Bone Marrow Aspirate and the Use of Autologous Cells and Cellular Products in Regenerative Medicine
Received: 21-Jan-2023 / Manuscript No. ECR-23-87584 / Editor assigned: 24-Jan-2023 / PreQC No. ECR-23-87584 (PQ) / Reviewed: 07-Feb-2023 / QC No. ECR-23-87584 / Revised: 10-Feb-2023 / Manuscript No. ECR-23-87584 (R) / Published Date: 17-Feb-2023 DOI: 10.4172/2161-1165.1000483
Bone Marrow Aspirate Basics
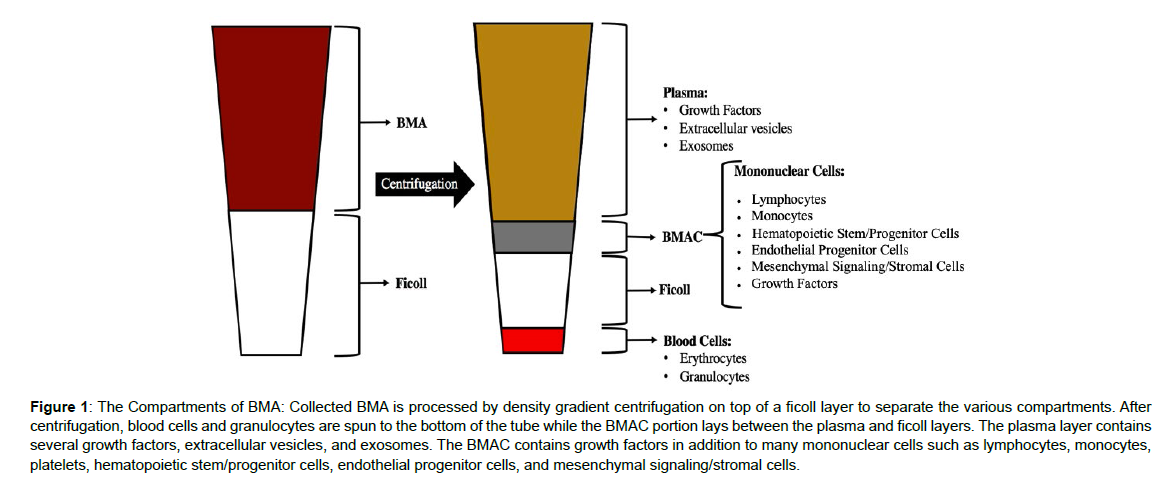
Bone marrow aspirates (BMA) have become an increasingly popular therapeutic source for regenerative medicine due to the relative ease and safety associated with collection. Often harvested from the iliac crest, bone marrow aspirates are one of the few means of acquiring progenitor cells and high concentrations of growth factors for use in tissue engineering and repair studie [1]. Bone marrow aspirates are heterogeneous mixtures containing tissue fragments, peripheral blood, mononuclear cells, and platelets and hematopoetic stem cells (HSCs) [2]. Much of the regenerative potential of BMA is afforded by the growth factors rather than the stem cells, as is often thought, as these HSCs are too naive, and don’t receive the requisite signaling to mature into effector cells, when harvested and reinjected into the patient. Minimal manipulation techniques using gradient centrifugation have been developed to create bone marrow aspirate concentrate (BMAC), which can contribute to the regenerative process by acting as an “irritant”, which is capable to stimulating or boosting immune system activation. In gradient centrifugation processes, whole BMA is layered on top of a Ficoll-Paque or lymphocyte separation media (LSM) and centrifuged to enable the depletion of red blood cells and granulocytes, while concentrating mononuclear cells such as lymphocytes, monocytes, platelets, hematopoietic progenitor/stem cells, and mesenchymal stromal cells (MSCs). Ficoll/LSM is formulated to have a high-density composition so that three layers of cellular products form when non-nucleated red blood cells pass freely through the Ficoll layer, mononuclear cell collect in the middle, and plasma is retained at the top (Figure 1). At the end of the procedure, the BMAC is collected from the middle “buffy coat” layer of the centrifugation, is then washed, aliquoted and cryopreserved for downstream use as the immune activating “irritant”. Aside from its cellular composition, BMAC contains a high concentration of several growth factors. Those growth factors and cytokines found to be enriched in BMAC include, vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), bone morphogenic protein 2/7 (BMP2, BMP7), platelet derived growth factor (PDGF), transforming growth factor-β2 (TGF-β2), fibroblast growth factors (FGF), and insulin-like growth factor (IGF) [2]. Each of the growth factors has been linked to specific pathways found to be beneficial for regenerative medicine across a broad range of therapeutic targets. Consequently, the use of BMAC has gained significant traction for regenerative use in orthopedics and musculoskeletal repair [3,4].
Figure 1: The Compartments of BMA: Collected BMA is processed by density gradient centrifugation on top of a ficoll layer to separate the various compartments. After centrifugation, blood cells and granulocytes are spun to the bottom of the tube while the BMAC portion lays between the plasma and ficoll layers. The plasma layer contains several growth factors, extracellular vesicles, and exosomes. The BMAC contains growth factors in addition to many mononuclear cells such as lymphocytes, monocytes, platelets, hematopoietic stem/progenitor cells, endothelial progenitor cells, and mesenchymal signaling/stromal cells.
Therapeutic Compartments within BMAC
Much of the regenerative potential of BMAC derives from the high concentration of growth factors captured within the mononuclear cell mixture. Together, the array of growth factors can trigger multiple downstream pathways that may induce tissue repair and recovery. In musculoskeletal injuries, the inability for natural cartilage regeneration leads to a progressive decline in function. However, the bone marrow is a rich source of cytokines and growth factors that work to restore cartilage homeostasis through the stimulation of endogenous repair mechanisms, but their abundance and activity declines with age [5].
VEGF is a pro-angiogenic growth factor that indirectly promotes the renewal of de-novo cartilage growth. Generally released in times of hypoxic stress by HIF-1 mediated signaling pathways, VEGF promotes the migration and proliferation of endothelial progenitor cells in the subchondral bone and spongiosa region [6]. These events trigger neoangiogenesis, the growth of new blood vesicle sprouting, that provides fresh nutrient supplies to the damage tissue, and helps remove waste products. Although most mature articular cartilage structures are avascular, the proximity of the vascular network allows for diffusion of required nutrients [7].
IL-8 is a potent chemotaxis cytokine involved in immune responses to injury. In injured tissue, IL-8 is released as a homing mechanism for the recruitment of neutrophils, macrophages, and other bone marrow derived inflammatory cells. Furthermore, IL-8 has been found to enhance the pro-angiogenic effect of bone marrow derived cells via the upregulation of VEGF [8]. Thus, the addition of IL-8 to an injury site may contribute to tissue repair by promoting the migration of endogenous bone marrow cells appropriately primed to contribute to an enhanced regenerative response [9].
TGFβ and BMPs have similar regenerative effects due to their ability to stimulate chondrocyte proliferation and differentiation. These growth factors can target endogenous MSCs, and promote chondrogenic differentiation pathways with the downstream production of new collagen [10]. TGFβ2 is an effective mediator of increased type II collagen and aggrecan production. However, significant research has been conducted regarding the regenerative potential of various subsets of TGF proteins and has shown TGFβ1, TGFβ2, and TGFβ3 to all have positive effects on chondrogenic differentiation and collagen production [11]. A head to head comparison of TGFβ1 and TGFβ2 found TGFβ2 to be a more potent stimulator of MSC activation and collagen production. However, TGFβ2 signaling also leads to an elevation in autrocrine production of TGFβ1, a finding that further highlights the potential benefit of TGFβ2 administration to a collagen depleted injury [12]. BMPs have a synergistic pro-collagen effect to TGFβ, via the stimulation of chondrogenic lineage commitment in resident MSCs. In vitro experiments with cultured MSCs confirm the potential of BMPs in the production of type II collagen and extra cellular matrix [13]. Specifically, BMP-7 is a potent inducer of collagen production that is not dependent upon the severity or any microenvironment cues from the site of injury [14].
PDGF has been well studied for its role in wound healing and angiogenesis, and more recently its role in collagen synthesis [15,16]. One mechanism by which PDGF has been shown to promote collagen production is via the suppression of IL1β-mediated cartilage degradation [17].
In addition to the direct therapeutic potential of BMAC, the cellular compartment of BMAC also offers substantial promise in regenerative research and tissue engineering. Three populations of cells that can be concentrated from bone marrow include the HSCs, endothelial progenitor cells (EPCs), and MSCs. These cell subsets have been shown to provide potential therapeutic benefits in musculoskeletal repair experimentation [18-20].
HSCs transplants were first found to be beneficial in radiationinduced tissue models where transplanted stem cells could re-populate the entire hematopoietic system within a mouse bone marrow niche [21]. This finding laid the framework for future work that established hematopoietic cell transplants as a revolutionary treatment for a range of hematopoietic diseases. HSCs are identified by the positive expression of CD34, CD117 (c-kit) and human leukocyte antigens (HLAs) [22]. It is the presence of HLA on hematopoietic linage cells that requires allogenic donor matching to prevent tissue rejection upon transplantation [23]. Although HSC have been traditionally used for blood disorders, it has been suggested that these cells may possess some level of stem cell plasticity that could mediate their differentiation into other non-hematopoietic cell types, such as neuronal cells, glial cells, hepatic cells, and endothelial cells via a process of transdifferentiation [24].
EPCs are a second progenitor cell type in the bone marrow that can differentiate into mature endothelial cells and contribute to neovascularization. Characterized by positive expression of CD34, CD31, and CD133, EPCs are often recruited from the bone marrow niche into the peripheral blood by secretion of VEGF and stromal-derived factor 1 [25,26]. EPC mobilization mechanisms makes these cells particularly effective in targeting injury specific locations. Increased cytokine production by inflammatory and damaged cells signals EPC recruitment, engraftment, and differentiation into new endothelial cells and repair of established vasculature [27,28].
A third cell type, MSCs, also reside within the bone marrow and play a significant role in musculoskeletal homeostasis. MSCs are often described as a cellular drug store, as these cells secrete many proregenerative cytokines [29]. These cytokines have systemic effects resulting in immune-modulatory, anti-fibrotic, and pro-angiogenic outcomes. In response to microenvironmental cues and stresses such a hypoxia and inflammation, MSC release a robust cocktail of cytokine signals that are beneficial for tissue recovery and cellular reprogramming [30]. Recent insights into the endogenous function of MSC has suggested that MSCs act as pericytes, or perivascular cells that aid in vascular maintenance and wound healing. The localization of MSCs in almost every organ in the body also points to the versatility of this cell type in contributing support to vascularization and tissue health and homeostasis [31-33].
The unique combination of growth factors and immune-activating “irritants” within BMAC makes for a potent mediator of cartilage, bone, and musculoskeletal repair. BMAC therapy has gained traction primarily due to clinical successes in the treatment of chondral injuries and osteoarthritis of the knee [34]. Published studies completed with placebo controlled groups all demonstrate BMAC safety, however clinical efficacy outcomes are often variable due to non-standardized BMAC preparation techniques, administration and follow up protocols [3]. Consequently, it remains unclear what the exact mechanism of action of BMAC is that mediates regeneration and repair at the site of injury. Progenitor cell populations within BMAC represent a small proportion of the total nucleated cells, with MSCs representing only 0.001% of the total population [35]. Such low abundance of progenitor cells has prompted investigators to employee methods for MSC enrichment and isolation as an alternative technique to BMAC therapy. However, direct comparative studies are needed to determine which of these methods hold the most promise.
MSC Production
MSCs can be isolated from BMAC by processing techniques that have been developed using in vitro cell culture. Centrifugation of whole bone marrow aspirate on top of a Ficoll or lymphocyte separation media enables the production of BMAC that can be plated on cell culture dishes. This process was developed by fundamental experiments where BMAC was cultured in the lab resulting in the expansion of adherent, fibroblast colony forming units [36]. After downstream expansion and characterization of these colonies, it was determined that these cells were in fact, MSCs. MSC populations are unique in the BMAC mononuclear cell mixture due to their affinity for attachment to cell culture treated plastic. Simple plating of isolated mononuclear cells onto cell culture dishes results in abundant MSC colony formation within 1-2 days. From mononuclear cell plating (passage zero), MSCs are grown to near confluency before passage with a dissociation reagent such as trypsin (0.5%). MSCs produced for clinical use typically undergo expansion and 2 to 3 passages in order to reach a therapeutic cell number in the millions to billions. However, as primary cell lines, the passaging of clinical products should be minimized to preserve the native phenotype of MSCs. Increasing passages and culture time results in a decrease in MSC potency and gradual loss of proliferative ability as shown by induction of senescence markers and decreased telomere length [37]. Cell culture expansion media has been specifically formulated to provide the necessary nutrients to preserve MSC growth and expansion in the lab. A typical media for MSC expansion includes a basal alpha MEM media with 10-20% fetal bovine serum (FBS). Although clinical trials have demonstrated success with FBS expanded MSCs, the use of animal-based serum has raised concerns regarding immune reactions to residual animal proteins in the final cell product. Therefore, manufacturing protocols are now moving to replace FBS with commercially available human derived platelet lysate and serumfree/ xeno-free media solutions [38, 39].
MSC expansion is typically limited only by the size of the production facility, available incubator space, and number of appropriately trained staff. Therefore, cell banking strategies serve as a reliable option to produce billions of passage-3 MSC products [40]. Cell banks generally consist of a master cell bank and a working cell bank. Master cell banks are typically where low passage number cells are expanded, harvested, and cryopreserved into aliquots at passage-0 or passage-1. This bank contains the most valuable cells that, once aliquoted, can be thawed and re-constituted to continue cell expansion almost without limit. Working cell banks are created for downstream expansion of cells from the master cell bank. The creation of master and working cell bank aliquots aids in the continuous production of batches without exhausting or overwhelming a single facility’s production capability. Bioreactors are increasingly being incorporated into production facilities to simplify the large-scale expansion with minimal manual effort. The development of bioreactors to load, feed, and harvest millions of cells automatically has minimized the manual effort required to produce large batches of MSCs. Although bioreactor formats vary in size and structure, hollow-fiber bioreactor set ups have become increasingly popular for MSC production [41]. These bioreactors operate as a closed system and retain the sterility and environmental regulation of a cGMP laboratory. The hollow-fiber composition of these bioreactors accommodates a large surface area size within a small and compact space.
Cryopreservation of the expanded cellular product is the final step in the cell production protocol for both BMAC and MSC therapies. Correct execution of this step is vital for the preservation of cellular viability and biological function of the expanded cells. Typically, prior to cell infusion, cells are stored in liquid nitrogen tanks for months to years. Cryopreservation medias may vary but generally contain 5-10% DMSO, a sulphur-based cryo-protectant [42]. The addition of DMSO is a critical component for cell membrane integrity during the cryopreservation process, however DMSO is known to be cytotoxic if not handled appropriately. Furthermore, the presence of DMSO in the delivered cell product may cause adverse reactions in a small subpopulation of sulphur-sensitive patients [43,44].
MSCs are currently being used in clinical trials for a wide variety of bone, cartilage, and joint injuries. Their immunomodulatory capabilities are complimented by a robust ability to remain immune evasive due to a lack of HLA expression on their surface. This feature has prompted the development of allogenic therapies, by which MSCs are derived from young, healthy donors or umbilical cords and expanded for use in multiple patients [45]. Head-to-head comparison studies of autologous and allogeneic MSC cell therapy has not only confirmed the safety of allogenic cells but has indicated a superior regenerative effect [46,47]. MSCs derived from patients with various diseases, such as atherosclerosis, have been found to carry regenerative defects throughout cell culture expansion. Increased reactive oxygen influences the laboratory expansion of these MSCs and inhibits their regenerative potency [48,49]. Therefore, allogenic sources of MSCs are increasingly being thought to be a superior source of therapeutic MSCs.
Despite many clinical successes, the precise mechanism(s) by which MSCs exert their therapeutic effect is still hotly debated. While the multipotent differentiation potential of MSCs has been robustly demonstrated in various research models, it is believed that transplanted MSCs do not engraft or differentiate into new tissues, in vivo [50]. In fact, most transplanted MSCs die within a few days due to harsh microenvironmental conditions, anoikis, and inflammation [51,52]. Yet, despite such a dramatic loss of cells within the first days after transplant, MSCs have been shown to induce effects that can last several months, if not years. Consequently, their primary mechanism of action is believed to comprise of paracrine signaling through the controlled release of growth factors, cytokines and extracellular vesicles (exosomes) that stimulate endogenous and exogenous repair mechanisms, both at the site of injury and remotely [53-56]. Most recently, MSCs have been found to be rapidly phagocytosed by endogenous monocytic cells [57]. Monocytes play a critical role in the modulation of immune responses and the regulation of inflammation. Phagocytosis of transplanted MSCs by can induce phenotypic and functional changes in macrophages that leads to adaptations away from a pro-inflammatory M1 phenotype, towards a more anti-inflammatory M2 state [57].
MSC-derived extracellular vesicles
The survival of transplanted cells can vary greatly among both donors and recipients. This inevitably contributes to the therapeutic success of cellular transplants and the observable variations reported in clinical applications. As previously stated, the primary mechanism of action of BMAC and MSCs is attributed to the high concentration of growth factors and the liberation of extracellular vesicles, called exosomes. Therefore, protocols have been developed to further concentrate this functional fraction of BMAC and MSCs to provide a greater, and more consistent, regenerative outcome. Exosomes are small membrane bound vesicles (typically about 50-150 nm) produced from the cell’s endosomal pathway and excreted into the extracellular space via the exocytosis of multi vesicular bodies [58,59]. Exosomes differ from other larger extracellular vesicles and apoptotic bodies not only from their cellular origins, but also in their contents. Comparative analysis of various extracellular vesicles shows an enrichment of many different proteins and nucleic acids in exosomes. Exosomes are enriched for growth factors including VEGF and stem cell factor (SCF), small non-coding RNAs such as micro RNA, and tetraspanin proteins including CD9, CD63, and CD81 [60]. These cargos are important for cell to cell paracrine inter-communication in the maintenance of homeostasis and during tissue repair [61,62]. As a mechanism of cell-cell communication, exosomes regulate many pathways including, but not limited to, angiogenesis, inflammation,and cellular migration [58]. Exosomes mediate these effects by direct fusion with, or endocytosis into, host cells [63]. The exosome itself may play a role in immunomodulation of the microenvironment at the site of inflammation or may indirectly contribute to the cascade of events that leads to functional recovery [64]. In addition to being a highly enriched source of pro-regenerative growth factors, exosomes exhibit additional features that make them attractive candidates for regenerative medicine. Being an acellular product, they are much easier to handle in a clinical setting, their size making them a more versatile product with regards to patient delivery. Storage and transport are also much easier compared to MSCs as cryogenic preservation and meticulous thawing protocols are not required.
Laboratory protocols including ultracentrifugation, size gradient centrifugation, and size exclusion chromatography have been developed to facilitate the isolation exosomes from culture expanded MSCs [65]. However, BMAC-derived plasma alone can yield an easily accessible reserve of exosomes with regenerative potential [66]. These exosomes are highly variable between donated lots compared to the homogeneous product from cultured MSCs. As such, further largescale trials are required before relative efficacy can be determined in a clinical setting.
The Cell Production Laboratory (cGMP/cGTP)
All manufacturers of cell and tissue products produced for clinical use must follow the current good manufacturing practice (cGMP) guidelines based on the Federal Food, Drug, and Cosmetic Act (FD&C). These products must be produced in accordance with the code of federal regulation (CFR), Title 21, Part 1271 and current good tissue practice (cGTP) [67]. These acts ensure that the proper methodology for processing, packaging, and storing be followed to meet FDA requirements for safety, identity, quality, and purity. These regulations are set in place to prevent the introduction and transmission of communicable disease such as viruses, parasites, fungus, and bacteria. Accredited facilities and clean rooms registered to produce human cells, tissue, and cellular- and tissue-based products (HCT/P) must abide by these regulations and follow all stipulations laid out by the FD&C act. However, regulatory distinctions are made between minimally processed tissue facilities and cell production facilities. Minimal processing and tissue transfusion facilities in which product is isolated and stored for future clinical use are not required to register and follow the same regulations as a HCT/P facility. Cellular products that are considered “minimally manipulated” cannot be combined with another product or device and must be used for autologous treatments or used for allogenic treatments for close relatives. The distinction between a minimally processed tissue versus a laboratory produced cell or tissue product has caused some debate across the cell therapy community. Generally, minimally manipulated tissue products undergo minimal ex vivo handling where processing does not alter their native cellular state. BMAC is considered a minimally manipulated product due to the single centrifugation step required to enrich for the final cellular product. There are no downstream laboratory manipulations or modifications to the cells and the isolate is immediately ready for reinfusion [68]. MSC isolation and expansion does not meet the requirements to be designated as a minimally manipulated product and is therefore classified by the FDA an investigational new drug (IND). Therefore, expanded MSCs and their exosomes are subjected to stricter regulatory and characterization guidelines compared to BMAC.
Cell product manufacturing requires a laboratory that employs strict environmental monitoring, record keeping, and product testing for quality and regulatory compliance [69,70]. These facilities are designed to minimize the risk of product contamination by incorporating a uni-directional work flow in and out of the laboratory, a single-pass HEPA air filtration system and a 100% exhaust from biological safety cabinets through dedicated vents. Additionally, the structural design of a cGMP facility often includes a positive air pressure system that directs air circulation from within the tissue culture safety cabinet outwards towards less controlled areas. Environmental stability and equipment function are connected to a central monitoring system that alerts employees remotely of power disruptions or out-of-range changes that may need attention.
Assessment of product safety and sterility begins with the establishment of donor eligibility and donor screening/testing methods. The production facility must review appropriate medical records to ensure the donor is free from communicable diseases. Furthermore, throughout product production, the facility must maintain step-by-step written batch records to document and standardize the manufacturing process. Sterility checkpoints are established and recorded throughout product production and any positive sterility cases must be investigated for source. These checkpoints begin at the receipt of tissue and continue throughout cell manufacturing, cryopreservation, and product thawing. Sterility testing of cell products after the cryopreservation process is a critical step for product release and acceptance by the end user. Sterility testing must be completed by FDA-registered, CLIAcertified labs using approved and cleared protocols. Testing of the final cell product must include tests for mycoplasma, endotoxin and a gram stain. The results of these tests should be recorded and filed along with detailed laboratory cleaning records and sterility practices to ensure work is completed under FDA compliance. These data will need to be presented to the FDA during any site visit.
In addition to safety, production laboratories should also establish scientifically based specification and standardizations tests to ensure consistency between production batches. These qualification methods should not only include cell product composition but also product stability for the determination of long-term storage and expiration. These qualifications are aggregated to create minimum release criteria that all cell products must pass before being released for therapy.
Cell Product Characterization and Release Criteria Techniques
All cell products must pass basic quality assurance requirements in order to meet minimum release criteria before leaving the laboratory [71]. Cell count and cell viability pre- and post- cryopreservation are two fundamental tests that must be performed. Cell characterization, typically implemented by flow cytometry, western blot, or RNA analysis are more advanced molecular methods that better categorize the final cell product. In the case of culture expanded MSCs an identification profile would include a positive surface expression of CD90, CD105, and CD73 combined with negative expression of CD45, CD34, and CD31 [72]. These markers are essential to demonstrate a pure MSC population free of hematopoietic, endothelial and fibroblast cells.
Advanced characterization of cellular products is an essential step for the examination of product potency, reproducibility, and effectiveness. In large clinical trials or in cell banking, the selection of the most “potent” cell products will increase clinical effectiveness. There are currently no guidelines established to define the effectiveness of cell therapy products and batch-to-batch or harvest-to-harvest variation is anticipated and currently unavoidable. The age of the donor cells and the accumulation of reactive oxygen species throughout ex vivo expansion can have substantial effects on BMAC-derived MSC function [48,49]. This can be exacerbated in vitro as media composition, handling technique and culture time can induce a number of changes in MSC biology. Therefore, the collection of advanced characterization data sets prior to and after expansion is essential to follow MSC batches for consistency and potency.
Cytokine analysis of the final product further allows for the predication and correlation of cell function to clinical outcome. Cytokine analysis is completed by ELISA-based assays by which conditioned media or BMAC sample is collected and tested. This data can be used in downstream analysis to select the most concentrated and potent products that best fit the clinical application.
BMAC-derived exosome product characterization can be completed in a similar manner. In general exosome products must be composed of particles of the expected size range (50-150nm), be enriched for exosome surface markers CD9, CD81, and CD63, and be absent of intracellular organelle proteins such as mitochondrial cytochrome C.
In vitro, functional assays can be combined with cytokine analysis and exosome characterization to establish causal effects of basic cell-to-cell interactions such as, but not limited to, cell migration, angiogenesis, and cell proliferation. Co-cultures with target cells and cell products can provide important scientific insight into mechanisms of action. These assays also allow for the identification of protein and miRNA mediators of the observed regenerative effect. For example, MSC have been demonstrated to promote T-cell suppression in vitro by co-culture assays that record T-cell activation markers and their proliferation potential. This observation has been further validated in clinical trials, where patients who received both allogenic and autologous MSC showed reduction in early T-cell activation [47].
Conclusion
BMAC and cellular- (or cyto-) therapy is a new paradigm in medicine that holds great promise for the treatment of chronic musculoskeletal injuries and diseases. However, the biggest challenge facing the advancement of these therapies is the need for standardization and consistency in product production and reporting. Basic science has provided consistent evidence that BMAC and MSC therapy hold regenerative potential as a consequence of their cellular and growth factor compositions. Thus, it is critical that comprehensive product characterization be pursued so that clinical successes, as well as failures, can be analyzed and examined at a cellular level. It is important to identify those fixed and flexible components of cellular therapy products that drive product efficacy if we are to move the technology forward. Demonstrable safety of BMAC and MSC cell therapy has ignited the drive for companies and academic institutions to test these treatments for a range of degenerative disease. As progress accelerates patient safety and product sterility standards must be held to the highest standards to ensure the success and continuation of this growing field.
References
- Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, et al. (2017) Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc Tech 6: e441-e445.
- Holton J, Imam M, Ward J, Snow M (2016) The Basic Science of Bone Marrow Aspirate Concentrate in Chondral Injuries. Orthop Rev (Pavia) 8: 6659.
- Piuzzi NS, Hussain ZB, Chahla J, Cinque ME, Moatshe G, et al. (2018) Variability in the Preparation, Reporting, and Use of Bone Marrow Aspirate Concentrate in Musculoskeletal Disorders: A Systematic Review of the Clinical Orthopaedic Literature. J Bone Joint Surg Am 100: 517-25.
- Imam MA, Holton J, Horriat S, Negida AS, Grubhofer F, et al. (2017) A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J 3: 58.
- Moatshe G, Morris ER, Cinque ME, Pascual-Garrido C, Chahla J, et al. (2017) Biological treatment of the knee with platelet-rich plasma or bone marrow aspirate concentrates. Acta Orthop 88: 670-674.
- Murata M, Yudoh K, Nakamura H, Kato T, Inoue K, et al. (2006) Distinct signaling pathways are involved in hypoxia- and IL-1-induced VEGF expression in human articular chondrocytes. J Orthop Res 24: 1544-1554.
- Maes C, Stockmans I, Moermans K, Van Looveren R, Smets N, et al. (2004) Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest 113: 188-199.
- Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH, et al. (2014) IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int 38: 1050-1059.
- Eseonu OI, De Bari C (2015) Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology (Oxford) 54: 210-218.
- Yu DA, Han J, Kim BS (2012) Stimulation of chondrogenic differentiation of mesenchymal stem cells. Int J Stem Cells 5: 16-22.
- Villiger PM, Lotz M (1992) Differential expression of TGF beta isoforms by human articular chondrocytes in response to growth factors. J Cell Physiol 151: 318-325.
- Joyce ME, Roberts AB, Sporn MB, Bolander ME (1990) Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110: 2195-2207.
- Scarfi S (2016) Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells 8: 1-12.
- Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ (2011) The role of growth factors in cartilage repair. Clin Orthop Relat Res 469: 2706-2715.
- Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A (1991) Role of platelet-derived growth factor in wound healing. J Cell Biochem 45: 319-326.
- Ataliotis P (2000) Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Dev 94: 13-24.
- Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, et al. (2011) IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One 6: e28663.
- Kamei N, Atesok K, Ochi M (2017) The Use of Endothelial Progenitor Cells for the Regeneration of Musculoskeletal and Neural Tissues. Stem Cells Int 2017: 1960804.
- Jones EA, Giannoudis PV, Kouroupis D (2016) Bone repair with skeletal stem cells: rationale, progress to date and clinical application. Ther Adv Musculoskelet Dis 8: 57-71.
- Xiong LL, Liu F, Deng SK, Liu J, Dan QQ, et al. (2017) Transplantation of Hematopoietic Stem Cells Promotes Functional Improvement Associated with NT-3-MEK-1 Activation in Spinal Cord-Transected Rats. Front Cell Neurosci 11: 213.
- Lupu M, Storb R (2007) Five decades of progress in haematopoietic cell transplantation based on the preclinical canine model. Vet Comp Oncol 5: 14-30.
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32: 1380-1389.
- Park M, Seo JJ (2012) Role of HLA in Hematopoietic Stem Cell Transplantation. Bone Marrow Res 2012: 680841.
- Ogawa M, LaRue AC, Mehrotra M (2015) Plasticity of hematopoietic stem cells. Best Pract Res Clin Haematol 28: 73-80.
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, et al. (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175-189.
- Hristov M, Weber C (2004) Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med 8: 498-508.
- Rennert RC, Sorkin M, Garg RK, Gurtner GC (2012) Stem cell recruitment after injury: lessons for regenerative medicine. Regen Med 7: 833-850.
- Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9: 11-15.
- Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45: e54.
- Caplan AI (2017) Mesenchymal Stem Cells: Time to Change the Name. Stem Cells Transl Med 6: 1445-1451.
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, et al. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301-313.
- Caplan AI (2008) All MSCs are pericytes. Cell Stem Cell 3: 229-230.
- Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, et al. (2016) Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J Sports Med 4: 2325967115625481.
- Gianakos AL, Sun L, Patel JN, Adams DM, Liporace FA (2017) Clinical application of concentrated bone marrow aspirate in orthopaedics: A systematic review. World J Orthop 8: 491-506.
- Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P(2006) Postnatal skeletal stem cells. Methods Enzymol 419: 117-48.
- Gharibi B, Hughes FJ (2012) Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med 1: 771-782.
- Oikonomopoulos A, van Deen WK, Manansala AR, Lacey PN, Tomakili TA, et al. (2015) Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep 5: 16570.
- Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, et al. (2015) Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther 6: 55.
- Cooper K, Viswanathan C (2011) Establishment of a mesenchymal stem cell bank. Stem Cells Int 2011: 905621.
- Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, et al. (2016) Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med.
- Stacey GN, Masters JR (2008) Cryopreservation and banking of mammalian cell lines. Nat Protoc 3: 1981-1989.
- Otrock ZK, Sempek DS, Carey S, Grossman BJ (2017) Adverse events of cryopreserved hematopoietic stem cell infusions in adults: a single-center observational study. Transfusion 57: 1522-1526.
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, et al. (2014) Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28: 1317-1330.
- Zhang J, Huang X, Wang H, Liu X, Zhang T, et al. (2015) The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther
- Glassberg MK, Minkiewicz J, Toonkel RL, Simonet ES, Rubio GA, et al. (2017) Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 151: 971-81.
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, et al. (2017) Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol 69: 526-237.
- Bellio MA, Khan A (2018) Improving Cell Production Techniques to Enhance Autologous Cell Therapy. Circ Res 122: 191-3.
- Kizilay Mancini O, Lora M, Cuillerier A, Shum-Tim D, Hamdy R et al. (2018) Mitochondrial Oxidative Stress Reduces the Immunopotency of Mesenchymal Stromal Cells in Adults With Coronary Artery Disease. Circ Res 122: 255-66.
- Lee S, Choi E, Cha MJ, Hwang KC (2015) Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev
- Ding Y, Zhang Y, Tse HF, Lian Q (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23: 1045-1059.
- Bao L, Meng Q, Li Y, Deng S, Yu Z, et al. (2017) C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J Card Fail 23: 403-415.
- Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther 23: 812-823.
- Fu Y, Karbaat L, Wu L, Leijten J, Both SK, et al. (2017) Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng Part B Rev 23: 515-528.
- de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A et al. (2018) Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 36: 602-615.
- Qin J, Xu Q (2014) Functions and application of exosomes. Acta Pol Pharm 71: 537-543.
- Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907-1920.
- Keerthikumar S, Gangoda L, Liem M, Fonseka P, Atukorala I et al. (2015) Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 6: 15375-15396.
- Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S (2015) Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15: 260-271.
- Barile L, Moccetti T, Marban E, Vassalli G (2017) Roles of exosomes in cardioprotection. Eur Heart J 38: 1372-1379.
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, et al. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654- 659.
- Tan L, Wu H, Liu Y, Zhao M, Li D (2016) Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity 49: 357-365.
- Colao IL, Corteling R, Bracewell D, Wall I (2018) Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med 24: 242-256.
- Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, et al. (2015) Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS One 10: e0145686.
- Freeman M, Fuerst M (2012) Does the FDA have regulatory authority over adult autologous stem cell therapies? 21 CFR 1271 and the emperor's new clothes. J Transl Med 10: 60.
- George B (2011) Regulations and guidelines governing stem cell based products: Clinical considerations. Perspect Clin Res 2: 94-99.
- Campbell A, Brieva T, Raviv L, Rowley J, Niss K, et al. (2015) Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med 4: 1155-1163.
- Belotti D, Gaipa G, Bassetti B, Cabiati B, Spaltro G, et al. (2015) Full GMP-compliant validation of bone marrow-derived human CD133(+) cells as advanced therapy medicinal product for refractory ischemic cardiomyopathy. Biomed Res Int 473159.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR (2014) MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14: 141-145.
- Hagmann S, Moradi B, Frank S, Dreher T, Kammerer PW, et al. (2013) Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet Disord 14: 223.
- Witwer KW, Soekmadji C, Hill AF, Wauben MH, Buzas EI, et al. (2017) Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles 6: 1396823.
- Landgraf K, Brunauer R, Lepperdinger G, Grubeck-Loebenstein B (2011) The suppressive effect of mesenchymal stromal cells on T cell proliferation is conserved in old age. Transpl Immunol 25:167.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google scholar , Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: White IA (2023) Bone Marrow Aspirate and the Use of Autologous Cellsand Cellular Products in Regenerative Medicine. Epidemiol Sci, 13: 483. DOI: 10.4172/2161-1165.1000483
Copyright: © 2023 White IA. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3184
- [From(publication date): 0-2023 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 2793
- PDF downloads: 391