Research Article Open Access
Biotransformation of Nitro Aromatic Compounds by Flavin-Free NADHAzoreductase
| Santosh A. Misal1,3*, Vivek T. Humne2, Pradeep D. Lokhande2 and Kachru R. Gawai3* | |
| 1Department of Chemistry, Indiana University Bloomington, Indiana, 47405, USA | |
| 2Center for Advance Studies, Department of Chemistry, University of Pune, Pune-411 007, India | |
| 3Biochemistry Division, Department of Chemistry, University of Pune, Pune-411 007, India | |
| Corresponding Authors : | Santosh A. Misal Department of Chemistry Indiana University Bloomington Indiana, 47405, USA Tel: +1 574 516 8259 E-mail: samisal@indiana.edu |
| Kachru R. Gawai Biochemistry Division Department of Chemistry University of Pune, Pune-411 007, India Tel: +91-020-25691395 Fax: +91-020-25691728 E-mail: krgawai@chem.unipune.ac.in |
|
| Received October 05, 2014; Accepted January 28, 2015; Published January 30, 2015 | |
| Citation: Misal SA, Humne VT, Lokhande PD, Gawai KR (2015) Biotransformation of Nitro Aromatic Compounds by Flavin-Free NADH-Azoreductase. J Bioremed Biodeg 6:272. doi:10.4172/2155-6199.1000272 | |
| Copyright: © 2015 Misal SA, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Nitro aromatic compounds are the potential toxic organic pollutant released into the environment and are often resistant to degradation under normal environmental conditions. The biotransformation and/or detoxification of these compounds can be possible by microbial azoreductase enzyme. Azoreductase enzyme has an ability to reduce the toxic nitro group to corresponding amino group. In present report, the flavin-free NADH azoreductase was isolated and purified from alkaliphilic bacteria Bacillus badius. The enzyme was purified by a combination of ammonium sulphate precipitation and size exclusion chromatography. The purified azoreductase has efficiently demonstrated both azoreductase and nitroreductase activities. The biochemical properties of the azoreductase including cofactor requirement, substrate specificity and enzyme inhibition have been studied. The biotransformation of some selected nitro aromatic compounds like 3-nitro benzoic acid, 4-nitro toluene, 3-nitro toluene and 1-chloro-2-nitro benzene by purified azoreductase was carried out at 37°C. The reduction products of these nitro aromatic compounds were analyzed by IR and NMR spectroscopy.
| Keywords |
| Azoreductase; Nitroaromatic; Biotransformation; Biodegradation |
| Introduction |
| Nitro aromatic compounds are major group of environmental pollutants released into the environment exclusively from anthropogenic sources. They are mainly produced from incomplete combustion of petroleum and natural gasses. Nitro compounds are mostly utilized as synthetic intermediate in chemical, pharmaceutical industries. Some of them are routinely used in industrial solvents, dyes, agrochemicals and explosives. The direct discharge of these compounds as an industrial waste into the ecosystem is harmful to the biological system as well as human beings. Many of these compounds have mutagenic and carcinogenic potential. These compounds can be converted to nontoxic compounds in the environment by microorganisms [1,2]. Nitro aromatic compounds such as nitrobenzene, nitrophenol, nitrotoluene and nitrobenzoate are common precursors for the synthesis of complex synthetic and industrial nitrogen containing aromatic organic compounds. Upon ingestion of nitro compounds into the human body, it can be converted to toxic metabolic intermediates. These metabolic intermediates can be further converted into the non-toxic compounds by various enzyme systems including some oxidases and reductases [3]. |
| It was previously shown that the nitro aromatic compounds can be easily reduced under anaerobic conditions to aromatic amines by different kinds of microorganisms [4]. Complete mineralization of the nitro aromatic compounds under aerobic condition has also been demonstrated in several bacterial strains. Moreover, the involvement of reductase enzymes in the aerobic/anaerobic reduction mechanism of the nitro aromatic compound remains to be noteworthy. The reduction is mediated by single or two-electron system in the organisms. Singleelectron reduction reactions of nitro aromatic compounds are catalyzed by flavin containing reductases such as NADPH: cytochrome P-450 reductase, ferredoxin: NADP+ reductase and bacterial oxygen-sensitive nitroreductases [5-7]. It was also well known that the two-electron reduction of nitro aromatic compounds to nitroso (NO) compounds and, subsequently, to hydroxylamines is specifically catalyzed by bacterial oxygen-insensitive nitroreductases [7,8]. Some of the specific nitroreductases have been isolated and characterized from anaerobic human intestinal bacteria [9]. |
| Azoreductases are widely present in the microorganisms which can specifically catalyze the reduction of azo (-N=N-) bond and NO2 group of the complex organic compound. The reduction and / or biodegradation of toxic azo dyes by microorganisms have been extensively studied, and the primary role of azoreductases is explored [10,11]. Raffi and Cerniglia (1993) demonstrated that the azoreductase and nitroreductase activities were indistinguishable [12]. The azoreductase catalyze the reduction of azo dyes in presence of flavin and/or nicotinamide adenine dinucleotide/ nicotinamide adenine dinucleotide phosphate (NADH /NADPH) as an electron equivalent [13,14]. In our previous reports, we demonstrated that some of the nitro aromatic compounds can also be reduced by neutrophilic azoreductase by two electron reaction mechanism to its respective amines [15,16]. |
| Alkaliphilic microorganisms are mostly ignored due to their rare occurrence and complex growth conditions. These microorganisms can grow in highly alkaline conditions and can also tolerate the elevated temperature. Many alkaliphilic bacterial strains have been isolated and proven to be vital for the biotechnological and industrial applications. The alkaliphiles are largely known for its highly stable proteolytic and hydrolytic enzymes; these enzymes have wide industrial applications [17]. The azoreductases from alkaliphilic microorganism were not studied in this point of view so far. |
| However, different forms of azoreductase and nitroreductases are also isolated from several aerobic and anaerobic bacteria [18-20]. Numerous azoreductases have been characterized from variety of fungal and bacterial strains, but alkaliphilic azoreductases are still not well explored. In our previous efforts, we isolated various alkaliphilic bacterial strains from alkaline Crater Lake of Lonar and studied different types of azoreductases and nitroreductases [13,14,21]. Since these enzymes are the key enzymes in biotransformation and biodegradation processes of xenobiotic, very little data is available on azoreductases which have the highly stable properties such as temperature and pH. |
| Thus, the present report demonstrates the properties of flavinfree NADH-azoreductase from alkaliphilic bacteria B. badius and its ability to transform some selected nitro aromatic compounds to its corresponding amines. |
| Materials and Methods |
| Chemicals |
| Yeast extract, peptone (Hi-Media, India), DEAE–cellulose (Sigma, USA), Sephadex G-100 (Pharmacia Fine Chemicals, Sweden), EDTA, DTT, NADH, NADPH, FADH2, FMNH2, Dichloromethane (DCM), 3-nitro benzoic acid, 4-nitro toluene, 3-nitro toluene, 1-chloro-2-nitro benzene were purchased from Sisco Research Laboratory, India. |
| Microorganism isolation and growth conditions |
| The B. badius D1 was isolated from alkaline Crater Lake of Lonar, India. The strain was identified by 16S rRNA gene sequencing method [13]. The bacterial culture was grown in 500 ml Erlenmeyer flask incubated in orbital shaker at 250 RPM and 37°C for overnight. The 1 L culture medium contains: 5.0 g yeast extract, 10.0 g peptone, 5.0 g NaCl with trace elements KH2PO4 170 mg, Na2HPO4 980 mg, (NH4)2SO4 100 mg , MgSO4 0.87 mg, MgO 0.1 mg, FeSO4 0.05 mg, CaCO3 0.2 mg, ZnSO4 0.08 mg, CuSO4 0.016 mg, CoCl3 0.015 mg and boric acid 0.006 mg. The pH of the medium was adjusted to 9.0 with sodium carbonate. Azo dyes were added from stock solutions at the final concentration of 1000 mg/l. |
| Enzyme purification |
| The bacterial cells were grown in 1 L cultivation medium for 24 hr at 37°C. The culture was centrifuged at 8000 x g for 15 min to obtain the pellet. This pellet was thoroughly washed twice with 100 mM sodium phosphate buffer (pH 7.4). The cells were resuspended in 100 mM sodium phosphate buffer, 1 mM of EDTA, DTT, lysozyme and 20% (v/v) glycerol and sonicated by Sartorius ultrasonic homogenizer for 30 s, 60 Hz, 6 times. The soluble fraction of cytosol was separated by centrifugation at 15000× g for 20 min at 4°C in a DuPont Sorvall RC- 5B refrigerated centrifuge. The clear yellowish supernatant obtained constitutes the crude bacterial extract and used for further studies. |
| The azoreductase enzyme was purified by ammonium sulphate precipitation and size exclusion chromatography with modifications in the procedure already described [13]. The crude extract was initially subjected to ammonium sulfate precipitation by addition of 40 % solid ammonium sulfate. The mixture was constantly stirred for 5-6 hrs at 4°C. The precipitated proteins were separated by centrifugation at 15,000 x g for 20 and then solid ammonium sulfate concentration was further increased up to 80 %. Each fraction was collected by centrifugation and dissolved in the fresh minimal volume of 100 mM sodium phosphate buffer, pH 7.4. The excessive salt was removed by dialyzing the precipitated proteins against the same buffer without salt for 12 hours. These fractions were analyzed by its enzyme activity assays and SDS PAGE. The fraction which showed azoreductase activity was further concentrated and loaded on a Sephadex G-100 column (2 cm x 30 cm) already equilibrated with two bed volume of 100 mM sodium phosphate buffer, pH 7.4. The protein was eluted at a flow rate of 1 ml/min and fractions of 2 ml were collected and analyzed for its UV absorbance and azoreductase activity. The fractions showed higher azoreductase activity were pooled together and concentrated for further characterization. |
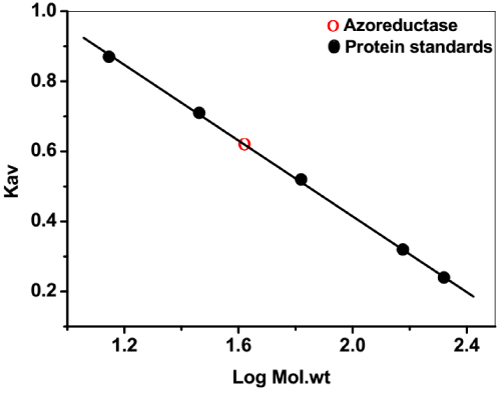
| In order to determine the apparent molecular mass of the purified azoreductase, the pre-calibrated Sephadex G-100 column (2×30 cm) was used. The elution volumes of the standard proteins were compared with the azoreductase. The molecular mass was estimated by plotting the graph Kav versus log of molecular mass of proteins. The protein standards used were β-amylase, alcohol dehydrogenase, bovine serum albumin, carbonic anhydrase and lysozyme [14]. |
| The total protein concentration during purification steps was measured by modified Lowry’s method [22] using BSA as a standard. The protein content in the fractions of size exclusion chromatography was determined by measuring its absorbance at 280 nm by UV-visible spectrophotometer. |
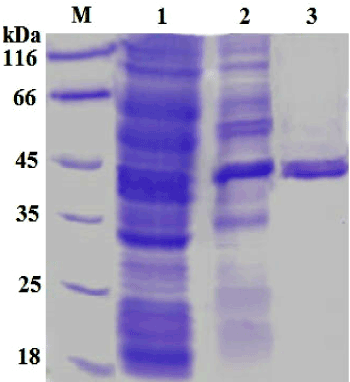
| The native and denatured gel electrophoresis was performed to determine the homogeneity of purified enzyme. In native and SDSPAGE, 10 % resolving polyacrylamide gel and 4.5 % stacking gel was used as described by Laemmli [23]. The relative molecular mass of the azoreductase was determined by using standard protein molecular mass markers (BioEra, India). The gels were stained with Coomassie brilliant blue R-250 dye. |
| Azoreductase activity assay |
| The azoreductase activity assays were performed with slight modifications in the procedure already described by Zimmermann et al. [18] with UV-visible spectrophotometer (Jasco V 630) at 37°C by monitoring the decrease in absorbance of amaranth dye at 520 nm (€= 21.5 mM /cm). The 1 ml reaction mixture contained 0.25 mM NADH, 0.05 mM amaranth dye and 50 μl of enzyme solution in 100 mM sodium phosphate buffer (pH 7.4). One unit of activity is defined as the amount of azoreductase required to reduce 1 μmol of amaranth dye per minute. |
| The other aliquots of a supernatant were analyzed by UV-visible spectrophotometer by recording the spectra at different concentrations to check the presence of bound flavin to the enzyme. |
| Effect of cofactor |
| The effect of cofactor on azoreductase activity was assayed using amaranth dye as a substrate. The activity assay for each cofactor was performed separately in 1 ml reaction mixture contained 0.25 mM cofactor (NADH/ NADPH/ FADH2 /FMNH2), 0.05 mM amaranth dye and 50 μl of enzyme solution in 100 mM sodium phosphate buffer (pH 7.4). Change in the absorbance of amaranth dye at 520 nm was monitored by UV-visible spectrophotometer at 37°C. |
| The effect of various metal ions on azoreductase activity was assayed with various metal ions such as MgSO4, MnSO4, FeCl3 CuSO4, and HgCl2 and different concentration of EDTA, SDS. The activity assay was performed with 2 mM of metal ions and keeping other components similar as described above. |
| Reduction of nitro aromatics |
| The reduction reaction was carried out at 37°C, in 20 ml of reaction mixture contained 1 mM nitro aromatic compounds, 1 mM NADH and 2 ml of enzyme solution (2 mg/ml) in 0.1 M sodium phosphate buffer (pH 7.4). The reaction was started with the addition of NADH and was monitored with constant stirring for 12 hrs. The absorbance of the reaction mixture was measured at each hour by UV-visible spectrophotometer. Immediately, after 12 h, the 20 ml reaction mixture was diluted with 20 ml of DCM. The dissolved organic compounds were recovered from DCM and separated on silica gel column chromatography. The purity of the transformed products were checked by thin layer chromatography with hexane/ethyl acetate (4:1, v/v) and visualized under UV light. The pure transformed products were analyzed by IR and NMR spectroscopy. |
| Results and Discussion |
| Purification of azoreductase from B. badius D1 |
| The flavin-free NADH-azoreductase has been purified from B. badius D1 by two-step procedure summarized in Table 1. The purified azoreductase appeared to be a single band on SDS and native- PAGE corresponding to a molecular mass of approximately 43 kDa (Figure 1a). Moreover, the single peak was obtained during the size exclusion chromatography elution of azoreductase corresponding to the molecular size of 43 kDa (Figure 1b). It suggests the monomeric nature of azoreductase. Previously, azoreductase was reported to be a monomeric in nature from Pseudomonas sp., Bacillus sp. bacterial strains. Furthermore, the homodimer and homotetramer form of azoreductase was shown from Shigella dysenteriae type 1 and Staphylococcus aureus respectively [13,19,24,25]. |
| Characterization of azoreductase |
| The effect of pH, temperature on azoreductase activity and thermal stability is already described [13]. The optimum activity of the purified enzyme was observed at pH 7.4 and 60°C. This enzyme has wide substrate specificity including mono and di azo dyes. The substrate specificity was further studied with some nitro aromatic compounds. |
| Analysis of cofactor requirements and substrate specificity of azoreductase |
| Some azoreductases are flavin containing, or they require flavins as a cofactor for electron transfer [26,27]. These types of azoreductases are categorized as flavin dependent azoreductases. In the present study, thorough analysis by TLC and UV-visible spectroscopy signified that the purified enzyme does not contain flavin as a cofactor. In addition, the externally added reduced flavins did not enhance the azoreductase activity (Table 2a). It clearly demonstrates that this azoreductase is neither flavo-protein nor flavin dependent. The flavin-free monomeric nature of azoreductase was first reported from P. kullae K24, but it showed a preference for NADPH as an electron donor [28]. |
| The effect of externally added NADH, NADPH, FADH2 and FMNH2 for the azo-reduction activity was analyzed using amaranth as a substrate (Table 2a). The decolorization rate was faster with NADH than NADPH. Moreover, the turnover rate with NADH is 1.2 fold more than NADPH, it clearly indicating that azoreductase preferred NADH as a source for reducing an equivalent. |
| Inhibition studies of azoreductase |
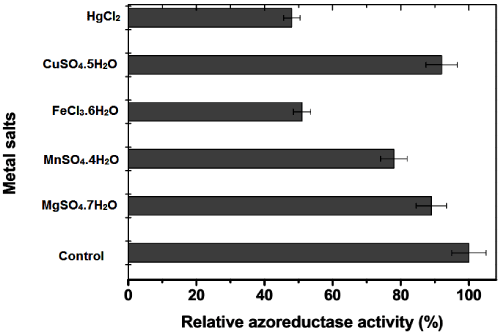
| The effect of various metal ions and its inhibitory action on azoreductase was studied. The activity assays were performed with various metal ions such as MgSO4, MnSO4, FeCl3 CuSO4, and HgCl2 and different concentration of EDTA, SDS. The summary of inhibition studies is given in Figure 2 and Table 2b. It was previously reported that the Hg has inhibitory action on azoreductase enzyme [29]. In our study, we found the 50 per cent inhibition in azoreductase activity at 2 mM of Hg and Fe ions. However, the addition of Cu and Mg ions has no significant inhibitory action (Figure 2). The addition of 2-Mercaptoethanol and DTT showed about 50 per cent azoreductase activity inhibition at 2 mM concentration. Moreover, the 2 mM Triton X-100 showed 20 percent inhibition. The EDTA and SDS at higher concentration resulted in concentration dependent decrease in the activity of azoreductase (Table 2b). |
| Analysis of transformed products |
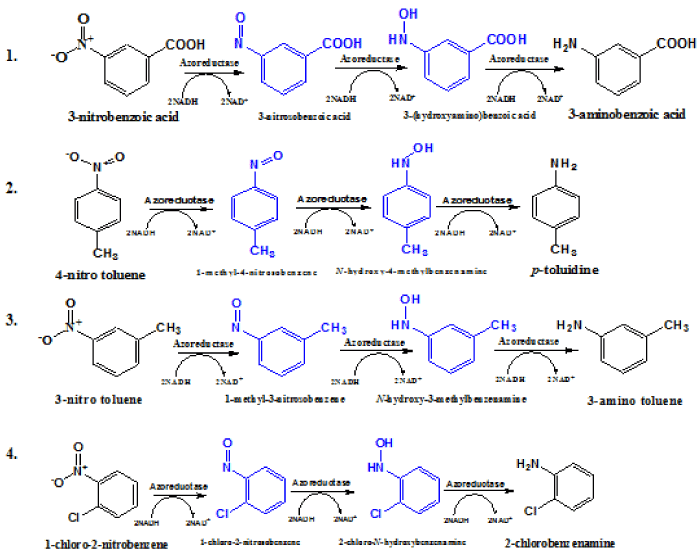
| The FTIR and NMR spectra of transformed products of nitro aromatic compounds clearly showed the presence of NH2 group. It conclusively demonstrating that under aerobic conditions the nitro aromatic compounds were converted efficiently into their corresponding amines by purified flavin-free NADH-azoreductase (Table 3 and Figure 3). Based on properties, the enzymes of monomeric flavin free azoreductases seem to have a different origin but have same chemical function of azo and nitro reduction, suggesting convergent evolution [30]. This flavin-free NADH azoreductase from B. badius contributes to nitro aromatic and azo dye degradation. The azoreductase is quite heat stable and use a wide range of azo dyes and nitro compounds as a substrate. These factors are of prime importance when choosing an enzyme for an industrial process. Stability of an enzyme at extreme conditions is economically and industrially significant. |
| Nitro aromatic compounds, which are priority pollutants and toxicant, sometimes become more reactive upon biotransformation. The toxicity of the nitro aromatic compounds is due to the nitroso and hydroxylamino groups. It has been previously shown that the nitro aromatics compounds can be easily reduced under anaerobic conditions to aromatic amines by different kinds of microorganisms [4,9]. In present study, we demonstrated the aerobic biotransformation of -nitro benzoic acid, 4-nitro toluene, 3-nitro toluene and 1-chloro-2- nitro benzene by purified azoreductase from alkaliphilic B. badius D1 in the presence of NADH at pH 7.4 and 37°C. |
| The nitro group consists of two highly electronegative elements, N and O making the N-O bond more polarized. The partially positive charge of nitrogen atoms, combine with its high electro-negativity, makes the nitro group easily reducible [9]. The established route of reduction of nitro groups occurs as a series of two electron transfer, which yields the nitroso compounds and subsequently, to hydroxyl amine intermediates to the corresponding amino derivatives of parent compounds. In the azoreductase catalyzed reduction reaction, the two pairs of electrons are donated by NADH (Figure 3). |
| Conclusion |
| In conclusion, nitroreductase activity is not only limited to nitroreductase enzymes as we have clearly demonstrated it with the flavin-free NADH-azoreductase. This enzyme has dual major roles in biotransformation, it can reduce (-N=N-) and N=O groups. The stability of azoreductase at higher temperature and pH makes it more favorable for wide biotechnological and industrial applications. In addition, it could also play a leading role in the detoxification mechanisms. The azoreductases could act as a potential biocatalyst for synthesis of valuable drug molecules. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2a | Table 2b | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1a | Figure 1b | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15989
- [From(publication date):
March-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 11233
- PDF downloads : 4756
