Research Article Open Access
Biosorption of some Heavy Metals by Deinococcus radiodurans Isolatedfrom Soil in Basra Governorate-Iraq
| Jaafar R1, Al-Sulami A1, Al-Taee A2*, Aldoghachi F3, Suhaimi N4 and Mohammed S5 | |
| 1College of Education for Pure Science, Basra University, Basra, Iraq | |
| 2Marine Science Center, Basra University, Basra, Iraq | |
| 3College of Science, Basra University, Basra, Iraq | |
| 4College of Biotechnology, Putra Malaysia University, Malaysia | |
| 5College of Medicine, Putra Malaysia University, Malaysia | |
| *Corresponding Author : | Al-Taee A Marine Science Center, Basra University, Basra, Iraq E-mail: amraltaee@yahoo.com |
| Received January 18, 2016; Accepted February 06, 2016; Published February 10, 2016 | |
| Citation: Jaafar R, Al-Sulami A, Al-Taee A, Aldoghachi F, Suhaimi N, et al. (2016) Biosorption of some Heavy Metals by Deinococcus radiodurans Isolated from Soil in Basra Governorate-Iraq. J Bioremed Biodeg 7:332. doi: 10.4172/2155-6199.1000332 | |
| Copyright: © 2016 Jaafar R, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The bacterium Deinococcus radiodurans has been isolated from Basra soil. On the basis of morphological, biochemical, 16S rRNA gene sequencing and phylogeny analysis, the isolates were authentically identified as D. radiodurans. The biosorption capabilities of D. radiodurans for cadmium (Cd+2) and lead (Pb+2) were monitored at different concentrations and contact times. The characterization of heavy metals around the cells of bacterial strains was observed using the Fourier Transform Infrared (FT-IR) spectrophotometer, X-ray powder diffraction analysis (XRD) and transmission electron microscope (TEM).
| Keywords |
| Deinococcus radiodurans; Biosorption; Fourier Transform Infrared; X-ray powder diffraction; Transmission electron microscope |
| Introduction |
| Bioremediation is a natural process which depends on bacteria, fungi, and plants to alternating pollutants as these organisms carry out their normal life functions. Thus, bioremediation offers a substitute tool to destroy or reduce the harmful pollutants through biological activity with an effective cost. In the early 1980s, certain microorganisms were found to accumulate metallic elements at a high capacity [1,2]. |
| There are a lot of studies about the bioremediation ability of D. radiodurans and its genetic engineering, for cleaning up heavy metals in nuclear waste contaminated sites [3-7]. The development of bioremediation strategies using Deinococcus spp is therefore vital for the cleanup of contaminated site with radioactive waste. Additional advantages of deinococci are that they are vegetative, easily cultured, and nonpathogenic. As these sites rarely contaminated by a single chemical, it is necessary to bio remediating strain to be multi resistant to various toxic agents. The present work describes the use of a combination of spectroscopic and microscopic methods to characterize the heavy metals around the cells of bacterial strains isolated from extreme habitats as well as to elucidate the interaction mechanisms of these bacteria with these metals. |
| Materials and Methods |
| Source of bacterial isolate |
| The tested isolates, D. radiodurans used in this study was isolated previously from the Um - Qasr district, south of Basra city- Iraq. The isolate was identified by biochemical tests and sequencing 16S rRNA gene and comparing the sequences online with GenBank database (www.ncbi.nlm.nih.gov). |
| Biosorption experiment |
| The equilibrium, kinetics data of the biosorbent D. radiodurans were obtained by performing batch experiments |
| Characterization study |
| FTIR analysis: The Fourier Transform Infrared (FT-IR) analysis was done with Perkin Elmer spectrometer model 100 series (sample preparation UATR). This analysis was done in the Chemistry department, University Putra Malaysia. |
| X-ray powder diffraction analysis (XRD): The powder X-ray diffraction analysis was performed using a Shimadzu diffractometer model XRD 6000. The diffractometer employed Cu-Kα radiation to generate diffraction patterns from powder crystalline samples at ambient temperature. The Cu-Kα radiation was generated by Philips glass diffraction, X-ray tube broad focus 2.7KW type. The crystallite size D of the samples was calculated by using the Debye–Scherer’s relationship. Where D is the crystallite size, λ is the incident X-ray wavelength, β is the (FWHM) Full Width at Half-Maximum, and θ is the diffraction angle, Deby -Scherrer equation: D= K λ /BCOS θ. This analysis was done in the Chemistry department, University Putra Malaysia. |
| Transmission Electron Microscope: By centrifuging samples broth culture for 10 min at 3000 rpm, and decanting the supernatant, fixing pallet with 4% gutaraldehyde for 4 h at 4°C and centrifuged again, decanting fixative and adding an appropriate quantity of animal serum to submerge sample then allowing serum to clot. Washing three times with 0.1M Cacodylate buffer for 10 min. Postng fix in 1% Osmium tetroxide for 2h at 4°C. Washing again three times with 0.1M Cacodylate buffer for 10 min. dehydrating in series of acetone at 35, 50, 75, 95, and 100% for 10, 10, 10, 10 and 15 min respectively (Table 1). |
| Embedding: Specimens were placed into the beam capsule fill with resin and polymerized in oven at 60 °C for 24-48h. Ultra sectioning was realized by choosing an area of interest, for ultrathin section, i.e. selecting the silver section, picking up sections with a grid, then drying with filter paper. Finally the sections were stained with Uranyal acetate for 15 min, and washed by double distilled water. Lead sections were stained for 10 min, washed twice with distilled water. This analysis was done in the Electron Microscope Laboratory, Institute of Bioscience, University Putra Malaysia. |
| Results and Discussion |
| FT-IR |
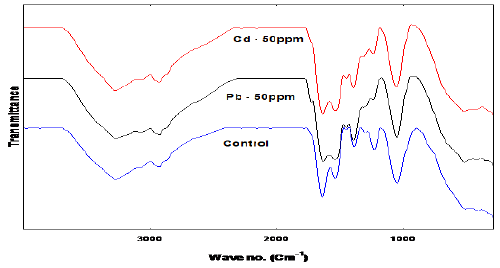
| One of the most important characteristics of a biosorbent is the presence of its surface functional groups, which are largely characterized by the FTIR spectroscopy method. This technique can only provide a qualitative description for biosorbent functional groups. The studies of FTIR spectra on Deinococcus provided the basis to interpret the results with Fucus vesiculosus [8], as shown in Figure 1 there was a shift in the bands corresponding to carboxyl (COOH) groups after the biosorption process. Following metal binding, the asymmetric carboxyl stretching band shifted from 1630 to 1636 and 1632 Cm−1 for Cd and Pb respectively [9], and there was an increase in the distance between this band and the symmetric stretching of the same groups at 1418 Cm−1, to lower wave numbers after biosorption, indicating that chelating complexes were formed [10,11]. Therefore, chelation was another important mechanism involved in the biosorption of Cd and Pb with Deinococcus; as reported by Sheng et al. [12] for Sargassum with FTIR spectroscopy studies. These changes in the FTIR spectra have also been observed in other biosorption studies [13,14]. Also the peak at 1387 attributed to C-O bond shifted to 1392 and 1390 Cm-1 when exposed to Cd and Pb respectively. |
| Other studies stated that cadmium biosorption was achieved by the formation of ionic bridges between the metal and two carboxyl groups or a bidentate chelating complex with one carboxyl group [15,16]. The symmetric carboxyl-stretching band’s intensity decreased after Cd and Pb binding. The bacteria formed stronger bonds with these two metals. Carboxyl groups are the most abundant functional groups and are the main functional groups involved in the biosorption of heavy metals with Deinococcus and other biomasses [17-19]. Cadmium biosorption with Deinococcus reduced after blocking these groups, the majority of these groups are located in the Deinococcus cell wall and their negative charge can attract metal cations [20,21]. |
| XRD |
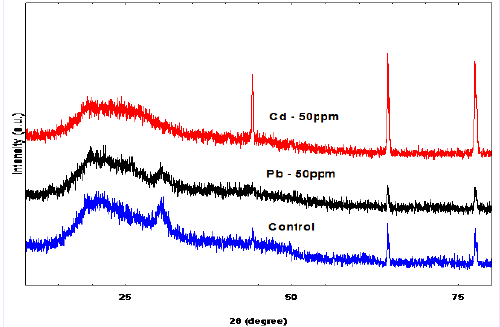
| X-ray diffraction is a non-destructive technique used to provide detailed information on the crystallographic structure of materials. This method offers several advantages e.g., non-destructive, high accuracy, capability to detect single crystals, polycrystalline or amorphous materials. Moreover, standards are readily accessible for thousands of material systems. Due to its versatility, XRD has been widely employed to assist in the characterization of biosorbent and in the verification of heavy metals biosorption mechanisms [22]. Figure 2 shows the biosorbent of D. radiodurans un-loaded and loaded with Cd (II) and Pb (II). The XRD profile of the unloaded biosorbent shows typical diffraction peaks. Broad peaks were obtained instead of sharp peaks indicating the sample was poorly crystalline. The peaks at 2-theta 30.2, 44, 64.2, and 77.5°corresponding to (201), (114), (641), and (811) planes. The XRD spectrum is compared with the exited spectrums of control that have been published by the Joint Committee on Powder Diffraction Standards (JCPDS file no. 00-001-0166). The XRD spectra of Cd (II) and Pb (II) exhibit strong peaks at 2-theta value 29.9, 44.1, 64.4 and 77.8° corresponding to (200), (114), (640) and (822) planes, respectively, for Cd (II), whereas, the value 44.2, 64.2 and 77.8° corresponding to (112), (211), and (422) for Pb (II). The XRD spectrum is compared with the exited spectrums of control that have been published by the Joint Committee on Powder Diffraction Standards (JCPDS file no. 00-002- 097) and (JCPDS file no. 00-002-085) respectively. |
| The average crystal size of the un-loaded control, loaded with Cd (II) 50 mgl-1, and Pb (II) 50 mgl-1 nanoparticle estimated from the highest peak by using the Debye-Scherrer Eq. is 20.0, 27.8, and 14.0 respectively. The results of XRD patterns of D. radiodurans show three sharp peaks with a decrease in the intensity of the these peaks for un-loaded biosorbent after exposing the bacteria to Pb (II) and Cd (II), which could explain the immobilization process. This is in agreement with the results reported in the literatures of [23-25]. And this may suggest that Pb (II) and Cd (II) immobilized on surface of D. radiodurans [26]. |
| Transmission electron microscope |
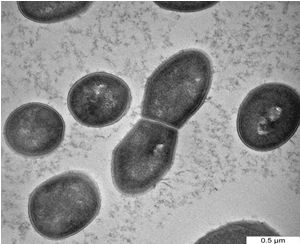
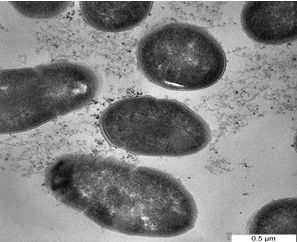
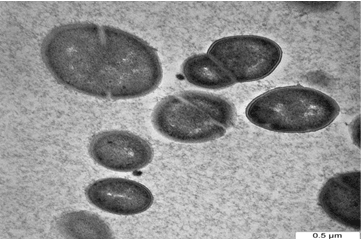
| One of the most important of TEM techniques has revealed the internal structure of the bacteria, especially when embedding bacteria in a resin and staining thin sections of the resulting block with uranyl acetate. Figure 3a shows the cells of D. radiodurans before interaction with metals while Figure 3b,3c show the bacteria after exposure to Pb and Cd solutions. Metals were mostly seen on the cell wall, looking almost like a crust around the cell in addition to the change in the size and the shape of cells. The cellular localization of the metals bound by the cells of study bacteria was located mainly within the cell wall. However, some intracellular metal accumulates associated were also identified in the cytoplasm of the bacterial cells [27]. Reported that, the cellular localization of the uranium bound by the cells was located mainly within the cell wall of three types of A. ferrooxidans using TEM. Electron microscopic observation carried out by Panak et al. [28] revealed the presence of Ag+2 as discrete particles at or near the cell wall of both gram positive and gram negative bacteria. Sinha and Mukherjee [29] showed that, Bacillus sp. cell wall components with phosphate residues i.e polysaccharides, teichoic and teichuronic acids or phospholipid layers of the membranes can bind U. While cellular functional groups can be responsible for the extracellular association of [30] showed localized intracellular site of accumulated Cd+2 by P. aerugenosa and showed electron dense grains in the cytosol and towards the cell envelope. Also, Sultan et al. and Singh et al. [31,32] reported that, the cell surfaces of cultures treated with cadmium chloride tended to be rough, suggesting that the cell increased its surface to improve the interaction of toxic substances with the cell surface. The results of the present study also indicate morphology and size changes of bacteria exposed to heavy metals, such changes may due to cell surface changes as a result of heavy metals exposure reported that, the cell surface morphological changes in Cryptococcus sp. after exposing to heavy metals, appeared as shrunken and distorted cell wall in the presence of Cd and depressions in the presence of Pb and Zn. Various factors may be responsible for such alterations in cell surface morphology of microbial biomass in the presence of heavy metals. Secretion of extracellular polymeric substance by Desulfovibrio desulfuricans during biosorption of Zn and Cu was reported to modify its cell surface morphology [34] Similarly, Murray [35] reported high dark dense cytoplasm due to Co+2 precipitations; partially emptied with a very thick cell wall; changing in the morphology of vegetative cells of B. firmus and B. subtilis. |
| Conclusion |
| Deinococcus radiodurans isolated from polluted soils in Basra city south of Iraq, showed high tolerance for Pb and Cd with the possibility of being exploited in bioremediation in two routes, bioaccumulation and biosorption. D. radiodurans survive the adverse effect of high concentration of Pb and Cd as expressed in morphological changes. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3a | Figure 3b | Figure 3c |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 11608
- [From(publication date):
March-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 10589
- PDF downloads : 1019
