germplasm conservation are principally ongoing in India, China, Thailand, Philippines, Mexico, Guatemala and Brazil. For breeding purposes, the selection of the best-performing trees in the location of interest is the best practice for finding parent plants. Several of the J. curcas genes involved in TAG biosynthesis have already been cloned [58,59].
The physicochemical properties of biodiesel produced from the seed oil of Jatropha have characteristics similar to fossil diesel. The kernels of J. curcas yield about 46 to 58% of semi-dry oil (iodine value 93 to 107) and contain mainly oleic (37 to 63%) as principal fatty acid [60]. J. curcas oil’s viscosity and density are appropriate for a biodiesel fuel [61].
Neem (Azadirachta indica) is an evergreen tree of the family Meliaceae, native to India and well established in other Asian countries, Africa, Central and South America and in the United States (small scale plantations). The tree can reach 15 to 20 m height and produce 37 to 55 kg of fruit (olive-like drupe) per year depending on the conditions [62]. The neem plant grows well in hot weather and on different types of soils and marginal lands but severe drought can make the leaves to fall off. The well-developed root system of the tree makes it suitable for erosion control and able to survive prolonged drought and poor nutrient composition in soils. It has a remarkable ability to tolerate high (up to 48°C) and low (up to 4°C) temperatures [63]. Neem seed has to be de-pulped immediately after harvest, sun dried and stored (up to 3 months) till crushed for oil extraction. The oil content of the kernels ranges from 30 to 60%. The diameter of the plant canopy may reach 15 to 20 m in old trees. The brownish oil with the strong odour contains mainly triglycerides and large amounts of triterpenoid compounds which give a bitter taste. The neem seed oil meets the values prescribed by the American Standard Testing Method for biodiesel [64]. The viscosity value of neem oil is between 20.5 and 48.5 mm2/s whereas that of neem biodiesel is between 3.2 and 10.7 mm2/s (diesel viscosity: 4.7) and it can be used as fuel in diesel engines directly and by blending it with methanol [65].
Camelina [Camelina sativa (L.) Crantz], also known as ‘false flax’ and ‘gold of pleasure’, is a broadleaf, annual and fast growing plant belonging to the Brassicaceae family. It attains heights of about 60 to 110 cm. Camelina seed oil can be used as non-food feedstock for the production of biofuel [66,67] while the lignocellulosic biomass of the plant for ethanol production (second generation biofuel). The probable origin of the plant is the southeastern Europe and southwestern Asia [68]. Recent molecular evidence, however, challenges this belief and shows high genetic diversity among the Russian-Ukrainian camelina populations suggesting that this region could be the centre of the species origin [69]. This could explain why the plant germinates at low temperatures around 5°C and is very tolerant to spring frost. Although, this cool-season crop has been grown in Eastern Europe and Russia for many years until the early 1940s, only recently it has received increased attention [70]. Camelina production cost is much lower than canola and mustard production cost [71] mainly because of the low requirements in fertilizers and pesticides. The low production cost in combination with the short period from seed to harvest (85 to 100 days) make it an ideal crop for biodiesel production. Camelina is able to grow under moist soil types [72] and cold semi-arid growing regions [68]. In addition, the plant grows well on marginal land and can tolerate drought conditions. Camelina is usually seeded in spring [72,73] in temperate climates when the soil temperature is about 10°C. In the Mediterranean or warmer regions, it is advised to seed camelina in the fall because the plant cannot tolerate well temperatures above 25°C during the flowering and seed set stage. Flowers are small, self-pollinated with four petals of paleyellow color in a raceme inflorescence. Winter and spring biotypes are available. The establishment of an even crop with row spacing 12 to 14 cm enhances the competitiveness of the crop against weeds. Generally, the seeding and harvesting equipment used for canola crop is suitable for camelina [72]. Harvest by direct cutting, using a standard combine, when the pods color changes from green to golden brown and seed moisture is no more than 8% is the preferred method. The plant is not as sensitive as rapeseed (canola) to seed loss due to pod shatter. The seeds are very small and the 1000-seed weight ranges between 0.8 and 1.8 g [74,75]. Broadcast seeding rate of 6 kg/ha (~500 seeds per square meter) is recommended for maximum yield. The seeds have about 43% oil per dry weight depending on the cultivar and on the region [74]. The expected yield of camelina ranges between 106 and 907 L/ha [76]. Nowadays, the plant is mainly cultivated in the Balkans and in Russia and to a lesser extends in northern France, Belgium and Holland. In North America, camelina is widely distributed in Saskatchewan, the Maritime Provinces and the northern US Great Plains Regions [72,74]. In 2004, camelina was introduced to Montana, initially in field trials, and since then production has expanded to commercial scale. The Montana State University developed two lines in 2007 the ‘Blaine Creek’ (shortseason, high-yield line) and the ‘Suneson’ (mid-season, average yield) while Blue Sun Biodiesel developed ‘Platte’ spring variety (good yield under dry conditions). In 2010, ‘Sustainable Oils Company’ developed the ‘SO-40’, ‘SO-50’, SO-60’ spring varieties with good adaptability and high yield. Camelina varieties from Europe include ‘Celine’, ‘Calena’ and ‘Epona’ and other varieties developed by the ‘Great Plains, The Camelina Company’.
Camelina oil has unique properties as it contains about 90% unsaturated fatty acids: 25 to 42% alpha-linolenic acid (18:3), 13 to 21% linoleic acid (18:2), 14 to 20% oleic acid (18:1), 12 to 18% eicosenoic acid (20:1) and 2 to 4% erucic acid (22:1) [75]. Camelina biodiesel performance appears to be similar to biodiesel produced from other oilseeds such as rapeseed and soybean [67] with the exception of its higher iodine value (140 to 160) [74].
Pongamia (Pongamia pinnata) also known as Karanj or Karanja is a medium size (8 to 25 m height) semi-deciduous nitrogen fixing tree that belongs to the family Fabaceae or Leguminaceae and it has probably originated from India. The plant can be also found in Pakistan, Sri Lanka, northern Australia, Fiji and Japan and recently in Egypt and United States (Florida and Hawaii). Pongamia pinnata can grow in arid and waste lands. It can withstand water logging and slight frost, draught, heat and salinity [77]. Generally, it grows fast in humid tropical lowlands and matures after 4 to 7 years to produce fruits with kidneyshaped kernels [78]. Pongamia oil content of kernels ranges between 30 and 40% by weight [78,79]. The oil has light yellow color and is nonedible due to the presence of toxic flavonoids such as karanjin and diketone pongamol [79]. Pongamia oil contains mostly oleic acid (45 to 70% by weight) followed by linoleic, palmitic, and stearic acids [16,79]. The fuel properties of Pongamia biodiesel are similar to conventional diesel, but with cleaner emissions, nonopolyaromatic, with lesser toxic smoke and soot. The oil is high in oleic (44.5 to 71.3%), linoleic (10.8 to 18.3) and eicosenoic acid (9.5 to 12.4%). On the basis of these fuel properties, Pongamia biodiesel is a good option for renewable energy.
Mahua (Madhuca indica) is a tropical tree found largely in the central and northern plains and forests of India. The tree of the Sapotaceae family has long productive life (up to 60 years), evergreen foliage, is well adapted to arid environments and grows fast [80,81]. The non-edible egg shaped fruit is obtained after 4 to 7 years from planting and contains 1 to 2 kidney-shaped kernels [78]. Commercial production of seeds ultimately begins from the tenth year and seed yield ranges from 20 to 200 kg/tree [62]. The oil content of dried mahua seeds is up to 50% [82]. Storage conditions can affect the quality of the oil as the kernels are susceptible to fungi and insect attack [83]. Mahua oil is characterized by an free fatty acid content of around 20% by weight [20,80] and a relatively high percentage of saturated fatty (35.8% by weight) acids such as palmitic (17.8% by weight) and stearic (14.0% by weight) acids [84]. The remaining fatty acids are primarily distributed among unsaturated components such as oleic (46.3% by weight) and linoleic (17.9% by weight) acids [84]. The biodiesel obtained from mahua oil has fuel properties comparable to diesel when tested in compression ignition diesel engine while the exhaust emission characteristics were even better than the diesel fuel [85].
Improvement of Biodiesel Feedstocks
From the viewpoint of the farmer, high profit is the most important factor which influences the decision to grow biofuel feedstocks. Feedstock supply is dependent upon production cost which includes the cost of land, labor, capital, management and agricultural subsidies. Supply is also influenced by varieties, weather, disease, insects and mechanization. To date, the tendency for biofuel feedstocks is to increase production volume to generate profit. Better varieties with stable production over the time and adaptability to different climatic conditions have the potential to increase profit per unit of land by reducing the cost of production. Plant oils derived mainly from TAGs in seed tissues (embryo or endosperm) are used in the production of biofuels. Genetic modification of existing oilseed feedstocks to yield higher oil content and optimal fatty acid composition is a strategy to improve the yield and fuel properties of biodiesel. Although, the pathways that control their biosynthesis are well understood, little is known about how plants regulate the composition of lipids produced in seeds. Molecular biology-based plant breeding, genetic and genome engineering technologies can accelerate the genetic improvement of bioenergy feedstocks and create superior feedstocks with increased seed oil content. Seed oil content is typically a quantitative trait in nature under polygenic control (trait controlled by two or more genes) influenced by the developmental stage and environmental conditions. One strategy is to explore the natural variation present in existing and potential feedstocks. In Arabidopsis and tobacco, overexpression of diacylglycerol acyltransferase (DGAT) catalyzing the final step of TAG synthesis, increased oil content [86,87]. Interestingly, the increased seed expression of DGAT gene correlated with the increased seed oil content [86]. Therefore, DGAT gene could serve as a molecular marker to evaluate potential plant feedstocks for breeding purposes. TAG synthesis occurs through complex pathways involving multiple metabolic steps and cell compartments. Proteins that bind to DNA and regulate the expression of genes are called transcription factors (TFs) and control a multitude of genes. Several TFs known as master regulators of embryogenesis and seed maturation have been shown to control directly or indirectly the expression of genes involved in fatty acid synthesis and composition, triacylglycerol assembly and packaging in seeds. These TFs include the LEAFY COTYLEDON1 (LEC1), LEAFY COTYLEDON1-LIKE (LEC1-LIKE), LEAFY COTYLEDON2 (LEC2), ABSCISIC ACID INSENSITIVE3 (ABI3), FUSCA3 (FUS3), WRINKLED 1 (WRI-1) and GLABRA2 (GL2) [88-95]. Another potential avenue to improve feedstocks is to modify the fatty acid composition. High oleic acid content is required for biofuel production. Optimization of fatty acid composition through genetic engineering of plant feedstocks, could improve the quality of biofuel (e.g. oxidative stability, low-temperature operability, lubricity, corrosiveness). Selection for breeding and genetic engineering approaches resulting in elevated oleic acid levels have been reported in many oilseed crops [96-98]. Conventional soybean lines with 80% oleic acid accompanied by a corresponding decrease in linoleic acid were obtained based on the contribution of only two genes (FAD2-1A and FAD2-1B) [99].
Benefits and Challenges of Bioethanol
Like biodiesel, bioethanol is a renewable resource of energy and burns more cleanly in air than petroleum. However, the use of high concentrations of ethanol as biofuel would require modification of the current engines. At present, commercial-scale bioethanol is produced exclusively via first generation feedstocks. First generation bioethanol is produced from food crops, primarily corn and sugarcane, but also sugar beet, cassava and cereals like wheat [1,100,101]. Sugar and starch carbohydrates coming from feedstocks are firstly hydrolyzed and then fermented by yeasts to produce bioethanol. Besides the traditional food crops as feedstocks for bioethanol production, non-food crops such as switchgrass, miscanthus, elephant grass, artichoke, poplar and willow can also be used, thus bypassing the fuel versus food controversy. Second generation biofuels derived from plant biomass rich in ligninocellulose utilize the waste parts of food crops such as stems, leaves and stems remaining after harvest [102]. The basic steps in lignocellulosic bioethanol production is the pretreatment (e.g. acid, alkaline) of the plant cell wall to release its structural polysaccharides cellulose and hemicellulose, the polysaccharide hydrolysis to simple sugars and the subsequent conversion of sugar molecules to ethanol by fermentation [103]. Currently, the conversion of the fermentable sugars contained within the lignocellulosic biomass into ethanol poses several technical and engineering challenges. The major problem in converting lignocellulosic biomass to ethanol is the presence of lignin in the plant cell well. Lignin molecules decrease the efficiency of saccharification by inhibiting the activity of hydrolytic and fermentation enzymes and preventing their access to the polysaccharides [104]. The high recalcitrance of lignocellulosic biomass to processing requires the use of a series of chemical, biochemical and physicochemical procedures which increase the cost of second generation bioethanol production. As a result, there are no commercial scale lignocellulosic ethanol facilities presently in operation [1,102]. Moreover, the most widely used industrial ethanologenic microorganisms are not capable of efficient assimilation of all the sugars released during the biomass processing substantially reducing product yields. The generation of improved cellulases (exogenously or within the plant) for degradation of the cellulosic biomass, and lignin and cell wall manipulations are expected to increase the efficiency of the biomass conversion to ethanol. Additional genetic efforts have been undertaken to increase ethanol yield and biomass both for first and second generation feedstocks.
Promising bioethanol producing plants
The quest for alternative bioenergy crops which should satisfy the requirements for high biomass and biofuel yields with low costs, have brought attention to several potential second generation bioethanol plants. These include C4 grasses which have high photosynthetic efficiencies and are capable of growing well in conditions of limited resources thus producing high biomass even in adverse environments. Conventional breeding or genetic engineering efforts are now focused on traits suitable for bioethanol production such as high yield, minimal agricultural input and increased efficiency of lignocellulosic conversion to ethanol. United States government mandates have set a target for 136 billion liters (Bnl) of renewable fuels by 2022 of which 79 Bnl should be cellulosic bioethanol [105]. Several C3 plants such as poplar (Populus) and willow (Salix) have been given substantial attention as potential bioethanol producers. These woody species can also produce high biomass but have long growing seasons and increased lignin content. The grasses selected below have agricultural traits that make them attractive as potential bioethanol feedstocks. In particular, they are short season and excellent convertors of solar energy to chemical energy in the form of biomass; they have high efficiency of water and nitrogen usage and high capability to grow in marginal lands and poor soils. These plants would have a competitive advantage over C3 plants in case of severe climatic change accompanied by elevated temperatures and increased drought.
Sweet sorghum [Sorghum bicolor (L.) Moench] of the Poaceae family has received a lot of attention lately as it seems to accumulate many attributes that would make it a first-class biofuel feedstock in terms of energy production and sustainability. Sorghum is a grass native to Africa and has been grown traditionally as food and forage worldwide [106]. It is the fifth most important cereal in the world and a major crop in arid and semi-arid regions mainly in sub-Saharan Africa and south Asia. The United States are the biggest producers of Sorghum, followed by India, China, Nigeria, Mexico and Argentine. Although it is predominately grown for its grain, it also constitutes a good sustainable source for lignocellulose-based biofuel production [107]. It is an annual C4 plant with high efficiency of photosynthesis and limited water use. Additionally, it has short crop duration of 4 months and 1 to 3 growing seasons depending on temperate or tropical growth. The annual sugar yields could range from 3600 to 45000 kg/ha and sugar fermentation can reach bioethanol yields of 3000 to 13000 L/ha about twice the capacity potential of corn and about 1/3 more than sugarcane [108-110]. In addition, it is well adapted to many different regions and local climates and can grow in areas that have become unsuitable for maize and rice due to increased temperatures and reduced precipitation. Many Sorghum varieties are drought-tolerant and have the advantage to grow in poor quality marginal lands requiring very low input of fertilizers [111]. This avoids competition for land allocated to food crops or forests and assures minimal negative impact on the environment. Alternatively, as an annual crop it is suitable for normal crop rotation with food crops-a preferable cultivation practice rather than consuming valuable cropland. About 4000 different traditional varieties across the world [112] constitute an enormously rich collection of germplasm to be assessed for biofuel potential and to be used as a basis for crop improvement [113,114]. Studies with different sorghum varieties are underway in different parts of the world in order to identify superior genotypes and achieve best bioethanol production performance [111,115-117]. Different sweet Sorghum cultivars were tested in northern Greece to determine their productivity in stress conditions of increased salinity and reduced irrigation [115]. Finally, the sequencing of the complete Sorghum genome that was deciphered a few years back as well as recent studies of population genome-wide associations of agroclimatic traits would serve as valuable tools towards molecular breeding for new improved varieties [118,119].
Sugarcane (Saccharum spp. hybrids) is a complex polyploid grass of the Poaceae family belonging to the tribe of Andropogoneae together with maize and Sorghum. Modern sugarcane varieties are derived from interspecific crosses between the noble cane Saccharum officinarum (2n=80) and the wild S. spontaneum (2n=40 to 128). It can grow on tropical or temperate climates and diverse soil conditions. It is an exquisite C4 photosynthesizer with dry matter yield of about 70 to 80 t/ha/year. Sugarcane accounts for approximately 70% of sugar and 35% alcohol production worldwide [120]. The world’s sugarcane top producer is Brazil with approximately 700 billion tonnes annually, followed by China and India, Thailand and Pakistan [121]. Sucrose accumulates in the internodes of the stem and accounts for 50% of the dry weight [122]. After stalk harvest the crop produces sugar juice and bagasse, the dry fibrous matter left after the juice is extracted. Bioethanol is produced primarily from the sugar juice whereas bagasse as well as green tops and leaf titter constitute a good source of lignocellulosic residue with potential for biomass-based bioethanol production. In this manner, bioethanol production from sugarcane per land area is expected to increase promoting in parallel environmental sustainability [117,123].
Switchgrass (Panicum virgatum L.) of the Poaceae family is a perennial warm season C4 grass, native to North America. It has been traditionally used for soil conservation and as a forage crop and since the last decade has been considered as a potential biofuel feedstock for the production of cellulosic ethanol [124-127]. Switchgrass is categorized in two ecotypes upland and lowland and has a wide adaptability over diverse types of soils and environmental condition [128]. Lowland type is tall with coarse leaves and adapted to flood plains whereas the upland type is shorter, displaying slower growth, higher cold tolerance and is adapted to northern United States. Due to a deep-rooting system switchgrass grows very well on lands of low water and nitrogen inputs [129,130]. Its natural and agronomic advantages including high C4 photosynthetic capacity, high biomass yields in marginal lands, amenability for easy harvesting, handling and storing both as wet or dry feedstock have made it a popular non-grain cellulosic alternative to other bioethanol crops [131]. Extensive studies have been undertaken in the past several years in evaluating the biomass and ethanol producing potential of switchgrass both in small scale and field trial experiments. In field trials in US marginal croplands with moderate levels of nitrogen (N) fertilizer input, switchgrass was found to have an annual biomass yield of 5200 to 11000 kg/ha and the mean ethanol production was estimated to be 2500 L/ha (Table 1) [126]. In addition, it was found to produce 500% more renewable energy than the nonrenewable energy that was consumed for its production and was estimated to have 94% less GHG emission than gasoline [126]. Trials were also conducted in other world parts, for example, 16 different upland and lowland varieties of switchgrass were evaluated over a 5 year period in different sites in Greece and Italy [132]. Overall, lowland varieties gave higher biomass yields with a mean over sites and years of 17000 kg/ha in Greece and 12000 kg/ha in Italy.
| Plant Species | Crop Duration (Months) | Growing seasons | Capacity of Bioethanol Yield (L/ha) |
|---|---|---|---|
| Sugarcane (Sacchrum hybrid) | 7-12 | 1 | ˜5000-10000 (Sanchez & Cardona, 2008, Somerville, et.al.,) |
| Sweet Sorghum (sorghum bicolor) | 4-6 | 1 in temperate areas, 2-3 in tropical areas | ˜3000-13000 (Zhao et al., Propheter et al.,) |
| Switchgrass (Panicum vigatum) | 2-7 | 1 | ˜500-3000 (Schmer et al., Propheter et al.,) |
| Miscanthus (miscanthus giganteus) | 3-4 | 1 | ˜5000-12000 (Heaton et al., Somerville, et.al.,) |
Adapted from Byrt et al.,
Table 1: Bioethanol feedstock agronomic characteristics and ethanol yields. Yields vary widely based on varieties, location, rainfall and cultivation practices.
Miscanthus (giganteus x giganteus) a sterile hybrid between M. sinensis and M. sacchariflorus is another perennial C4 grass, native to Asia and introduced to Europe in the 1930s. It is a highly efficient photosynthesizer with low water and fertilizer requirements and the ability to store carbon in the soil [133]. Field trials have been conducted for many decades in Europe and recently in the United States in order to evaluate Miscanthus biomass potential which depends on genotype, environmental conditions, soil types and harvesting times. A large collection of data from different countries of North and South Europe evaluated Miscanthus biomass potential from different genotypes. Dry mass yield was estimated in the range of 400 to 44000 kg/ha and was higher when harvesting was performed in the autumn for all countries [134]. Southern Europe had consistently higher yields reaching maxima of 44000 kg/ha in northern Greece when Miscanthus × giganteus was harvested in September and about 35000 kg/ha in Portugal when Miscanthus × giganteus and M. sacchariflorus were harvested in the autumn [134-136]. Ethanol production has been estimated to be in the range of 5000 to 12000 L/ha depending on location [108,123].
Pretreatment of Lignocellulosic Biomass In Bioethanol Production
As stated above, the biochemical route for the production of second generation bioethanol begins with a pretreatment step designed to overcome some or all of the barriers posed to the enzymatic hydrolysis of cellulose and incur to a greater or lesser degree the fractionation of the wood components. These barriers are predominantly the presence of hemicellulose and lignin that create a matrix encasing and protecting the cellulose fibrils as well as the crystallinity of cellulose itself. Disruption of these structures as well as increasing the available surface area and biomass porosity are essential to the successful enzymatic hydrolysis of cellulose in the next process step [137].
Among the most widely utilized pretreatment methods is the acid hydrolysis of biomass with the use of dilute mineral acids. Sulfuric acid is most commonly used [138], but other acids such as nitric [139], hydrochloric [140] and phosphoric [141] have also been investigated. The pretreatment is performed at elevated temperatures and concentrations of acid as high as 4% by weight resulting in the hydrolysis of hemicellulose and partial solubilization of lignin. The acid hydrolysis shows good sugar yields and can be performed in various types of biomass such as hardwoods, agricultural byproducts and energy crops [142] as well as softwoods [143]. Due to the acidic conditions and elevated temperatures sugar degradation reactions also occur leading to products such as furfural and HMF which are formed by the acid dehydration of pentoses and hexoses, respectively. The need to neutralize the process liquid also adds to sugar loss and operating cost due to the extra process step.
Steam or hydrothermal pretreatment utilizes steam or hot water under pressure to hydrolyze the hemicellulose, whereas lignin is solubilized to a lesser extent and cellulose remains in the biomass [144]. When steam explosion is used, the sugars produced are monomers and the concentration of degradation products is higher, whereas in the case of water, a mixture of sugar oligomers and monomers is produced and the degradation reaction is better controlled [145]. Process times can be up to a few hours and most usual temperature range applied is 170 to 210°C. The mode of action here is similar to that of dilute acid but the protons required to catalyze the hydrolysis of hemicellulose come from the acetic acid released from the biomass itself and therefore no additional catalyst is added. Key to the success of the process, therefore, is the degree of hemicellulose acetylation and for that reason it has been most successfully implemented in the case of hardwoods as well as agricultural byproducts where the acetyl content of biomass can be as high as 4% by weight and not in the case of softwoods where it is usually around 1% [146].
In alkaline pretreatment, an inorganic base NaOH and Ca(OH)2, or ammonia are used to remove lignin whereas hemicellulose and cellulose are not greatly affected and remain within the biomass particle [147]. Alkaline treatments can be performed at low temperatures and longer treatment times -as low as room temperature and as long as weeks of pretreatment have been reported- compared to the acidic pretreatment that utilizes higher temperature and relatively short times, up to a few hours. Alkaline pretreatments have been most successfully implemented in the case of agricultural residues due to the less recalcitrant nature of their lignin [148].
The use of an oxidizing agent, such as ozone, hydrogen peroxide, or oxygen, has also been used for the pretreatment of biomass. Ozonation degrades lignin more specifically [149], whereas alkaline hydrogen peroxide pretreatment leads to the removal of significant amounts of both lignin and hemicellulose. Oxygen pretreatment (wet oxidation) combines the lignin degradation action of oxygen with the acid hydrolysis action from the solubilized acetic acid of the biomass leading to the oxidative degradation of lignin and the acidic hydrolysis of hemicellulose, but degradation of hemicellulose sugars to organic acids can also take place under the oxidative regime of the process [150].
In organosolv pretreatment, the mixture of water and an organic solvent such as methanol, ethanol and acetone is used to remove lignin [151]. Hemicellulose is also removed and its sugars remain in the aqueous face after distillation for separation and reuse of the organic solvent. Lignin present in the aqueous phase is precipitated and obtained as a solid stream from the process liquor. The pretreated biomass fiber consists mainly of cellulose and also contains varying amounts of hemicellulose and lignin. The main benefit of organosolv processes is that they achieve a good fractionation of all three lignocellulose components in relatively clean streams. However, the use of expensive and hazardous organic solvents which need to be recycled and reused add to the cost and environmental risk of the process.
Ammonia Fiber Expansion (AFEX) is a promising pretreatment method that utilizes the rapid decompression of ammonia pretreated biomass in order to open up the fiber structure [152]. The effects here are mainly the decrystallization of cellulose and the increase of the accessible surface area of the biomass as well as partial deacetylation and hemicellulose removal [153]. These changes enable the efficient enzymatic hydrolysis of the cellulose at low enzyme loadings, which represents the major advantage of the method.
Enzymatic Hydrolysis of Cellulose Towards Fermentable Sugars in Bioethanol Production
Enzymatic hydrolysis of the pretreated biomass represents the second step in the biochemical conversion route towards ethanol. The efficient enzymatic conversion of cellulose to glucose requires the combined use of various activities including the exoglucanases that can hydrolyse the cellulose chain from the ends and produce cellobiose, the endoglucanases that can produce new ends within the cellulose chain and the β-glucosidases that hydrolyze cellobiose to glucose. The most widely studied cellulase system is that of the filamentous fungus T. reesei [154]. The anaerobic bacterium Clostridium thermocellum is another interesting source of cellulolytic enzymes with a different strategy to T. reesei. Here, the various enzymes required to hydrolyze cellulose are organized to a protein called scaffoldin and form a complex structure known as the cellulosome which is attached to the bacterial membrane [155].
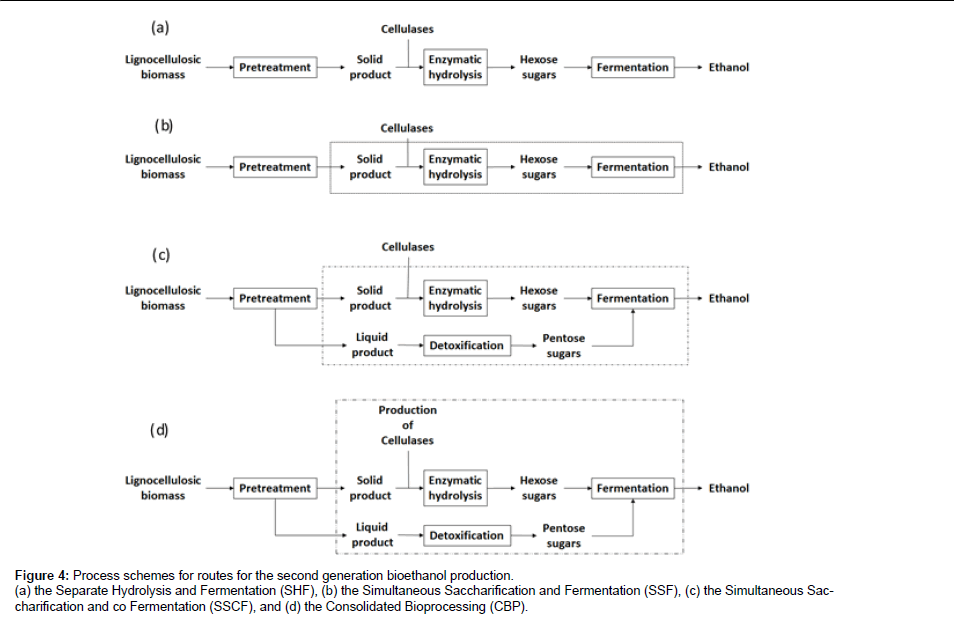
Enzymatic hydrolysis can be performed in tandem with the consequent fermentation in which case the Separate Hydrolysis and Fermentation strategy (SHF) is employed (Figure 4a). This enables the operation of the separate steps of hydrolysis and fermentation to be run at optimal conditions which are 55°C for the hydrolysis with T. reesei cellulases and 30°C for the fermentation with S. cerevisiae yeast, and thus achieve maximum yields for both steps. This, however, requires two separate unit operation which increases the overall complexity and cost of the process. A different approach is the Simultaneous Saccharification and Fermentation (SSF) where the enzymatic hydrolysis and the fermentation of produced sugars is done simultaneously in the same vessel but at suboptimal conditions for both operations i.e. around 37°C (Figure 4b). The advantages of SSF include lower capital cost and higher yield [156], reduced end product inhibition of enzymatic hydrolysis by the produced glucose [157] and a greater tolerance to the inhibitors formed in the pretreatment step since they can be partially metabolized by the fermenting microorganisms [158].
Figure 4: Process schemes for routes for the second generation bioethanol production.
(a) the Separate Hydrolysis and Fermentation (SHF), (b) the Simultaneous Saccharification and Fermentation (SSF), (c) the Simultaneous Saccharification
and co Fermentation (SSCF), and (d) the Consolidated Bioprocessing (CBP).
The co-fermentation of the pentose sugars produced in the pretreatment step (Simultaneous Saccharification and co-Fermentation, SSCF) can be achieved with the co-culture of a hexose and a pentose fermenting microorganism such as P. stipitis and Brettanomyces clausennii [159] (Figure 4c). The Consolidated Bioprocessing (CBP) would allow the simultaneous production of cellulolytic and hemicellulolytic enzymes and the fermentation of pentoses and hexoses to ethanol in a one pot approach [160] (Figure 4d). This would require the genetic engineering of a cellulase producing microorganism to ferment ethanol, or a natural sugar fermenter to express a cellulase system [160]. Alternatively a naturally occurring microorganism such as Fusarium oxysporum, able to produce cellulase and hemicellulase enzymes and perform ethanolic fermentation, can also be utilized [161].
Thermochemical Processing of Lignocellulosic Biomass for Biofuels Production
The thermo chemical route for the conversion of biomass into biofuels comprises four major strategies: (a) the gasification of biomass and the production of hydrocarbons [162] via a process known as Biomass to Liquid (BTL), (b) the fast pyrolysis of biomass for the production of bio-oil, which can be performed with or without the addition of catalysts [163], (c) the liquefaction of biomass at in a water or water-solvent mixture at sub or supercritical conditions for the production of bio-oil [164] and (d) the production of hydrocarbons from oxygen containing degradation products of sugars, such as furans and acids, via catalytic routes [165].
The BTL process begins with the gasification of biomass at high temperatures in the presence of an oxidizer (oxygen, air, etc.) for the production of syngas (CO+H2). The synthesis gas is then cleaned from the char and tar which are also formed during the gasification reactions and steam reformed to control the CO to H2 ratio. The synthesis gas is then used as a feed for the Fischer-Tropsch synthesis of high molecular weight linear hydrocarbons (waxes). Typical catalysts used in the lower temperature Fischer-Tropsch reaction (220 to 240°C) are cobalt (Co) catalysts supported on high surface area oxides, such as silica and alumina, and may also contain various amounts of metal or oxide promoters [166]. When iron (Fe) catalysts are used at higher temperatures (200 to 350°C) olefinic hydrocarbons and alcohols are mainly produced, but the iron catalysts exhibit lower conversion rates and lifetime [167]. Finally, the waxes produced in the Fischer-Tropsch can be converted to green diesel and gasoline range hydrocarbons through catalytic hydro cracking over NiMo catalysts supported over a silica-alumina oxide [168]. Alternatively conventional cracking over various acidic microporous (zeolites H-Y and H-ZSM-5) and mesoporous (amorphous silica-alumina and Al-MCM-41) catalysts can be implemented for the production of hydrocarbons in the gasoline and LPG range [169].
The fast pyrolysis of biomass is performed at temperatures between 400 and 550°C and yields a complex mixture of chemicals termed pyrolysis oil or bio-oil which comprises of an aqueous phase and an organic phase. Char and gases are also formed at lower yields. In slow pyrolysis lower temperatures are employed (typically 250 to 350°C), carbonization takes place and higher char yields are achieved [163]. The bio-oil is a complex mixture of various oxygenated compounds derived from the fragmentation, depolymerization and degradation of the major biomass components cellulose, hemicellulose and lignin. These include acids, alcohols, aldehydes, esters, ketones, sugars, phenols, guaiacols, syringols, furans, lignin derived phenols and extractible terpene [170]. The major problems with bio-oil are the high water content, high oxygen content, high acidity, low heating value and low stability which make its storage, transportation and use problematic. The catalytic fast pyrolysis aims at improving the bio-oil properties with the introduction of various catalysts such as mesoporous aluminosilicate AlMCM-41 [171] that lowered acids and increased aromatic concentrations. It was shown however that increased catalyst acidity reduced the organic phase and increased the aqueous phase yields [172,173]. However, the relative high deoxygenation and the increased concentration of aromatics and/or phenols of bio-oil are considered as the major advantages of catalytic fast pyrolysis and can be achieved by selecting the appropriate catalytic systems [174-176]. The produced bio-oil can be further upgraded in situ during pyrolysis by the use of metal zeolites, such as iron modified zeolites affecting the yields and composition of bio-oil [177] or can be upgraded down-stream via hydrodeoxygenation to produce non-oxygenated hydrocarbons, and subsequently via coprocessing (cracking) of the deoxygenated hydrocarbon-rich stream in mixtures with gas-oil [178-181] to produce green gasoline. The aqueous phase of bio-oil which is enriched in lower molecular weight oxygenates (i.e acetic acid) can be catalytically upgraded towards hydrogen (as fuel, also for fuel cells) via catalytic steam reforming [182,183].
The hydrothermal biomass liquefaction is a thermo chemical biomass transformation route for the production of bio-oil. The complexity of the bio-oil composition is similar to that from the fast pyrolysis route. The temperatures employed are usually higher than those employed in the pretreatment technologies in the biochemical route (i.e 150 to 200°C) and can reach as high as 375°C [184]. The solvents used can be water [185], an alcohol-water mixture [186] or acetone [184] under subcritical or supercritical conditions [186]. Non catalytic liquefaction as well as the use of a variety of catalysts such as alkalis [184] and ZnCl2 [187] have been reported. Solvolysis (hydrolysis) and depolymerization of the biomass polymers, as well as reduction of the oxygen content of the hydrolysis and degradation products, through dehydration and decarboxylation reactions are thought to occur during the liquefaction [164].
A more recent approach, that of carbohydrate to hydrocarbons process is based on the conversion of C5 (xylose) and C6 (glucose, fructose) to their respective furans, furfural and HMF, via acid catalyzed dehydration reactions in aqueous reaction media. The catalysts employed in this reaction can be, for example, zeolites and ion exchange resins [188,189] and modified mesoporous materials [190]. These furans can be further dehydrated to yield levulinic acid and levulinate esters which in turn can be converted to g-valerolactone via hydrogenation and then via decarboxylation and oligomerization reactions can give C8 alkenes [191]. Another route is the production of alcohols from the hydrogenation or hydrodeoxygenation of sugars, which can be dehydrated to form alkenes and then to olefins via oligomerization.
Improvement of Bioethanol Feedstocks
Dedicated energy crops with traits suitable for bioethanol production are needed to meet the current and future expectations of the industry. Bioethanol production is currently based on sucrose or starch derived from grains or vegetative biomass. Genetic improvement of sucrose or starch yield can be accomplished by traditional breeding and new biotechnological approaches to allow greater efficiency and precision. However, breeding efforts to develop improved varieties with increased ethanol yield have been limited by the lack of understanding of the factors involved in alcohol production as there is evidence for both genetic and environmental contribution.
Further down, the lignocellulosic bioethanol technology is not as mature as the starch or sucrose based bioethanol technology. As stated above, the main challenge in lignocellulosic bioethanol is not so much the yield since lignocellulosic biomass is the most abundant carbon source in the planet, but its conversion to fermentable sugars due to lignin presence. Therefore, reducing the levels of lignin or modifying lignin composition could increase the biorefinery efficiencies. Towards this aim, two main approaches can be employed: 1) the classical breeding strategy; and 2) the genetic manipulation approach. The traditional approach to decrease lignin levels is to take advantage of the natural genetic variability in the quantity and quality of lignin of the plant cell wall. It has been reported that lignin content varied from 23 to 34% in Populus, while the S/G ratio ranged from 1.8 to 2.3 [192,193]. So, it remains to be demonstrated whether this variability in lignin composition can have an effect on the conversion efficiency. Moreover, modern molecular biology-based techniques can efficiently exploit the natural genetic diversity of feedstocks and benefit breeding efforts. Genomic regions associated with lignin content were detected in Populus and Eucalyptus, providing markers for the identification and development of plants with reduced lignin content [194-196]. However, cultivation conditions can also alter lignin content and composition, as it has been demonstrated for nitrogen supply [197,198]. Therefore, the use of a feedstock with reduced lignin content or modified lignin composition in combination with optimized cultivation conditions will generate an energy crop with enhanced conversion of biomass.
At a molecular level, the modification of the lignin biosynthetic machinery can be achieved by manipulation of the genes involved in lignin biosynthesis or the key transcription factors regulating lignin pathway. In Populus, several genes of the lignin biosynthetic pathway have been perturbed, such as 4-COUMARATE CoA LIGASE (4CL1), CINNAMYL ALCOHOL DEHYDROGENASE (CAD), CAFFEIC ACID 3-O-METHYLTRANSFERASE (COMT), S-ADENOSYL-METHIONINE CAFFEOYL-CoA (CCoAOMT), FERULATE 5-HYDROXYLASE (F5H) resulting in different levels of lignin reduction [199-203], for review [204]. The over expression of the pine cytosolic gene GLUTAMINE SYNTHETASE (GS1a) in Populus and the ectopic expression of the Eucalyptus EgMYB1 gene encoding a transcription factor gave also some promising results [205,206].
In Sorghum, a gene operating in the flowering pathway and determining flowering time was reported recently as a good molecular marker to employ when breeding for Sorghum varieties suitable for biofuel production. Specifically, activation of the Maturity Locus 1(ML1) gene (called PRR37) resulted in delayed flowering but increased plant height, producing plants with as much as three times the amount of stem and leaf matter [207]. This would be desirable when growing Sorghum for high biomass to produce second generation lignocellulosic-based biofuel. Furthermore, in a recent study in sugarcane, genes involved in lignin biosynthesis were RNAi-suppressed resulting in 8 to 12% lignin reduction and consequently 28 to 32% increase in saccharification efficiency [208].
In switchgrass, a great number of investigations have focused on improving bioethanol relevant traits by the aforementioned approaches of classical breeding or genomic technologies [209,210]. Notably, crosses between tetraploid upland and lowland switchgrass resulted in hybrid vigor and progeny displayed increase of biomass yields in the range of 30-60% pointing to exploiting heterosis for generating elite cultivars [211]. Recently a genetic linkage map was developed by crossing upland and lowland switchgrass ecotypes which will provide a complete switch grass map and enable the identification of quantitative trait loci associated with biomass quantity and quality characters as well as cold tolerance and abiotic stress traits [212]. At the level of specific gene targeting, efforts to reduce lignin content focused on the down regulation of the 4CL1, COMT and CAD genes that are involved in lignin biosynthesis [213-215] and the over expression of the R2R3-MYB transcription factor [216,217]. Interestingly, a transgenic switch grass that over expresses the maize Corngrass1 (Cg1) microRNA, which is a micro RNA that locks the plant in the juvenile stage has reduced lignin levels, 250% more starch and higher saccharification efficiency [218].
Genetic improvement of Miscanthus has focused on exploring the natural germplasm, as well as creating hybrids and increasing polyploidation, towards cultivars and hybrids suitable for bioethanol production [219,220] Different Miscanthus genotypes have been assessed for low lignin content as high lignin content compromises fermentation efficiencies and increases pretreatment costs [221]. Transgenic approaches in this plant are limited since transformation is not developed as yet.
Reflections on Future Biofuel Feedstock Development
ographic distribution and dedicated to biofuel production will eventually increase feedstock quantities and stabilize prices. Currently, most of the feedstocks have multiple uses and the demand of the different end consumers keeps the prices high. A broad portfolio of plant feedstocks suitable for biofuel production will minimize dependency on one feedstock and potential biofuel market instability due to the impact of yield shocks on biofuel prices. Using new non-food feedstocks able to grow on marginal lands with low input may be another way to reduce the cost of biofuels and increase biofuel feedstock supply without competing with food markets. With the majority of the world’s poor population depending on agriculture for living, producing biofuels locally can create new jobs and lead to rural development. Land availability constraints to produce the feedstocks required to comply with the blending mandates set in Europe and other countries will require efforts to continue increasing feedstock yield, efficiency in biomass conversion processes and making use of abandoned land. In case agricultural and forestry residues are used in second-generation biofuel industries, no additional land is needed for cultivation since the currently available feedstock sources can be used in the initial stage of production. This in turn, could be beneficial to smallholder farmers and the domestic economy. Establishing woody energy crops is a long term project but has positive environmental attributes as it requires little tillage, reduces runoff of fertilizers and pesticides and supports habitat diversity for plant and animal species. Investment on the identification of the most suitable biofuel feedstocks for a given country, region and local climate will entail characterization of existing plant germplasm collections and molecular biologybased plant breeding and/or genetic modification of feedstocks. Other advances in technologies related to harvesting, processing and transportation of plant feedstocks would play a significant role in reducing costs and increasing biofuel production.
References
- Saunders J, Izydorczyk M, Levin DB (2011) Limitations and Challenges for Wheat-Based Bioethanol Production. Economic Effects of Biofuel Production, Dr Marco Aurelio Dos Santos Bernardes (Ed), Canada.
- Holzman DC (2008) The carbon footprint of biofuels: can we shrink it down to size in time? Environ Health Perspect 116: A246-252.
- de Vries SC, van de Ven GWJ, van Ittersum MK, Giller KE (2010) Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass and Bioenergy 34: 588-601.
- Pahl G (2005) Biodiesel: Growing a New Energy Economy. Chelsea Green Publishing, pp. 368.
- Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresource Technology 70: 1-15.
- Fjerbaek L, Christensen KV, Norddahl B (2009) A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol Bioeng 102: 1298-1315.
- Graboski MS, McCormick RL (1998) Combustion of fat and vegetable oil derived fuels in diesel engines. Progress in Energy and Combustion Science 24:125-164.
- Yamane K, Ueta A, Shimamoto Y (2001) Influence of physical and chemical properties of biodiesel fuels on injection, combustin and exhaust emission characteristics in a direct injection compression ignition engine. International Journal of Engine Research 2:249-261.
- Knothe G, Steidley KR (2005) Lubricity of Components of Biodiesel and Petrodiesel. The Origin of Biodiesel Lubricity. Energy & Fuels 19: 1192-1200.
- Stournas S, Lois E, Serdari A (1995) Effects of fatty acid derivatives on the ignition quality and cold flow of diesel fuel. Journal of the American Oil Chemists' Society 72: 433-437.
- DeOliveira E, Quirino RL, Suarez PAZ, Prado AGS (2006) Heats of combustion of biofuels obtained by pyrolysis and by transesterification and of biofuel/diesel blends. Thermochimica Acta450: 87-90.
- Knothe G (2008) Designer Biodiesel: Optimizing Fatty Ester Composition to Improve Fuel Properties. Energy & Fuels 22: 1358-1364.
- Patil PD, Gude VG, Deng S (2009) Biodiesel Production from Jatropha Curcas, Waste Cooking, and Camelina Sativa Oils. Industrial & Engineering Chemistry Research 48:10850-10856.
- Kumar Tiwari A, Kumar A, Raheman H (2007) Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass and Bioenergy 31: 569-575.
- Naik M, Meher LC, Naik SN, Das LM (2008) Production of biodiesel from high free fatty acid Karanja (Pongamia pinnata) oil. Biomass and Bioenergy 32: 354-357.
- Giannelos PN, Zannikos F, Stournas S, Lois E, Anastopoulos G (2002) Tobacco seed oil as an alternative diesel fuel: physical and chemical properties. Industrial Crops and Products 16: 1-9.
- Usta N (2005) Use of tobacco seed oil methyl ester in a turbocharged indirect injection diesel engine. Biomass and Bioenergy 28: 77-86.
- Veljkovic VB, Lakicevic SH, Stamenkovic OS, Todorovic ZB, Lazic ML (2006) Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 85: 2671-2675.
- Ghadge SV, Raheman H (2005) Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass and Bioenergy 28: 601-605.
- Jena PC, Raheman H, Prasanna Kumar GV, Machavaram R (2010) Biodiesel production from mixture of mahua and simarouba oils with high free fatty acids. Biomass and Bioenergy 34: 1108-1116.
- Azam MM, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass and Bioenergy 29: 293-302.
- de Oliveira D, Di Luccio M, Faccio C, Rosa CD, Bender JP, et al. (2004) Optimization of enzymatic production of biodiesel from castor oil in organic solvent medium. Appl Biochem Biotechnol 113-116: 771-80.
- Georgogianni KG, Kontominas MG, Pomonis PJ, Avlonitis D, Gergis V (2008) Alkaline Conventional and in Situ Transesterification of Cottonseed Oil for the Production of Biodiesel. Energy & Fuels 22: 2110-2115.
- Moser BR, Vaughn SF (2010) Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra low-sulfur diesel fuel. Bioresour Technol 101: 646-653.
- Peterson CL, Reece DL, Hammond BL, Thompson J, Beck SM (1997) Processing, characterization, and performance of eight fuels from lipids. Applied Engineering in Agriculture 13: 71-79.
- Else MA, Coupland D, Dutton L, Jackson MB (2001) Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiologia Plantarum111: 46-54.
- Gui MM, Lee KT, Bhatia S (2008) Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy33: 1646-1653.
- Anjani K (2010) Extra-early maturing germplasm for utilization in castor improvement. Industrial Crops and Products 31: 139-144.
- Baldanzi M, Fambrini M, Pugliesi C (2003) Redesign of the castorbean plant body plan for optimal combine harvesting. Annals of Applied Biology 142: 299-306.
- Babita M, Maheswari M, Rao LM, Shanker AK, Rao DG (2010) Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environmental and Experimental Botany 69: 243-249.
- Auld DL, Rolfe RD, McKeon TA (2001) Development of Castor with Reduced Toxicity. Journal of New Seeds 3: 61-69.
- Halling KC, Halling AC, Murray EE, Ladin BF, Houston LL, et al. (1985) Genomic cloning and characterization of a ricin gene from Ricinus communis. Nucleic Acids Res 13: 8019-8033.
- Tregear JW, Roberts LM (1992) The lectin gene family of Ricinus communis: cloning of a functional ricin gene and three lectin pseudogenes. Plant Mol Biol 18: 515-525.
- Rojas-Barros P, De Haro A, Munoz J, Fernandez-Martinez JM, Maria J (2004) Isolation of a Natural Mutant in Castor with High Oleic/Low Ricinoleic Acid Content in the Oil. Crop Science 44: 76-80.
- Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, et al. (2010) Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol 28: 951-956.
- Allan G, Williams A, Rabinowicz PD, Chan AP, Ravel J, et al. (2008) Worldwide genotyping of castor bean germplasm (Ricinus communis L.) using AFLPs and SSRs. Genetic Resources and Crop Evolution 55: 365-378.
- Foster JT, Allan GJ, Chan AP, Rabinowicz PD, Ravel J, et al. (2010) Single nucleotide polymorphisms for assessing genetic diversity in castor bean (Ricinus communis). BMC Plant Biol 10: 13.
- Severino LS, Auld DL, Baldanzi M, Candido MJD, Chen G, et al. (2012) A Review on the Challenges for Increased Production of Castor. 104: 853-880.
- Comar V, Tilley D, Feliz E, Turdera M, Chagas Neto M (2004) Comparative emergy evaluation of castorbean (Ricinus communis) production systems in Brazil and the US. In. Ortega E, Ulgiati S (Eds), Proceedings of the IV Biennial International Workshop “Advances in Energy Studiesâ€, Unicamp, Campinas, SP, Brazil, pp. 227-237.
- Albuquerque MCG, Machado YL, Torres AEB, Azevedo DCS, Cavalcante Jr CL (2009) Properties of biodiesel oils formulated using different biomass sources and their blends. Renewable Energy 34: 857-859.
- Refaat AA (2009) Correlation between the chemical structure of biodiesel and its physical properties. International Journal of Environmental Science and Technology 6: 677-694.
- Canoira L, GarcÃa Galeán J, Alcántara R, Lapuerta M, GarcÃa-Contreras R (2010) Fatty acid methyl esters (FAMEs) from castor oil: Production process assessment and synergistic effects in its properties. Renewable Energy 35: 208-217.
- Berman P, Nizri S, Wiesman Z (2011) Castor oil biodiesel and its blends as alternative fuel. Biomass and Bioenergy 35: 2861-2866.
- Scholz V, da Silva JN (2008) Prospects and risks of the use of castor oil as a fuel. Biomass and Bioenergy 32: 95-100.
- ConceiçÃo MM, Candeia RA, Silva FC, Bezerra AF, Fernandes Jr VJ, et al. (2007) Thermoanalytical characterization of castor oil biodiesel. Renewable and Sustainable Energy Reviews 11: 964-975.
- Ceasar SA, Ignacimuthu S (2011) Applications of biotechnology and biochemical engineering for the improvement of Jatropha and Biodiesel: A review. Renewable and Sustainable Energy Reviews 15: 5176-5185.
- Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): A review. Industrial Crops and Products 28: 1-10.
- Carels N (2009) Chapter 2 Jatropha curcas. A Review. In: Advances in Botanical Research. 50: 39-86.
- Fairless D (2007) Renewable energy: energy-go-round. Nature 447: 1046-1048.
- Kaushik N, Kumar K, Kumar S, Kaushik N, Roy S (2007) Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass and Bioenergy 31: 497-502.
- Sunil N, Varaprasad KS, Sivaraj N, Suresh Kumar T, Abraham B, Prasad RBN (2008) Assessing Jatropha curcas L. germplasm in-situ-A case study. Biomass and Bioenergy 32: 198-202.
- Divakara BN, Upadhyaya HD, Wani SP, Gowda CLL (2010) Biology and genetic improvement of Jatropha curcas L: A review. Applied Energy 87: 732-742.
- Basha SD, Sujatha M (2009) Genetic analysis of Jatropha species and interspecific hybrids of Jatropha curcas using nuclear and organelle specific markers. Euphytica 168: 197-214.
- Mastan SG, Sudheer PD, Rahman H, Ghosh A, Rathore MS, et al. (2012) Molecular characterization of intra-population variability of Jatropha curcas L. using DNA based molecular markers. Mol Biol Rep 39: 4383-4390.
- Sun Q-B, Li L-F, Li Y, Wu G-J, Ge X-J (2008) SSR and AFLP Markers Reveal Low Genetic Diversity in the Biofuel Plant Jatropha curcas in China. Crop Science 48: 1865-1871.
- Tong L, Shu-Ming P, Wu-Yuan D, Dan-Wei M, Ying X, et al. (2006) Characterization of a new stearoyl-acyl carrier protein desaturase gene from Jatropha curcas. Biotechnol Lett 28: 657-662.
- Ye J, Qu J, Bui HT, Chua NH (2009) Rapid analysis of Jatropha curcas gene functions by virus-induced gene silencing. Plant Biotechnol J 7: 964-976.
- Banerji R, Chowdhury AR, Misra G, Sudarsanan G, Verma SC, Srivastava GS (1985) Jatropha seed oils for energy. Biomass 8: 277-282.
- Akbar E, Yaakob Z, Kamarudin SK, Ismail M, Salimon J (2009) Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. European Journal of Scientific Research 29: 396-403.
- Padhi SK, Singh RK (2011) Non-edible oils as the potential source for the production of biodiesel in India: A review. Journal of Chemical and Pharmaceutical Research 3: 39-49.
- Karmakar A, Karmakar S, Mukherjee S (2012) Biodiesel production from neem towards feedstock diversification: Indian perspective. Renewable and Sustainable Energy Reviews 16: 1050-1060.
- Fazal MA, Haseeb A, Masjuki HH (2011) Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renewable & Sustainable Energy Reviews 15: 1314-1324.
- Karmakar A, Karmakar S, Mukherjee S (2012) Biodiesel production from neem towards feedstock diversification: Indian perspective. Renew Sust Energ Rev 16: 11-11
- Budin JT, Breene WM, Putnam DH (1995) Some compositional properties of camelina (Camelina sativa L. Crantz) seeds and oils. JAOCS, Journal of the American Oil Chemists' Society 72: 309-315.
- Fröhlich A, Rice B (2005) Evaluation of Camelina sativa oil as a feedstock for biodiesel production. Industrial Crops and Products 21: 25-31.
- Francis A, Warwick SI (2009) The Biology of Canadian Weeds. 142. Camelina alyssum (Mill.) Thell.; C. microcarpa Andrz. ex DC.; C. sativa (L.) Crantz. Canadian Journal of Plant Science 89: 791-810.
- Ghamkhar K, Croser J, Aryamanesh N, Campbell M, Kon'kova N, et al. (2010) Camelina (Camelina sativa (L.) Crantz) as an alternative oilseed: molecular and ecogeographic analyses. Genome 53: 558-567.
- Pavlista AD, Isbell TA, Baltensperger DD, Hergert GW (2011) Planting date and development of spring-seeded irrigated canola, brown mustard and camelina. Industrial Crops and Products 33: 451-456.
- Berti M, Wilckens R, Fischer S, Solis A, Johnson B (2011) Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Industrial Crops and Products 34: 1358-1365.
- Gugel RK, Falk KC (2006) Agronomic and seed quality evaluation of Camelina sativa in western Canada. Canadian Journal of Plant Science 86: 1047-1058.
- Urbaniak SD, Caldwell CD, Zheljazkov VD, Lada R, Luan L (2008) The effect of seeding rate, seeding date and seeder type on the performance of Camelina sativa L. in the Maritime Provinces of Canada. Canadian Journal of Plant Science 88: 501-508.
- Zubr J (1997) Oil-seed crop Camelina sativa. Industrial Crops and Products 6: 113-119.
- Vollmann J, Moritz T, Kargl C, Baumgartner S, Wagentristl H (2007) Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Industrial Crops and Products 26: 270-277.
- Moser BR (2010) Camelina (Camelina sativa L.) oil as a biofuels feedstock: Golden opportunity or false hope? Lipid Technology 22: 270-273.
- Vivek, Gupta AK (2004) Biodiesel production from Karanja oil. Journal of Scientific and Industrial Research 63: 39-47.
- Mohibbe Azam M, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass and Bioenergy 29: 293-302.
- Karmee SK, Chadha A (2005) Preparation of biodiesel from crude oil of Pongamia pinnata. Bioresour Technol 96: 1425-1429.
- Ghadge SV, Raheman H (2006) Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresour Technol 97: 379-384.
- Kumari V, Shah S, Gupta MN (2006) Preparation of Biodiesel by Lipase-Catalyzed Transesterification of High Free Fatty Acid Containing Oil from Madhuca indica. Energy & Fuels 21: 368-372.
- Bhatt YC, Murthy NS, Datta RK (2004) Use of mahua oil (Madhuca indica) as a diesel fuel extender. Journal of the Institution of Engineers (India): Agricultural Engineering Division 85: 10-14.
- Puhan S, Vedaraman N, Rambrahamam BV, Nagarajan G (2005) Mahua (Madhuca indica) seed oil: A source of renewable energy in India. Journal of Scientific and Industrial Research 64: 890-896.
- Singh A, Singh IS (1991) Chemical evaluation of mahua (Madhuca indica) seed. Food Chemistry 40: 221-228.
- Puhan S, Vedaraman N, Ram BVB, Sankarnarayanan G, Jeychandran K (2005) Mahua oil (Madhuca Indica seed oil) methyl ester as biodiesel-preparation and emission characterstics. Biomass and Bioenergy 28: 87-93.
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, et al. (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126: 861-874.
- Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, Spitsin S, Flynn J, Matyszczuk P, Andryszak K, Laurelli M et al (2010) Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnology Journal 8: 277-287.
- Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13: 624-630.
- Mu J, Tan H, Zheng Q, Fu F, Liang Y, et al. (2008) LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol 148: 1042-1054.
- Shen B, Allen WB, Zheng P, Li C, Glassman K, et al. (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol 153: 980-987.
- Tan H, Yang X, Zhang F, Zheng X, Qu C, et al. (2011) Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol 156: 1577-1588.
- Berger N, Dubreucq B, Roudier F, Dubos C, Lepiniec L (2011) Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell 23: 4065-4078.
- Liu J, Hua W, Zhan G, Wei F, Wang X, et al. (2010) Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol Biochem 48: 9-15.
- Baud S, Wuillème S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60: 933-947.
- Shi L, Katavic V, Yu Y, Kunst L, Haughn G (2012) Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J 69: 37-46.
- Stoutjesdijk PA, Hurlestone C, Singh SP, Green AG (2000) High-oleic acid Australian Brassica napus and B. juncea varieties produced by co-suppression of endogenous Delta12-desaturases. Biochem Soc Trans 28: 938-940.
- Hu X, Sullivan-Gilbert M, Gupta M, Thompson SA (2006) Mapping of the loci controlling oleic and linolenic acid contents and development of fad2 and fad3 allele-specific markers in canola (Brassica napus L.). Theor Appl Genet 113: 497-507.
- Liu Q, Singh SP, Green AG (2002) High-stearic and High-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol 129: 1732-1743.
- Pham AT, Lee JD, Shannon JG, Bilyeu KD (2010) Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol 10: 195.
- CRFA-2009: Industry information. Canadian Renewable Fuels Association (2009).
- Sanchez OJ, Montoya S (2013) Biofuel Technologies: Production of Bioethanol from Biomass: An Overview. Springer Berlin Heidelberg pp 397-441.
- Jordan DB, Bowman MJ, Braker JD, Dien BS, Hector RE, et al. (2012) Plant cell walls to ethanol. Biochem J 442: 241-252.
- Berlin A, Balakshin M, Gilkes N, Kadla J, Maximenko V, et al. (2006) Inhibition of cellulase, xylanase and beta-glucosidase activities by softwood lignin preparations. J Biotechnol 125: 198-209.
- Kimber C (2000) Sorghum: Origin, History, Technology, and Production. John Wiley and Sons, New York.
- Xin Z, Wang ML (2011) Sorghum as a versatile feedstock for bioenergy production. Biofuels 2: 577-588.
- Byrt CS, Grof CP, Furbank RT (2011) C4 plants as biofuel feedstocks: optimising biomass production and feedstock quality from a lignocellulosic perspective. J Integr Plant Biol 53: 120-135.
- Almodares A, Hadi MR (2009) Production of bioethanol from sweet sorghum: A review. African Journal of Agricultural Research 4: 772-780.
- Zhao YL, Dolat A, Steinberger Y, Wang X, Osman A, Xie GH (2009) Biomass yield and changes in chemical composition of sweet sorghum cultivars grown for biofuel. Field Crops Research 111: 55-64.
- Rutto L, Xu Y, Brandt M, Ren S, Kering M (2013) Juice, Ethanol, and Grain Yield Potential of Five Sweet Sorghum (Sorghum bicolor [L.] Moench) Cultivars. Journal of Sustainable Bioenergy Systems 3: 113-118.
- Grassi G, Tondi G, Helm P (2004) Small sized Commercial Bioenergy Technologies as an Instrument of Rural Development Biomass and Agriculture: Sustainability, Markets and Policies OECD Publication Service, Paris pp 277-287.
- Deu M, Rattunde F, Chantereau J (2006) A global view of genetic diversity in cultivated sorghums using a core collection. Genome 49: 168-180.
- Upadhyaya HD (2009) Developing a mini core collection of sorghum for diversified utilization of germplasm. Crop Science 49: 1769-1780.
- Vasilakoglou I, Dhima K, Karagiannidis N, Gatsis T (2010) Sweet sorghum productivity for biofuels under increased soil salinity and reduced irrigation. Field Crops Research 120: 38-46.
- Ratnavathi CV, Suresh K, Kumar BSV, Pallavi M, Komala VV, Seetharama N (2010) Study on genotypic variation for ethanol production from sweet sorghum juice. Biomass and Bioenergy 34: 947-952.
- Propheter JL, Staggenborg SA, Wu X, Wang D (2010) Performance of Annual and Perennial Biofuel Crops: Yield during the First Two Years. Agron J 102: 806-814.
- Morris GP, Ramu P, Deshpande SP, Hash CT, Shah T, et al. (2013) Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc Natl Acad Sci U S A 110: 453-458.
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551-556.
- Goldemberg J, Guardabassi P: Ethanol (2010) Can the success of Brazil be replicated? Biofuels 1: 663-665.
- Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM (2010) Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol J 8: 263-276.
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329: 790-792.
- Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100: 1515-1523.
- McLaughlin SB, Adams Kszos L (2005) Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass and Bioenergy 28: 515-535.
- Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A 105: 464-469.
- Sanderson MA, Reed RL, McLaughlin SB, Wullschleger SD, Conger BV, Parrish DJ, Wolf DD, Taliaferro C, Hopkins AA, Ocumpaugh WR et al (1996) Switchgrass as a sustainable bioenergy crop. Bioresource Technology 56: 83-93.
- Moser LE, Vogel KP (1995) Forages An Introduction to Grassland Agriculture: Switchgrass, big bluestem, and indiangrass. Iowa State University Press 1: 409-420.
- Vogel K (2004) Switchgrass. In Warm-season (C4) grasses: Agronomy Monograph 45 Madison: ASA, CSSA, and SSSA pp 561-588.
- Parrish DJ, Fike JH (2005) The Biology and Agronomy of Switchgrass for Biofuels. Critical Reviews in Plant Sciences pp 423-459.
- Wullschleger SD, Davis EB, Borsuk ME, Gunderson CA, Lynd LR (2010) Biomass Production in Switchgrass across the United States: Database Description and Determinants of Yield. Agron J 102: 1158-1168.
- Alexopoulou E, Sharma N, Papatheohari Y, Christou M, Piscioneri I, Panoutsou C, Pignatelli V (2008) Biomass yields for upland and lowland switchgrass varieties grown in the Mediterranean region. Biomass and Bioenergy 32: 926-933.
- Heaton EA, Dohleman FG, Long SP (2008) Meeting US biofuel goals with less land: the potential of Miscanthus. Global Change Biology 14: 2000-2014.
- Brosse N, Dufour A, Meng X, Sun Q, Ragauskas A (2012) Miscanthus: a fast-growing crop for biofuels and chemicals production. Biofuels, Bioproducts and Biorefining 6: 580-598.
- Danalatos NG, Dalianis C, Kyritsis S (1996) Growth and biomass productivity of Miscanthus sinensis “giganteus†under optimum cultural management in north-eastern Gree. Biomass for Energy and the Environment:Proceedings of the 9th European Bioenergy Conference, Copenhagen, Denmark, June 24-27, 1996, ed by Chartier P, Ferrero GL, Henius UM, Hultberg S, Sachau J and Wiinblad M Pergamon, New York ,NY, USA pp 548-553.
- Lewandowski I, Clifton-Brown JC, Andersson B, Basch G, Christian DG, JΓ?rgensen U, Jones MB, Riche AB, Schwarz KU, Tayebi K et al (2003) Environment and Harvest Time Affects the Combustion Qualities of Miscanthus Genotypes. Agron J 95: 1274-1280.
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour Technol 101: 4851-4861.
- Chen R, Zhangwen W, Lee YY (1998) Shrinking-bed model for percolation process applied to dilute-acid pretreatment/hydrolysis of cellulosic biomass. Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology 70-72: 37-49.
- Zhang R, Lu XB, Sun YS, Wang XY, Zhang ST (2010) Modeling and optimization of dilute nitric acid hydrolysis on corn stover. Journal of Chemical Technology and Biotechnology 86: 306-314.
- Heredia-Olea E, Perez-Carrillo E, Serna-Saldivar SO (2012) Effects of different acid hydrolyses on the conversion of sweet sorghum bagasse into C5 and C6 sugars and yeast inhibitors using response surface methodology. Bioresource Technology 119: 216-223.
- Gamez S, Gonzalez-Cabriales JJ, Ramirez JA, Garrote G, Vazquez M (2006) Study of the hydrolysis of sugar cane bagasse using phosphoric acid. Journal of Food Engineering 74: 78-88.
- Esteghlalian A, Hashimoto AG, Fenske JJ, Penner MH (1997) Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresource Technology 59: 129-136.
- Conner AH, Wood BF, Hill CG, Harris JF (1985) Kinetic-Model for the Dilute Sulfuric-Acid Saccharification of Lignocellulose. Journal of Wood Chemistry and Technology 5: 461-489.
- Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quimica Nova 26: 863-871.
- Garrote G, Dominguez H, Parajo JC (1999) Hydrothermal processing of lignocellulosic materials. Holz als Roh- und Werkstoff 57: 191-202.
- Pettersen RC (1984) The chemistry of solid wood: The chemical composition of wood. American Chemical Society.
- Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83: 1-11.
- McIntosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass and Bioenergy 35: 3094-3103.
- Vidal PF, Molinier J (1988) Ozonolysis of lignin-Improvement of in vitro digestibility of poplar sawdust. Biomass 16: 1-17.
- McGinnis GD, Wilson WW, Mullen CE (1983) Biomass pretreatment with water and high-pressure oxygen. The wet-oxidation process. Industrial & Engineering Chemistry Product Research and Development 22: 352-357.
- Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82: 815-827.
- Holtzapple M, Jun J-H, Ashok G, Patibandla S, Dale B (1991) The ammonia freeze explosion (AFEX) process. Applied Biochemistry and Biotechnology28: 59-74.
- Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE (2005) Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour Technol 96: 2014-2018.
- Bernhard Seiboth CI, Verena Seidl-Seiboth, Trichoderma reesei (2011) Biofuel Production-Recent Developments and Prospects: A Fungal Enzyme Producer for Cellulosic Biofuels M AdS: INTECH, Brazil
- Demain AL, Newcomb M, Wu JH (2005) Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 69: 124-154.
- Wingren A, Galbe M, Zacchi G (2003) Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog 19: 1109-1117.
- Olofsson K, Bertilsson M, Lidén G (2008) A short review on SSF - an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1: 7.
- Tengborg C, Galbe M, Zacchi G (2001) Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzyme Microb Technol 28: 835-844.
- Olsson L, Hahn-HÄgerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme and Microbial Technology18: 312-331.
- Lynd LR, van Zyl WH, McBride JE, Laser M (2005) Consolidated bioprocessing of cellulosic biomass: an update. Curr Opin Biotechnol 16: 577-583.
- Panagiotou G, Kekos D, Macris BJ, Christakopoulos P (2003) Production of cellulolytic and xylanolytic enzymes by Fusarium oxysporum grown on corn stover in solid state fermentation. Industrial Crops and Products 18: 37-45.
- Kumar A, Jones D, Hanna M (2009) Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2: 556-581.
- Kersten S, Garcia-Perez M (2013) Recent developments in fast pyrolysis of ligno-cellulosic materials. Current Opinion in Biotechnology 24: 414-420.
- Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renewable and Sustainable Energy Reviews 15: 1615-1624.
- Elif Gurbuz JQB, James A Dumesic, Roman-Leshkov Y(2013) The Role Of Catalysis For The Sustainable Production Of Bio-Fuels And Bio-Chemicals: Role of Acid Catalysis in the Conversion of Lignocellulosic Biomass to Fuels and Chemicals. Elsevier, UK.
- Diehl F, Khodakov AY (2009) Promotion of Cobalt Fischer-Tropsch catalysts with noble metals: A review. Oil and Gas Science and Technology 64: 11-24.
- Demirbas A (2007) Converting biomass derived synthetic gas to fuels via Fisher-Tropsch synthesis. Energy Sources, Part A: Recovery, Utilization and Environmental Effects29: 1507-1512.
- Rosyadi E, Priyanto U, Suprapto, Roesyadi A, Nurunnabi M, Hanaoka T, Miyazawa T, Sakanishi K (2011) Biofuel production by hydrocracking of biomass FT wax over NiMo / Al 2O 3-SiO 2 catalyst. Nihon Enerugi Gakkaishi/Journal of the Japan Institute of Energy90: 1171-1176.
- Triantafyllidis KS, Komvokis VG, Papapetrou MC, Vasalos IA, Lappas AA (2007) Microporous and mesoporous aluminosilicates as catalysts for the cracking of Fischer-Tropsch waxes towards the production of "clean" bio-fuels. 170: 1344-1350.
- Zhang Q, Chang J, Wang T, Xu Y (2007) Review of biomass pyrolysis oil properties and upgrading research. Energy Conversion and Management48: 87-92.
- Iliopoulou EF, Antonakou EV, Karakoulia SA, Vasalos IA, Lappas AA, Triantafyllidis KS (2007) Catalytic conversion of biomass pyrolysis products by mesoporous materials: Effect of steam stability and acidity of Al-MCM-41 catalysts. Chemical Engineering Journal 134: 51-57.
- Triantafyllidis KS, Iliopoulou EF, Antonakou EV, Lappas AA, Wang H, Pinnavaia TJ (2007) Hydrothermally stable mesoporous aluminosilicates (MSU-S) assembled from zeolite seeds as catalysts for biomass pyrolysis. Microporous and Mesoporous Materials 99: 132-139.
- Aho A, Kumar N, ErÄnen K, Salmi T, Hupa M, Murzin DY (2007) Catalytic Pyrolysis of Biomass in a Fluidized Bed Reactor: Influence of the Acidity of H-Beta Zeolite. Process Safety and Environmental Protection 85: 473-480.
- Carlson T, Tompsett G, Conner W, Huber G (2009) Aromatic Production from Catalytic Fast Pyrolysis of Biomass-Derived Feedstocks. Top Catal 52: 241-252.
- Stephanidis S, Nitsos C, Kalogiannis K, Iliopoulou EF, Lappas AA, Triantafyllidis KS (2011) Catalytic upgrading of lignocellulosic biomass pyrolysis vapours: Effect of hydrothermal pre-treatment of biomass. Catalysis Today167: 37-45.
- Iliopoulou EF, Stefanidis SD, Kalogiannis KG, Delimitis A, Lappas AA, Triantafyllidis KS (2012) Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Applied Catalysis B: Environmental 127: 281-290.
- Aho A, Kumar N, Lashkul AV, ErÄnen K, Ziolek M, Decyk P, Salmi T, Holmbom B, Hupa M, Murzin DY (2010) Catalytic upgrading of woody biomass derived pyrolysis vapours over iron modified zeolites in a dual-fluidized bed reactor. Fuel 89: 1992-2000.
- Bui VN, Toussaint G, Laurenti D, Mirodatos C, Geantet C (2009) Co-processing of pyrolysis bio oils and gas oil for new generation of bio-fuels: Hydrodeoxygenation of guaïacol and SRGO mixed feed. Catalysis Today 143: 172-178.
- Thegarid N, Fogassy G, Schuurman Y, Mirodatos C, Stefanidis S, Iliopoulou EF, Kalogiannis K, Lappas AA: Second-generation biofuels by co-processing catalytic pyrolysis oil in FCC units. Applied Catalysis B: Environmental 145: 161-166.
- Fogassy G, Thegarid N, Toussaint G, van Veen AC, Schuurman Y, Mirodatos C (2010) Biomass derived feedstock co-processing with vacuum gas oil for second-generation fuel production in FCC units. Applied Catalysis B: Environmental96: 476-485.
- Lappas AA, Bezergianni S, Vasalos IA (2009) Production of biofuels via co-processing in conventional refining processes. Catalysis Today145: 55-62.
- Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA (2006) Hydrogen Production via Steam Reforming of the Aqueous Phase of Bio-Oil in a Fixed Bed Reactor. Energy & Fuels 20: 2155-2163.
- Garcia La, French R, Czernik S, Chornet E (2000) Catalytic steam reforming of bio-oils for the production of hydrogen: effects of catalyst composition. Applied Catalysis A: General201: 225-239
- çaglar A, Demirbas A (2001) Conversion of cotton cocoon shell to liquid products by supercritical fluid extraction and low pressure pyrolysis in the presence of alkalis. Energy Conversion and Management42: 1095-1104.
- Tekin K, Karagöz S, Bektas S (2012) Hydrothermal liquefaction of beech wood using a natural calcium borate mineral. Journal of Supercritical Fluids 72: 134-139.
- Yuan XZ, Li H, Zeng GM, Tong JY, Xie W (2007) Sub- and supercritical liquefaction of rice straw in the presence of ethanol–water and 2-propanol–water mixture. Energy32: 2081-2088.
- Cemek M, Küçük MM(2001) Liquid products from Verbascum stalk by supercritical fluid extraction. Energy Conversion and Management 42: 125-130.
- Karinen R, Vilonen K, NiemelÄ M (2011) Biorefining: heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 4: 1002-1016.
- Shimizu K-i, Uozumi R, Satsuma A (2009) Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods. Catalysis Communications 10: 1849-1853.
- Dias AS, Pillinger M, Valente AA (2005) Dehydration of xylose into furfural over micro-mesoporous sulfonic acid catalysts. Journal of Catalysis229: 414-423.
- Gürbüz E, Bond JQ, Román-Leshkov Y, Dumesic JA (2013) The role of Catalysis for the Production of Bio-fuels and Bio-chemicals: Role of acid catalysis in the conversion of lignocellulosic biomass to fuels and chemicals Elsevier.
- Zhou G, Taylor G, Polle A (2011) FTIR-ATR-based prediction and modelling of lignin and energy contents reveals independent intra-specific variation of these traits in bioenergy poplars. Plant Methods 7: 9.
- Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP (2006) Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl Biochem Biotechnol 129-132: 427-35.
- Yin T, Zhang X, Gunter L, Priya R, Sykes R, et al. (2010) Differential detection of genetic Loci underlying stem and root lignin content in Populus. PLoS One 5: e14021.
- Kirst M, Myburg AA, De León JP, Kirst ME, Scott J, et al. (2004) Coordinated genetic regulation of growth and lignin revealed by quantitative trait locus analysis of cDNA microarray data in an interspecific backcross of eucalyptus. Plant Physiol 135: 2368-2378.
- Porth I, Klápště J, Skyba O, Lai BS, Geraldes A, et al. (2013) Populus trichocarpa cell wall chemistry and ultrastructure trait variation, genetic control and genetic correlations. New Phytol 197: 777-790.
- Euring D, Löfke C, Teichmann T, Polle A (2012) Nitrogen fertilization has differential effects on N allocation and lignin in two Populus species with contrasting ecology. Trees - Structure and Function 26: 1933-1942.
- Pitre FE, Pollet B, Lafarguette F, Cooke JE, MacKay JJ, et al. (2007) Effects of increased nitrogen supply on the lignification of poplar wood. J Agric Food Chem 55: 10306-10314.
- Jouanin L, Goujon T, de Nadaï V, Martin MT, Mila I, et al. (2000) Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol 123: 1363-1374.
- Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, et al. (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 17: 808-812.
- Tian X, Xie J, Zhao Y, Lu H, Liu S, et al. (2013) Sense-, antisense- and RNAi-4CL1 regulate soluble phenolic acids, cell wall components and growth in transgenic Populus tomentosa Carr. Plant Physiol Biochem 65: 111-119.
- Voelker SL, Lachenbruch B, Meinzer FC, Jourdes M, Ki C, Patten AM, Davin LB, Lewis NG, Tuskan GA, Gunter L et al (2010) Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiology 154: 874-886.
- Pilate G, Guiney E, Holt K, Petit-Conil M, Lapierre C, et al. (2002) Field and pulping performances of transgenic trees with altered lignification. Nat Biotechnol 20: 607-612.
- Polle A, Janz D, Teichmann T, Lipka V (2013) Poplar genetic engineering: promoting desirable wood characteristics and pest resistance. Appl Microbiol Biotechnol 97: 5669-5679.
- Coleman HD, Cánovas FM, Man H, Kirby EG, Mansfield SD (2012) Enhanced expression of glutamine synthetase (GS1a) confers altered fibre and wood chemistry in field grown hybrid poplar (Populus tremula X alba) (717-1B4). Plant Biotechnol J 10: 883-889.
- Legay S, Sivadon P, Blervacq AS, Pavy N, Baghdady A, et al. (2010) EgMYB1, an R2R3 MYB transcription factor from eucalyptus negatively regulates secondary cell wall formation in Arabidopsis and poplar. New Phytol 188: 774-786.
- Murphy RL, Klein RR, Morishige DT, Brady JA, Rooney WL, Miller FR, Dugas DV, Klein PE, Mullet JE (2011) Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proceedings of the National Academy of Sciences108: 16469-16474.
- Jung JH, Vermerris W, Gallo M, Fedenko JR, Erickson JE, Altpeter F (2013) RNA interference suppression of lignin biosynthesis increases fermentable sugar yields for biofuel production from field-grown sugarcane. Plant Biotechnology Journal 11(6):709-16
- Nageswara-Rao M, Soneji JR, Kwit C, Stewart CN Jr (2013) Advances in biotechnology and genomics of switchgrass. Biotechnol Biofuels 6: 77.
- Shen H, Poovaiah CR, Ziebell A, Tschaplinski TJ, Pattathil S, et al. (2013) Enhanced characteristics of genetically modified switchgrass (Panicum virgatum L.) for high biofuel production. Biotechnol Biofuels 6: 71.
- Bartley L, Wu Y, Saathoff A, Sarath G (2013) Bioenergy Feedstocks: Breeding and Genetics: Switchgrass Genetics and Breeding Challenge John Wiley & Sons, Inc, USA.
- Serba D, Wu L, Daverdin G, Bahri B, Wang X, Kilian A, Bouton J, Brummer EC, Saha M, Devos K (2013) Linkage Maps of Lowland and Upland Tetraploid Switchgrass Ecotypes. BioEnergy Research pp.1-13.
- Xu B, Escamilla-Trevino LL, Sathitsuksanoh N, Shen Z, Shen H, Zhang YH, Dixon RA, Zhao B (2011) Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. The New phytologist192: 611-625.
- Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, et al. (2011) Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc Natl Acad Sci U S A 108: 3803-3808.
- Saathoff AJ, Sarath G, Chow EK, Dien BS, Tobias CM (2011) Downregulation of cinnamyl-alcohol dehydrogenase in switchgrass by RNA silencing results in enhanced glucose release after cellulase treatment. PLoS One 6: e16416.
- Shen H, He X, Poovaiah CR, Wuddineh WA, Ma J, et al. (2012) Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol 193: 121-136.
- Shen H, Poovaiah CR, Ziebell A, Tschaplinski TJ, Pattathil S, et al. (2013) Enhanced characteristics of genetically modified switchgrass (Panicum virgatum L.) for high biofuel production. Biotechnol Biofuels 6: 71.
- Chuck GS, Tobias C, Sun L, Kraemer F, Li C, Dibble D, Arora R, Bragg JN, Vogel JP, Singh S et al (2011) Over expression of the maize Corngrass1 microRNA prevents flowering, improves digestibility, and increases starch content of switchgrass. Proceedings of the National Academy of Sciences of the United States of America 108: 17550-17555.
- Dwiyanti MS, Stewart JR, Yamada T (2013) Bioenergy Feedstocks: Breeding and Genetics: Germplasm Resources of Miscanthusand Their Application in Breeding. John Wiley & Sons, Inc, USA.
- Brown JC, et al (2013) Bioenergy Feedstocks: Breeding and Genetics: Breeding Miscanthusfor Bioenergy. John Wiley & Sons, Inc, USA.
- Hodgson EM, Lister SJ, Bridgwater AV, Clifton-Brown J, Donnison IS (2010) Genotypic and environmentally derived variation in the cell wall composition of Miscanthus in relation to its use as a biomass feedstock. Biomass and Bioenergy34: 652-660.
Citation: Kapazoglou A, Drosou V, Nitsos CK, Bossis I, Tsaftaris A et al. (2013) Biofuels Get in the Fast Lane: Developments in Plant Feedstock Production and Processing. Adv Crop Sci Tech 1:117. DOI: 10.4172/2329-8863.1000117
Copyright: © 2013 Kapazoglou A et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 21178
- [From(publication date): 11-2013 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 16286
- PDF downloads: 4892