Calcium Dysregulation in Alzheimer's Disease: A Target for New Drug Development
Received: 17-Aug-2017 / Accepted Date: 08-Sep-2017 / Published Date: 15-Sep-2017 DOI: 10.4172/2161-0460.1000374
Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder and the most common cause of dementia among aged people whose population is rapidly increasing. AD not only seriously affects the patient’s physical health and quality of life, but also adds a heavy burden to the patient’s family and society. It is urgent to understand AD pathogenesis and develop the means of prevention and treatment. AD is a chronic devastating neurodegenerative disease without effective treatment. Current approaches for management focus on helping patients relieve or delay the symptoms of cognitive dysfunction. The calcium ion (Ca2+) is an important second messenger in the function and structure of nerve cell circuits in the brain such as neuronal growth, exocytosis, as well as in synaptic and cognitive function. Increasing numbers of studies suggested that disruption of intracellular Ca2+ homeostasis, especially the abnormal and excessive Ca2+ release from the endoplasmic reticulum (ER) via the ryanodine receptor (RYR), plays important roles in orchestrating the dynamic of the neuropathology of AD and associated memory loss, cognitive dysfunction. Dantrolene, a known antagonist of the RYR and a clinically available drug to treat malignant hyperthermia, can ameliorate the abnormal Ca2+ release from the RYR in AD and the subsequent pathogenesis, such as increased β-secretase and γ-secretase activities, production of Amyloid-β 42 (Aβ 42) and its oligomer, impaired autophagy, synapse dysfunction, and memory loss. However, more studies are needed to confirm the efficacy and safety repurposing dantrolene as a therapeutic drug in AD.
Keywords: Alzheimer’s disease; Calcium; Ryanodine receptor; Dantrolene
Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder and the most common cause of dementia among aged people [1,2]. AD may also result in abnormality in mood and personality [3,4]. Alzheimer’s disease is named after Dr. Alois Alzheimer, who first described this disease in his patient in 1906 [5]. AD represents one of the biggest diseases without effective treatment confronting human beings during this millennium [6]. Today, a new AD patient is diagnosed every 66 s in the United States. By 2050, one new case of AD is expected to develop every 33 s, resulting in nearly 1 million new cases per year [2]. In the US alone, an estimated 5.5 million patients are diagnosed with AD, a devastating neurodegenerative disease without effective treatment [4,7]. By 2050, the number of AD patient is expected to grow to 13.8 million [2,4]. Death usually occurs within 5 to10 years after a clinical diagnosis [8]. The total estimated worldwide financial burden of dementia was $604 billion in 2010 [9]. AD not only seriously affects the patient’s physical health and quality of life, but also adds a heavy burden to their family and society. It is urgent to understand AD pathogenesis and develop the means of prevention and treatment. Considering the rapidly increased elderly population, AD has become a major health problem for human beings.
AD Pathophysiology and Treatment Status
AD is a chronic devastating neurodegenerative disease without effective treatment [10]. Current approaches for management focus on helping patients slow or delay the symptoms of cognitive dysfunction [11,12]. There are many hypotheses about the pathogenesis of AD such as amyloid hypothesis, tau protein hypothesis, genetic hypothesis, excitatory amino acid hypothesis, chronic inflammation hypothesis, oxygen free radicals leading to neurodegenerative disease hypothesis, and neuronal apoptosis hypothesis. Amyloid cascade hypothesis is widely presumed to cause AD pathogenesis [13-15]. The autopsy of the pathological features is amyloid-β (Aβ) aggregates composed of senile plaques, intracellular neurofilament consisting of hyperphosphorylated tau protein deposits neurofibrillary tangles of tau (NFT) and loss of cerebral cortex caused by atrophy [16-18]. Many potential treatments for AD focused mainly on reducing levels of amyloid-β (Aβ) burden in the brain and inhibiting Aβ aggregation and promotion of Aβ clearance [19-21]. Despite the tremendous research looking into the molecular mechanisms of Aβ pathology, it is still unclear about the root causes of the AD related cognitive dysfunction [22,23]. Unfortunately, no drug of the amyloid-targeting the cascade is in the process to be approved for treatment of AD in patients [22,24]. Because Tau pathology play important roles in neurodegeneration, which is usually seen together with amyloid pathology, researchers also tried to develop new drugs targeting hyperphosphorylated NFT [22,25]. Studies have shown that there is a strong link between NFT deposition and neuronal loss related cognitive dysfunction [26-28]. Recent studies have suggested some tau genetic markers are associated with AD [29,30]. Unfortunately, there are no new drugs targeting tau pathology successful in patients up to now, although efforts are continued [22].
In order to block the progression of the disease in AD, we need to interfere with the pathogenic steps responsible for the clinical symptoms. Beside amyloid and tau pathology, alternative theories have been proposed for the pathogenesis of AD, such as inflammation, oxidative damage, iron deregulation, and cholesterol metabolism, etc. [31,32].
Role Of Calcium Signalling In Physiological Neural Processes And Dysregulation Of Calcium Signalling In The Pathogenesis Of AD
The calcium ion (Ca2+) is an important second messenger in the function and structure of nerve cell circuits in the brain. Ca2+ signalling regulates multiple neuronal functions, such as neuronal growth, exocytosis, synaptic plasticity and cognitive function [33-35]. Therefore disturbances in Ca2+ homeostasis can affect the neuron normal function and structure. A number of studies have shown that disruption of intracellular Ca2+ homeostasis plays important roles in orchestrating dynamic of the neuropathology of AD and associated memory loss, cognitive dysfunction [36-41].
Studies show that Ca2+ level in those neurons close to amyloid deposits is higher than normal resting level [42]. The elevated resting Ca2+ environment cloud promotes mechanisms of negative plasticity [43]. The mechanisms are an increase in calcineurin (CaN) expression and activity by elevated intracellular level. CaN is a Ca2+ signalling protein activated calmodulin (CaM), which is sensitive to subtle rises in intracellular Ca2+ levels. When CaN is activated, it is able to activate additional phosphatases, such as PP1, which further induce the longterm depolarization (LTD) that erases memories [44,45]. With blinding of Ca2+/CaM, CaMKII holenzymes can be activated. CaMKII also plays an important role in synaptic plasticity and memory formation. T286- autophosphorylation of αCaMKII is impaired at synapses in AD using post-mortem analyses and studies. The T286-autophosphorylation of αCaMKII in the hippocampus rescues deficits in contextual memory formation [46]. Studies suggested that neurotrophin-induced enhancement of p(T286)-αCaMKII leads to rescue of Aβ-induced deficits in LTP at hippocampal synapses [47]. Further, CaMKII has also been suggested to be a tau kinase. Studies with AD brain find that αCaMKII expression in cells usually co-localises with tau mRNA or NFT [48-50]. So, CaMKII dysregulation may therefore be closely related with Alzheimer’s disease. Small dose of sAβ1-42 impaired Ca2+ clearance from presynaptic terminals and increased the basal Ca2+ concentration in cultured rat hippocampal neurons. This caused an increase in the phosphorylation of Ca2+/calmodulin-dependent protein kinase IV (CaMKIV) and its substrate synapsin, which markedly inhibited synaptic vesicle (SV) trafficking along axons between synapses. sAβ1-42 prevents neurons from forming new synapses or adjusting strength and activity among neighboring synapses [51]. CaMKIV is crucially involved in Ca2+ induced CREB phosphorylation. Neural activity dependent CaMKIV signalling in the neuronal nucleus plays an important role in the consolidation/retention of hippocampusdependent long-term memory [52].
Researches demonstrated that hTau accumulation caused remarkable dephosphorylation of cAMP response element binding protein (CREB) in the nuclear fraction both in vivo and in vitro studies. Activity-dependent activation of the transcription factor CREB is at a central converging point of pathways and mechanisms activated during the processes of synaptic strengthening and memory formation, as CREB phosphorylation leads to transcription of memory-associated genes [53]. Disruption of these mechanisms in AD results in a reduction of CREB activation with accompanying memory impairment [54]. hTau accumulation impairs synapse and memory by CaN-mediated suppression of nuclear CaMKIV/CREB signalling [55].
Due to spatial and temporal patterns of amyloid deposition, which does not correlate very well with the clinical degree of dementia in Alzheimer disease, the amyloid hypothesis remains controversial. In contrast, cognitive decline correlates very well with synapse loss [56]. It is actually the occurrence of ‘negative’ lesions such as synaptic loss which precedes neuronal loss that best correlates with the advancement of cognitive decline. Several reports have noted the progressive loss of synaptic boutons and other synaptic elements in brains of patients with symptoms ranging from mild cognitive impairment (MCI) to earlymild AD [57,58]. In vitro studies have shown that Aβ oligomers can directly bind to synaptic sites [59] and reduce long-term potentiation (LTP) [60,61].
In early AD, mild cognitive impairment may be due to synaptic dysfunction with no widespread synaptic loss and neurodegeneration. Soluble Aβ oligomers can adversely affect synaptic structure and plasticity even at extremely low concentrations. In many cases, AD transgenic mice show abnormal synaptic transmission and impaired LTP usually before plaque formation [62,63]. Ca2+ is an essential mediator of basal synaptic transmission, short and long forms of synaptic plasticity, and dendritic spine morphology [64]. In AD mouse models at asymptomatic or early disease stages, the increased Ca2+ affects the synaptic pathophysiological processes by increasing both frequency of spontaneous synaptic potentials and negative plasticity [65,66].
Negative plasticity was proposed to explain cognitive decline in older people. Their framework describes a self-reinforcing, downward spiral of negative brain plasticity whereby declining brain function is attributable to a combination of disuse reduced quality of sensoryperceptual processing and weakened neuromodulatory control. In combination, these factors increase reliance on simplified cognitive processing at the expense of more complex processing capacity [67].
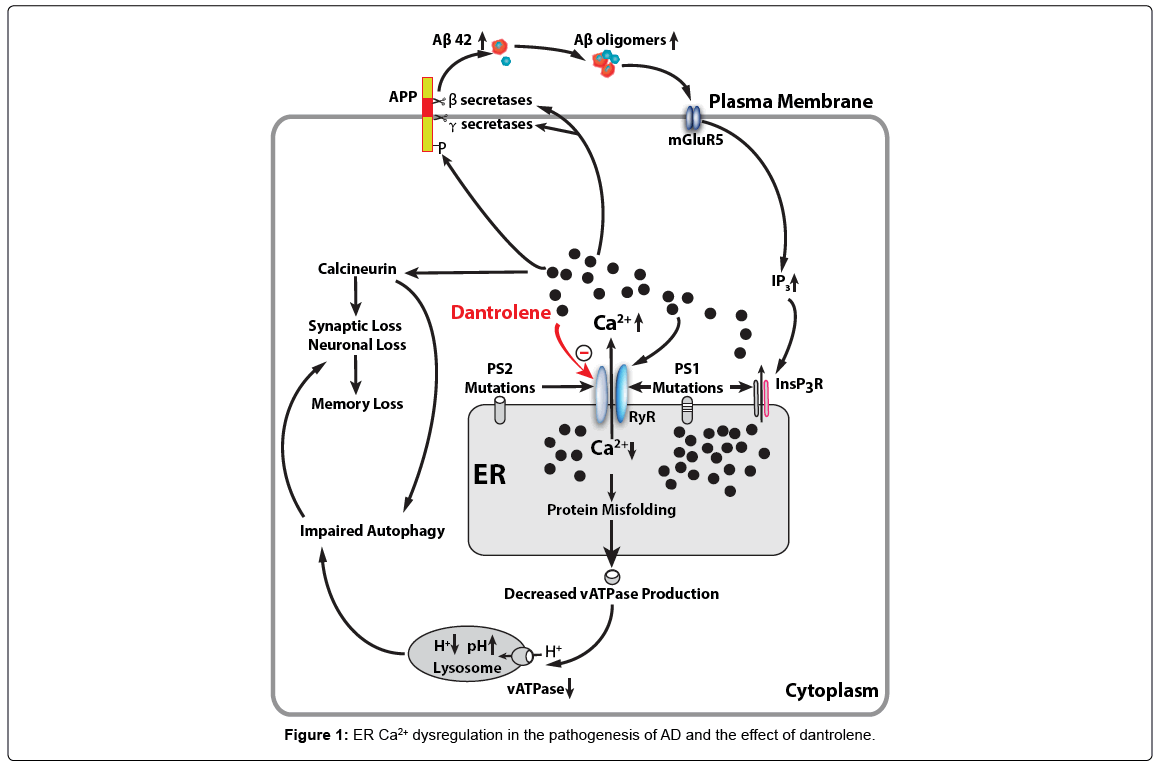
Additionally, Aβ plaque deposition was needed to induce calcium overload [42]. Aβ oligomers can increase cytosolic calcium through forming novel pores on plasma membranes and can stimulate mGluR5 which increases InsP3 production and Ca2+ release [68-71]. Recent studies showing that intracellular Aβ oligomers can stimulate G-protein-mediated Ca2+ release from the Endoplasmic reticulum (ER) through InsP3 [72]. The ER is a particularly intriguing organelle that actively removes Ca2+ from the cytoplasm and can release stored Ca2+ into cytosolic space through ER membrane calcium channel receptors, Inositol 1,4,5-Trisphosphate receptor (InsP3R) or the ryanodine receptor (RYR). Excessive Ca2+ release from the ER via activation of RYR and/or InsP3R is associated with amyloid and tau pathology and contributes to memory and learning loss in AD40 [73,74], while RYR can be activated by Ca2+ itself and may amplify the function of InsP3R via a calcium activated calcium release mechanism [75,76]. This may decrease or deplete Ca2+ levels in the ER. The abnormally low Ca2+ level will cause a decrease in vATPase production due to the protein-folding reaction depending on high concentrations of Ca2+ in the ER [77]. When vATPase maturation in the ER is disturbed, the proper pH value in lysosomes can’t be maintained due to decreased vATPase, which leads to impaired lysosomal acidification and function and subsequent autolysosome and autophagy function. It is interesting to note that ER Ca2+signaling abnormalities; plasticity and memory deficits precede detectable amyloid and tau pathology in AD [36].
ER is an important subcellular organelle for protein synthesis, modification and folding. ER stress and associated unfolded protein accumulation is triggered by the disruption of Ca2+homeostasis. ER stress can stimulate cells to cope with unfolded protein responses, which promote protein folding or degradation of abnormal folding proteins [78]. Protein misfolding and aggregation are common pathogenic mechanisms in a number of human diseases, including AD. Perturbations of the function or integrity of the ER such as the accumulation of misfolded proteins in the ER lumen, results in a condition-termed ER stress. To avert this condition, cells activate an integrated array of adaptive intracellular signaling cascades known as the unfolded protein response (UPR). ER stress is induced during AD, and has been indirectly implicated as a mediator of Aβ neurotoxicity. In neurodegenerative diseases like AD, these abnormal reactions may play an important role [79]. ER stress could be the consequence of aberrant cellular signaling induced by the interaction of Aβ oligomers with membrane receptors, although these mechanisms are possible contributors to Aβ neuropathology.
Aβ42 expression induces strong ER stress response and the strongly activated UPR failure to buffer the misfolded protein load, leading to cellular dysfunction and a shorter chronological life span (CLS) [80]. Multiple studies have demonstrated that Aβ oligomers can activate PKR and induce ER stress by eliciting the TNF-α pathway [81,82]. Additionally, Aβ may stimulate ER Ca2+ release through ryanodine receptors and IP3 receptors, which triggers ER stress, neuronal apoptosis and mitochondrial fragmentation [72,83]. ER stress and hyperphosphorylated tau could be induced by each other in a cycle to propagate AD pathology [84]. Furthermore, studies have shown that mutations in PS1 inhibit ER stress-induced lREla PERK autophosphorylation and eIF2α phosphorylation in ER membranes .It has been suggested that familial AD-linked PS1 mutations suppress the activation of IRE-1α. This predisposes cells to become more susceptible to ER stress due, in part, to decreases in protein chaperone synthesis as a result of reduced UPR induction [85,86]. The aberrantly spliced isoform of PS2 (PS2V) is also linked to AD. Similar to the PS1 mutations, this isoform increases the vulnerability of the cell to ER stress [87].
The most abundant microtubule-associated protein is the Tau protein. In healthy brains, the combination of tau protein and tubulin promotes its polymerization to form microtubulins. Tau proteins then combine with microtubulins to maintain microtubule stability and induce microtubules into bundles. However, tau protein in the brain of AD patients is abnormally hyper phosphorylated, which leads to biological function loss [88]. Temporarily increased intracellular calcium signaling would induce prolonged increased tau phosphorylation via glycogen synthase kinase 3-β (GSK-3β) pathway in human neuroblastoma SH-SY5Y cells [89]. On the other hand, when the hippocampal and cortical neurons were cultured with tau protein, significantly increased intracellular calcium through muscarinic receptor was observed [90]. The cytoplasmic protein tau normally serves to stabilize microtubules which form ‘tracks’ that facilitate intracellular vesicle trafficking and axonal elongation and maturation. This is highlighted by the finding that knocking down tau leads to severe neurite growth defects in primary cerebellar neurons [91]. However, certain insults cause an imbalance between the activities of tau kinases and phosphatases that lead to the abnormal phosphorylation of tau [92]. In its hyperphosphorylated state, tau becomes soluble and, in turn, polymerizes to form oligomers and/or NFTs [93].
Emerging evidence indicate that many calcium-related proteins are involved in the phosphorylation of tau. In vivo experiment CaKMII-α and hyper phosphorylated tau protein in hippocampus slices using double-labeling immunofluorescence methods, indicats that CaKMII-α might be involved in tau phosphorylation [48]. In the meantime, an N-methyl-D-aspartate (NMDA) receptor antagonist has been clinically used as an effective symptomatic treatment. Another in vitro experiment further confirmed phosphorylation of tau protein that was catalyzed by phosphatidylserine and phophatidylethanolamine via CaKM, which was identified by sodium dodecyl sulfate-polyacrylaide gel electrophoresis [94]. Calcium phosphatase calcineurin influenced tau metabolism. Reduced calcineurin activity would increase extracellular phosphorylated tau [95]. Similarly, the calcium-induced phosphorylation of tau mediated by glycogen synthase kinase 3 (GSK3) and cyclin-dependent kinase 5 (CDK5) could be dephosphorylated by calcineurins [96]. Meanwhile, increased activity of calpains regulated GSK3 and Cdk5 from the initial too late stages of the disease leads to hyperphosphorylated tau, synaptic degeneration and memory loss [97-99]. It was proposed that calpain inhibitor could be a novel treatment for the disease. Rao et al. reported CDK5 activation, tau hyperphosphorylation, and tau accumulation in brains of Tau P301L mice that were rescued when the mice were treated with selective calpain inhibitor [100].
The presenilin-1 (PS1) and Presenilin-2 (PS2) genes have been identified in AD pathogenic most related to early onset, autosomal dominant type [101]. Mutations in PS1 that cause early-onset inherited AD increased Ca2+ release through the ER InsP3R and RYR [102-104]. The number and function of RYRs are abnormally increased in different brain regions of AD mice and patients, which may exaggerate Ca2+ signalling in synaptic terminals and thereby render them vulnerable to dysfunction and degeneration in the settings of aging and amyloid accumulation in AD [105-107]. Recent studies suggested that mutated PS2 or amyloid precursor protein (APP) also contributed to the calcium dysregulation and pathogenesis of AD by over activation of RYR37 [104,108-110]. Obviously, the ryanodine receptor over activation and abnormal Ca2+ release from the ER play important roles in AD pathogenesis and the adequate inhibition RYRs over activation may be a new therapeutic target for the treatment of AD.
Dantrolene is a known antagonist of the RYR and is used clinically to treat malignant hyperthermia, muscle spasms and neuroleptic malignant syndrome. Dantrolene has been demonstrated to mitigate the amyloid pathology, synapse and memory loss in various AD tissue culture and animal models [73,75,111,112]. Therefore, dantrolene is theoretically a potential drug to reverse the calcium dysregulation and neuropathology in AD and restore cognitive dysfunction. In fact, our previous study has demonstrated that that long-term oral treatment with dantrolene in aged 3xTg-AD mice significantly decreased intraneuronal amyloid accumulation in the hippocampus. Studies show that dantrolene through the modulation of RyR-mediated Ca2+ release from ER and β- and γ-secretases activities leads to the reduction of Aβ production to prevent learning and memory decline [113]. It has been recently proposed that intraneuronal free oligomer of amyloid, rather than aggregated plaques, play important roles in synapse dysfunction and loss, as well as neurodegeneration [114-116]. The exact molecular mechanism of inhibitory effects of dantrolene on RYR is not clear, while recent studies suggested that certain cytosolic calcium concentration of magnesium ions are needed for effective RYR inhibition by dantrolene [117]. Overall, recent pilot studies suggested that calcium dysregulation in AD may be a potential therapeutic treatment for AD. Considering the earlier development of calcium dysregulation than amyloid pathology and importance of early treatment even before clinical symptoms, drugs targeting calcium dysregulation, such as dantrolene, may have good potential to facilitate the preclinical treatment in AD as possible effective therapeutic drugs (Figure 1).
Mutations in presenilin cause increased Ca2+ release from the ER through InsP3 (InsP3R) and ryanodine (RYR) two primary calcium channels. Abnormal elevation of cytosolic Ca2+ increase phosphoarylation of APP protein and activities of β- and γ-secretases, resulting in increased production of Aβ42 and Aβ oligomers, which in turn, further promote InsP3R-mediated Ca2+ release from ER by activating postsynaptic mGluR5 mediated InsP3 production. RYR can be activated by Ca2+ itself and therefore may function as an amplifier for Ca2+ release from the ER triggered by initial InsP3R activation. Abnormal decrease or depletion of ER Ca2+ level result in accumulation of misfolded proteins in the ER and decreased normal protein synthesis and secretion, including vATPase for lysosome, which then lead to decreased hydrogen concentration and elevated pH in lysosome. Dysfunctional lysosome lead to impaired function of autolysosome and overall autophagy function. On the other hand, the increased cytosolic Ca2+ activates calciuneurin which induces the synaptic loss and memory loss directly or via impaired autophagy. Dantrolene is a known antagonist of the RYR and inhibit excessive Ca2+ release from ER to cytosolic space and subsequent detrimental effects from abnormal elevation of cytosolic Ca2+ and depletion of ER Ca2+ in AD pathology.
Future Strategy for New Drug Development
Alzheimer’s disease has shown insidious onset and a progressive dementia. It is a multifactorial complex disorder of the brain. So the treatment is equally complex and is a huge challenge. From a clinical perspective, interventions that target treatment AD through early disease diagnosis, combination therapies, lifestyle changes, stem cell therapy and exercise interventions show promise for brain health [32,118-121]. Studies have shown that if the treatment is performed before the diagnosis, the outcome is better. So, the hope is, treatments in the future should be initiated in its earliest stages, such as when calcium dysregulation start which is earlier than amyloid pathology, before occurrence of irreversible brain damage or mental decline. Research on new strategies for earlier diagnosis seems to be among the most advanced areas in AD research. An effective approach of detecting early calcium dysregulation in AD brain will help the effectivity of early treatment by drugs targeting calcium dysregulation pathology.
Several potential biomarkers are being studied for their ability to indicate early stages of Alzheimer’s disease. For examples, beta-amyloid and tau levels in cerebrospinal fluid and brain changes detectable by imaging. PET scan is one of these imaging technologies which utilize a radioactive tracer to look for pathological markers of the disease, and it has made it possible to isolate tau tangles in the brain. PET scan imaging is a relatively non-invasive detection method that may help with earlier diagnosis. Recent research shows that these markers may change at different stages of AD process [122,123].
Researchers are looking for new ways to treat Alzheimer’s. Current Alzheimer’s treatments temporarily help relieve the symptoms of memory loss and cognitive dysfunction with thinking and reasoning, but do not treat the underlying disease, and delay of its progression.
Future AD treatments may include a combination of medications, similar to the strategies of treatments for many cancers or HIV/AIDS. Dendritic spine defects clearly contribute to cognitive decline observed in AD. These defects are considered an early event in memory circuit’s destabilization and should be taken into account for future development of investigational drugs. Novel pharmacotherapies should not be limited to the postulates of the amyloid cascade hypothesis. Events occurring at the synapse may prove to be instrumental in understanding the underlying pathology of this devastating disease.
Funding
Supported by grants to HW from the NIH (R01GM084979, 3R01GM084979- 02S1, 2R01GM084979-06A1).
Acknowledgement
We would like to thank Matan Y Ben Abou from Drexel University, Philadelphia, PA for editing of the manuscript.
References
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, et al. (2012) The natural history of cognitive decline in Alzheimer's disease. Psychol Aging 27: 1008-1017.
- Alzheimer's Association (2016) 2016 Alzheimer's disease facts and figures. Alzheimers Dement 12: 459-509.
- Cummings JL, Victoroff JI (1990) Noncognitive neuropsychiatric syndromes in Alzheimer's disease. Cogn Behav Neurol 3: 140-158.
- Association A (2017) 2017 Alzheimer's disease facts and figures. Alzheimers Dement 13: 325-373.
- Sherman MA, Lesné SE (2011) Detecting aß* 56 oligomers in brain tissues. Methods Mol Biol 670: 45-56.
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, et al. (2001) BACE knockout mice are healthy despite lacking the primary ß-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet 10: 1317-1324.
- Allgaier M, Allgaier C (2014) An update on drug treatment options of Alzheimer's disease. Front Biosci (Landmark Ed) 19: 1345-1354.
- Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7: 137-152.
- Wimo A, Jönsson L, Bond J, Prince M, Winblad B; Alzheimer Disease International (2013) The worldwide economic impact of dementia 2010. Alzheimers Dement 9: 1-11.
- Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, et al. (2017) Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer's disease: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16: 123-134.
- Holtzman DM, Morris JC, Goate AM (2011) Alzheimer's disease: The challenge of the second century. Sci Transl Med 3: 77sr1.
- Korolev IO (2014) Alzheimer’s disease: A clinical and basic science review. Med Stud Res J 4: 24-33.
- Watts JC, Prusiner SB (2017) ß-amyloid prions and the pathobiology of Alzheimer’s disease. Cold Spring Harb Perspect Med 2017: a023507.
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, et al. (2013) Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue. Cell 154: 1257-1268.
- Yankner BA, Lu T (2009) Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem 284: 4755-4759.
- Johnson AB, Blum NR (1970) Nucleoside phosphatase activities associated with the tangles and plaques of Alzheimer's disease: A histochemical study of natural and experimental neurofibrillary tangles. J Neuropathol Exp Neurol 29: 463-478.
- Wood SJ, Maleeff B, Hart T, Wetzel R (1996) Physical, morphological and functional differences between pH 5.8 and 7.4 aggregates of the Alzheimer's amyloid peptide A ß. J Mol Biol 256: 870-877.
- Jack CR, Petersen RC, Xu Y, O'Brien PC, Smith GE, et al. (1998) Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurol 51: 993-999.
- Rosenblum WI (2014) Why Alzheimer trials fail: Removing soluble oligomeric beta amyloid is essential, inconsistent and difficult. Neurobiol Aging 35: 969-974.
- Panza F, Logroscino G, Imbimbo BP, Solfrizzi V (2014) Is there still any hope for amyloid-based immunotherapy for Alzheimer's disease? Curr Opin Psychiatry 27: 128-137.
- Swerdlow RH, Burns JM, Khan SM (2014) The Alzheimer's disease mitochondrial cascade hypothesis: Progress and perspectives. Biochimica et Biophysica Acta 1842: 1219-1231.
- Giacobini E, Gold G (2013) Alzheimer disease therapy--moving from amyloid-β to tau. Nat Rev Neurol 9: 677-686.
- Drachman DA (2014) The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer's disease. Alzheimers Dement 10: 372-380.
- Canter RG, Penney J, Tsai LH (2016) The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature 539: 187-196.
- Finder VH (2010) Alzheimer's disease: a general introduction and pathomechanism. J Alzheimers Dis 22 Suppl 3: 5-19.
- Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology 42: 1681-1681.
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, et al. (2003) Tangle and neuron numbers, but not amyloid load, predicts cognitive status in Alzheimer's disease. Neurology 60: 1495-1500.
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, et al. (2004) Early Abeta accumulation and progressive synaptic loss, gliosis and tangle formation in AD brain. Neurology 62: 925-931.
- Uronen, Eveliina RL, Huttunen HJ (2016) Genetic risk factors of Alzheimer’s disease and cell-to-cell transmission of Tau. J Neurol Neuromedicine 1: 17-22.
- Cauwenberghe CV, Broeckhoven CV, Sleegers K (2016) The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genetic Med 18: 421-430.
- Edmondson C, Davies JC (2016) Current and future treatment options for cystic fibrosis lung disease: Latest evidence and clinical implications. Ther Adv Chronic Dis 7: 170-183.
- Folch J, Petrov D, Ettechto M, Abad S, Sanchez-lopez E, et al. (2016) Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast 2016: 8501693.
- Zhao X, Yang Z, Liang G, Wu Z, Peng Y, et al. (2013) Dual effects of isoflurane on proliferation, differentiation and survival in human neuroprogenitor cells. Anesthesiology 118: 537-549.
- Bezprozvanny I, Mattson MP (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci 31: 454-463.
- Ferreiro E, Oliveira CR, Pereira C (2004) Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1, 4, 5-triphosphate receptors in the neurotoxic effects induced by the amyloid-ß peptide. J Neurosci Res 76: 872-880.
- Chakroborty S, Goussakov I, Miller MB, Stutzmann GE (2009) Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci 29: 9458-9470.
- LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci 3: 862-872.
- Bezprozvanny I (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89-100.
- Popugaeva E, Bezprozvanny I (2013) Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci 6: 29.
- Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, et al. (2008) Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58: 871-883.
- Stutzmann GE, Mattson MP (2011) Endoplasmic reticulum Ca2+ handling in excitable cells in health and disease. Pharmacol Rev 63: 700-727.
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, et al. (2008) Aß plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59: 214-225.
- Foster TC (2007) Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6: 319-325.
- Mulkey RM, Endo S, Shenolikar S, Malenka RC (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486-488.
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335.
- Jiang X, Chai GS, Wang ZH, Hu Y, Li XG, et al. (2015) Spatial training preserves associative memory capacity with augmentation of dendrite ramification and spine generation in Tg2576 mice. Sci Rep 5: 9488.
- Zeng Y, Zhao D, Xie CW (2010) Neurotrophins enhance CaMKII activity and rescue amyloid-ß-induced deficits in hippocampal synaptic plasticity. J Alzheimers Dis 21: 823-831.
- Wang YJ, Chen GH, Hu XY, Lu YP, Zhou JN, et al. (2005) The expression of calcium/calmodulin-dependent protein kinase II-a in the hippocampus of patients with Alzheimer's disease and its links with AD-related pathology. Brain Res 1031: 101-108.
- Xiao J, Perry G, Troncoso J, Monteiro MJ (1996) apha-Calcium-calmodulin-dependent kinase II is associated with paired helical filaments of Alzheimer's disease. J Neuropathol Exp Neurol 55: 954-963.
- Yamamoto H, Hiragami Y, Murayama M, Ishizuka K, Kawahara M, et al. (2005) Phosphorylation of tau at serine 416 by Ca2+/calmodulin-dependent protein kinase II in neuronal soma in brain. J Neurochem 94: 1438-1447.
- Park D, Na M, Kim JA, Lee U, Cho E, et al. (2017) Activation of CaMKIV by soluble amyloid-ß1–42 impedes trafficking of axonal vesicles and impairs activity-dependent synaptogenesis. Sci. Signal 10: eaam8661.
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, et al. (2001) An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 106: 771-783.
- Teich AF, Nicholls RE, Puzzo D, Fiorito J, Purgatorio R, et al. (2015) Synaptic therapy in Alzheimer's disease: A CREB-centric approach. Neurotherapeutics 12: 29-41.
- Hardingham GE, Arnold FJ, Bading H (2009) Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neurosci 4: 261-267.
- Yin Y, Gao D, Wang Y, Wang ZH, Wang X, et al. (2016) Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci U S A 113: E3773-E3781.
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, et al. (1991) Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30: 572-580.
- Gaffney-Stomberg E, Cao JJ, Lin GG, Wulff CR, Murphy NE, et al. (2014) Dietary protein level and source differentially affect bone metabolism, strength, and intestinal calcium transporter expression during ad libitum and food-restricted conditions in male rats. J Nutr 144: 821-829.
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML (2004) Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol 33: 377-387.
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, et al. (2004) Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci 24: 10191-10200.
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, et al. (2008) Diffusible, non-fibrillar ligands derived from Aß1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95: 6448-6453.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. (2008) Amyloid ß-protein dimers isolated directly from Alzheimer brains impair synaptic plasticity and memory. Nat Med14: 837-842.
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, et al. (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2: 271-276.
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, et al. (2011) Amyloid-ß/Fyn–induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci 31: 700-711.
- Berridge MJ (2010) Calcium hypothesis of Alzheimer's disease. Pflugers Arch 459: 441-449.
- Briggs CA, Schneider C, Richardson JC, Stutzmann GE (2013) Beta amyloid peptide plaques fail to alter evoked neuronal calcium signals in APP/PS1 Alzheimer's disease mice. Neurobiol Aging 34: 1632-1643.
- Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, et al. (2012) Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer's disease. PLoS ONE 7: e52056.
- Tomaszczyk JC, Green NL, Frasca D, Colella B, Turner GR, et al. (2014) Negative neuroplasticity in chronic traumatic brain injury and implications for neurorehabilitation. Neuropsychol Rev 24: 409-427.
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, et al. (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280: 17294-17300.
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, et al. (2013) Metabotropic glutamate receptor 5 is a co-receptor for Alzheimer aß oligomer bound to cellular prion protein. Neuron 79: 887-902.
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, et al. (2010) Deleterious effects of amyloid ß oligomers acting as an extracellular scaffold for mGluR5. Neuron 66: 739-754.
- Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: Physiology, pharmacology and disease. Annu Rev Pharmacol Toxicol 50: 295-322.
- Demuro A, Parker I (2013) Cytotoxicity of intracellular aß42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J Neurosci 33: 3824-3833.
- Peng J, Liang G, Inan S, Wu Z, Joseph DJ, et al. (2012) Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516: 274-279.
- Wu Z, Yang B, Liu C, Liang G, Eckenhoff MF, et al. (2015) Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice. Alzheimer Dis Assoc Disord 29: 184-191
- Del Prete D, Checler F, Chami M (2014) Ryanodine receptors: Physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9: 21.
- Chen SR, Leong P, Imredy JP, Bartlett C, Zhang L, et al. (1997) Single-channel properties of the recombinant skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys J 73: 1904-1912.
- Görlach A, Klappa P, Kietzmann T (2006) The endoplasmic reticulum: Folding, calcium homeostasis, signaling and redox control. Antioxid Redox Signal 8: 1391-1418.
- Kudo T, Katayama T, Imaizumi K, Yasuda Y, Yatera M, et al. (2002) The unfolded protein response is involved in the pathology of Alzheimer's disease. Ann N Y Acad Sci 977: 349-355.
- Paschen W, Mengesdorf T (2005) Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38: 409-415.
- Chen X, Bisschops MMM1, Agarwal NR2, Ji B1, Shanmugavel KP, et al. (2017) Interplay of energetics and ER stress exacerbates Alzheimer's amyloid-ß (Aß) toxicity in yeast. Front Mol Neurosci 10: 232.
- Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, et al. (2013) TNF-a mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s ß-amyloid oligomers in mice and monkeys. Cell Metab 18: 831-843.
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzelet JC, et al. (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aß oligomers. J Clin Invest 122: 1339-1353.
- Paula-Lima AC, Adasme T, SanMartÃn C, Sebollela A, Hetz C, et al. (2011) Amyloid ß-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid Redox Signal 14: 1209-1223.
- Ho YS, Yang X, Lau JC, Hung CH, Wuwongse S, et al. (2012) Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: Implication in Alzheimer's disease pathogenesis. J Alzheimers Dis 28: 839-854
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, et al. (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1: 479-485.
- Katayama T, Imaizumi K, Honda A, Yoneda T, Kudo T, et al. (2001) Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J Biol Chem 276: 43446-43454.
- Sato N, Imaizumi K, Manabe T, Taniguchi M, Hitomi J, et al. (2001) Increased production of ß-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J Biol Chem 276: 2108-2114.
- Fyfe I (2017) Alzheimer disease: Tau pathology in atomic detail. Nat Rev Neurol 13: 513.
- Hartigan JA, Johnson GV (1999) Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3ß-dependent pathway. J Biol Chem 274: 21395-21401.
- Gómez-Ramos A, DÃaz-Hernández M, Rubio A, Miras-Portugal MT, Avila J (2008) Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci 37: 673-681.
- Caceres A, Kosik KS (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in priary cereellar Neurons. Nature 343: 461.
- Stoothoff WH, Johnson GV (2005) Tau phosphorylation: Physiological and pathological consequences. Biochimica et Biophysica Acta (BBA) 1739: 280-297.
- Gong CX, Iqbal K (2008) Hyperphosphorylation of microtubule-associated protein tau: A promising therapeutic target for Alzheimer disease. Curr Med Chem 15: 2321-2328.
- Baudier J, Cole RD (1987) Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem 262: 17577-17583.
- Karch CM, Jeng AT, Goate AM (2013) Calcium phosphatase calcineurin influences tau metabolism. Neurobiol Aging 34: 374-386.
- Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, et al. (2006) Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-ß accumulation. J Biol Chem 281: 39907-39914.
- Kurbatskaya K, Phillips EC, Croft CL, Dentoni G, Hughes MM, et al. (2016) Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol Commun 4: 34.
- Gao L, Tian S, Gao H, Xu Y (2013) Hypoxia increases Aß-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci 51: 138-147.
- Jin N, Yin X, Yu D, Cao M, Gong CX, et al. (2015) Truncation and activation of GSK-3ß by calpain I: A molecular mechanism links to tau hyperphosphorylation in Alzheimer's disease. Sci Rep 5: 8187.
- Rao MV, McBrayer MK, Campbell J, Kumar A, Hashim A, et al. (2014) Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci 34: 9222-9234.
- Hutton M, Hardy J (1997) The presenilins and Alzheimer's disease. Hum Mol Genet 6: 1639-1646.
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP (2000) Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275: 18195-18200.
- Cedazo-Minguez A, Popescu BO, Ankarcrona M, Nishimura T, Cowburn RF (2002) The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations J Biol Chem 277: 36646-36655.
- Toglia P, Cheung KH, Mak DO, Ullah G (2016) Impaired mitochondrial function due to familial Alzheimer's disease-causing presenilins mutants via Ca2+ disruptions. Cell Calcium 59: 240-250.
- Kelliher M, Fastbom J, Cowburn RF, Bonkale W, Ohm TG, et al. (1999) Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer's disease neurofibrillary and beta-amyloid pathologies. Neurosci 92: 499-513.
- Antonell A, Lladó A, Altirriba J, Botta-Orfila T, Balasa M, et al. (2013) A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer's disease. Neurobiol Aging 34: 1772-1778.
- Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, et al. (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 33: e1001-1006.
- Leissring MA, Yamasaki TR, Wasco W, Buxbaum JD, Parker I, et al. (2000) Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc Natl Acad Sci U S A 97: 8590-8593.
- Lee SM, Lee JW, Song YS, Hwang DY, Kim YK, et al. (2005) Ryanodine receptor-mediated interference of neuronal cell differentiation by presenilin 2 mutation. J Neurosci Res 82: 542-550.
- Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P (2008) The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium 44: 507-518.
- Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE (2015) Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer's disease mice. J Neurosci 35: 6893-6902.
- Liang L, Wei H (2015) Dantrolene,a treatment for alzheimer's disease? Alzheimer Dis Assoc Disord 29: 1-5.
- Oulès B, Del Prete D, Greco B, Zhang X, Lauritzen I, et al. (2012) Ryanodine receptor blockade reduces amyloid-ß load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32: 11820-11834.
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Aß causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45: 675-688.
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol 119: 523-541.
- Youmans KL, Tai LM, Kanekiyo T, Stine WB Jr, Michon SC, et al. (2012) Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol Neurodegener 7: 8.
- Choi RH, Koenig X, Launikonis BS (2017) Dantrolene requires Mg2+ to arrest malignant hyperthermia. Proc Natl Acad Sci USA 114: 4811-4815.
- McGough E, Kirk-Sanchez N, Liu-Ambrose T (2017) Integrating health promotion into physical therapy practice to improve brain health and prevent Alzheimer Disease. J Neurol Phys Ther 41: S55-S62.
- Kamphuis PJ, Scheltens P (2010) Can nutrients prevent or delay onset of Alzheimer's disease? J Alzheimers Dis 20: 765-775.
- Song HJ, Kim TH, Lee HH, Kim JM, Park YJ et al. (2017) Cell therapy products in Alzheimer Disease. J Menopausal Med 23: 1-4.
- Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, et al. (2013) Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 12: 167-179.
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, et al. (2014) Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med 6: 226ra230.
- Forlenza OV, Diniz BS, Teixeira AL, Stella F, Gattaz W (2013) Mild cognitive impairment (part 2): Biological markers for diagnosis and prediction of dementia in Alzheimer's disease. Revista Brasileira de Psiquiatria 35: 284-294.
Citation: Wang Y, Shi Y, Wei H (2017) Calcium Dysregulation in Alzheimer’s Disease: A Target for New Drug Development. J Alzheimers Dis Parkinsonism 7: 374. DOI: 10.4172/2161-0460.1000374
Copyright: © 2017 Wang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11693
- [From(publication date): 0-2017 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 10435
- PDF downloads: 1258