Cefixime, in General, Class 4 Drug but Individually Class 2 Drug
Received: 20-Apr-2017 / Accepted Date: 27-Apr-2017 / Published Date: 05-Feb-2017
Abstract
Poor oral bioavailability of BCS class 4 drug is due to poor aqueous solubility and poor permeability across intestine. Present work was undertaken to increase oral bioavailability of BCS class 4 drug i.e. Cefixime using P-glycoprotein inhibitor hydrophile i.e. Pluronic and complexing agent beta cyclodextrin.
Comparative study was carried out using Pluronic as solid dispersion and beta cyclodextrin as inclusion complexation in different drug to polymer ratios through physical mix, kneading, solvent evaporation and spray drying technique. Physicochemical characterisation was done using saturated solubility studies, in vitro dissolution, ATR, DSC, SEM, and XRD study.
Prepared formulations were also evaluated for % drug permeation using non-everted rat intestinal sac method which showed that Pluronic was not involved in permeability enhancement of Cefixime.
Solid dispersion prepared with Pluronic found to show marked increment in solubility and dissolution as well as in ex-vivo permeation study.
In vivo study was carried out using Pluronic sd in male Wister rats (n=6) and which showed enhanced absorption of Cefixime with AUC 1.9 times greater than of the aqueous plain Cefixime suspension. Thus, BCS class 4 Cefixime can be treated as bcs class 2 by increasing its solubility instead of permeability as evident by ex-vivo and in vivo study.
Keywords: Cefixime; Pluronic; Cyclodextrin; Permeability; Solubility; Dissolution
78270Introduction
Two factors govern the total oral absorption of drug i.e. drug’s solubility and permeability across intestine. The major challenge in the oral drug delivery of poorly permeable drug is their transport across intestine [1,2]. Intestinal wall is made up of tight-fitting single layer of epithelial cell where drug is transported by active transport or passive transport. In passive mechanism, diffusion of drug through close junctions of epithelial cells occurs depending on its size and charge while in active transport, uptake of drug is mediated by carrier i.e. active proteins [3-5].
In third mechanism of drug transport where drug is permeated transcellularly due to its lipophilicity straightaway crosswise at epithelial cell’s apical and basolateral membranes. For usual absorption of drug it is soluble then it will reach intestinal wall in dissolved state and later absorbed in gut membranes combining all above transport mechanisms.
Different permeation assay techniques, cost efficient and less effortful are utilized where solvent simulating intestinal fluid with some membranes are used. The system is having donar and receiver compartment for studying permeation of drug across the membrane. In vitro permeation assay techniques such as Caco-2, MDCK cell lines, PAMPA, tissue based assays like the intestinal everted sac, intestinal single pass perfusion, Thiry–Vella loops are being used [6-17].
Intestinal transporters play important role in drug’s absorption and in turn its oral bioavailability. Two types of transporters are identified in intestine i.e. uptake and efflux transporters. It has been reported that solute carrier (SLC) and the ATP-binding cassette (ABC) transporter super families are the major class of membrane transporters [14,17].
An extremely rich in quantity at the apical (luminal) membrane of enterocytes, and acting as barriers for many frequently used drug like anticholesteremic, antibacterial, antiretrovirals, immunosuppressive drug, chemotherapeutic drugs and anticardiac and finally limit their oral bioavailability. This ABC family of efflux transporters includes P-glycoprotein (P-gp), multidrug resistance-associated protein 2 (MRP 2) and breast cancer resistance protein (BCRP) [15,17]. It has been reported that p-glycoprotein is having large no. of substrate specificity and criteria for the substrates are that it should be hydrophobic, amphipathic or cationic with a ring structure, planar in nature and range of 200-1900 Da [16,17].
As BCS class 2 drugs are highly permeable they easily get into intestinal membranes but their low solubility causes less concentration at enterocytes and hence efflux transporters are less saturated. Thus, total oral absorption is decreased due to less quantity of BCS class 2 drugs at the enterocytes [17]. When drug is administered orally, usually transporters from intestine and liver get activated and finally determining the pharmacokinetics of drug in body [18]. Drug molecules are passing through membranes via passive diffusion and/ or via transporter processes.
Different factors are affecting the permeability of drug through intestine where drug is diffusing transversely through different mucosal layers of epithelial cell wall depending on drug’s size as well as charge. As indicated by work done for permeability enhancement, different approaches are being used in formulation development such as Nano-Emulsion Technique, Co-solvency, Spray freeze drying, pH adjustment, Liposome, Micro Encapsulation, Self-emulsifying drug delivery systems (SEDDS), Permeation enhancer, Cyclodextrin Inclusion Complexation [19,20].
BCS class 4 drugs are having poor oral bioavailability due to poor solubility and poor permeability. Poor oral bioavailability may be due to poor aqueous solubility or poor permeability, the reason is unknown for various BCS class 4 drugs. While, BCS class 3 drugs are highly soluble but poorly permeable. Hence, permeability enhancement strategies are often used for these class of drugs viz., by affecting various physicochemical mechanisms using lipid vesicles or using permeation enhancers [21,22].
Various permeation model membranes are being used for estimating permeation rate and hence, thereby assessing the oral absorption in humans namely, in vivo: Animal model; Ex vivo: Everted intestinal rings, Isolated sheet, Caco-2 cell monolayer, Membrane vesicles, Non-everted intestinal sac model, isolated sheet; In vitro: Synthetic membrane [23-26].
Highly permeable but poorly soluble drugs falls into BCS class 2. Hence, many studies were undertaken to increase its poor oral bioavailability by increasing BCS class 2 drug’s poor solubility using different solubility enhancing approaches like solid dispersion, inclusion complexation, solid lipid nanoparticle, nanoemulsion, SMEDDS, etc. [27].
It’s been reported that by increasing the solubility of poorly soluble BCS class2 drug, consequentially its pharmacodynamic activity is also increased [28]. However, it may happen that during drug development if judging is not proper it may lead to misclassification in BCS, which should be avoided using careful assessment and proper techniques [29]. Many BCS class 4 drugs are effluxed by p-glycoproteins in intestine hence the approach of using its inhibitor leads to increase in its oral bioavailability.
The explanation for inadequate permeableness of drugs are numerous such as less lipophilicity, charge, bigger size of it, little hydrogen ion potentiality [30]. But efflux by p- glycoprotein dominates in efflux transporting of drug molecules. Pluronic is p-glycoprotein inhibitor as indicated by earlier studies [31-34]. So, Cefixime is having poor oral bioavailability but its reason is unknown i.e. it is unclear whether its poor oral bioavailability is due to poor solubility or combination of poor solubility and poor permeability [35]. So, we tried to find this possibility of interacting Cefixime with P-gp by using p-glycoprotein inhibitor Pluronic. The other polymer used for comparison was BCD (beta-cyclodextrin).
Many reports state that BCD is not having any permeability modifying effect [36]. Hence, BCD can be possibly utilized in determining the other polymer’s permeability enhancing potential for the drug under consideration.
The studies on Cefixime indicated that solid dispersion as well as inclusion complexation is successful in enhancing the apparent solubility of Cefixime. The physical mixing, solvent evaporation method have been utilized along with different hydrophilic polymers [37-41]. But the effect of solid dispersion or inclusion complexation on oral bioavailability has not been studied. There is still lack of knowledge regarding application of pharmaceutical interventions along with best suited method for oral bioavailability of Cefixime.
Accordingly, to investigate the reason behind Cefixime’s poor oral bioavailability, present study was constructed with two approaches for enhancing oral absorption of Cefixime. Solid dispersion was formed with Pluronic (P-glycoprotein inhibitor) as hydrophilic polymer while inclusion complexation was prepared with BCD (complexing agent). Out of 2 approaches used; the one with enhanced solubility was checked for the enhanced oral absorption of Cefixime.
The formulations were formed by employing different solubility enhancing techniques viz., mixing physically, kneading method, evaporation of solvent, using microwave irradiation, lyophilization/ freeze drying and spray drying method. The most appropriate method with enhanced solubility and dissolution was then further characterized and used for pharmacokinetic study.
Materials and Methods
Materials
Cefixime (CFX) and Beta-cyclodextrin (BCD) were obtained as gift sample from Glenmark pharmaceuticals, Nashik, India. Pluronic from Sigma-Aldrich was used for the experiment. All other reagents and chemicals (analytical grade) were utilized as received.
Physical mixing (PM)
The binary system was prepared by physical mixing and grinding drug and polymer together for 4-5 min. Different ratios (1:1, 1:2, 1:3) were used for mixing Cefixime with polymer in a mortar until uniform mixture was obtained. The obtained mixtures were sieved (100 μm mesh) and stored in a desiccator at normal temperature for further analysis.
Kneading method (KD)
The formulations were prepared by kneading Cefixime and polymer in ratios like (1:1, 1:2, 1:3). The Cefixime was triturated with little quantity of methanol in mortar that followed polymer’s totaling to get slurry (till 1 hr). The prepared slurry was then dried up in atmosphere at room temperature followed by sieving and storing in desiccator.
Solvent evaporation method (SE)
Polymer was made soluble in aqueous solvent followed by adding organic solution of Cefixime in Methanol to it by constant stirring with add-on rate of 7-9 ml/min. Organic phase was evaporated from the solution completely using oven at 45-50°C. The product was sieved (sieve no.100) and kept in desiccator at room temperature prior to analysis by UV.
Lyophilization/Freeze-Drying (LY)
The solvent is evaporated by freeze drying to form amorphous powder of formulation. The Cefixime (CFX) and polymer (Pluronic/ BCD) were weighed accurately and solubilized in methanol and aqueous solvent, respectively. Methanolic solution was put in water solution with uninterrupted stirring rate of 5-6 ml/min. The organic phase was removed by evaporation at normal temperature. Lyoprotectant was added in formulation for freeze drying in Labconco Lyophilizer (USA) with initial freezing (vacuum 0.04 mbar). The lyophilized products were later stored in desiccator at normal temperature.
Microwave irradiation method (MW)
The more intimate mixing of Cefixime and polymers was carried out using microwaves. A homogeneous paste was formed by mixing Cefixime (CFX) and polymer with minimal quantity of solvents (Ethyl alcohol: H2O, 1:1 v/v). The paste was subjected to microwave irradiation for 7-8 min (power 510 watt) in a microwave (oven) synthesizer (CATA-R, catalyst systems, Pune, India). The products were kept up at normal temperatures to cool down. The formulations were kept in desiccator for 24 h prior to pulverization by mortar and pestle. The powdered product was sieved and put in desiccators over combined calcium chloride
Spray drying (SY)
The organic phase was evaporated by spraying the drug-solution through nozzle and hot air to form amorphous powder. Cefixime and polymer were made soluble in methanol followed by ultrasonication (bath sonicator, 10 min). The Cefixime-polymer Methanolic solution was spray dried by lab spray dryer model LU-222 Advanced (Labultima, Mumbai, India). The parameters set for spray drying were 4 ml/min flow rate, 80°C of inlet temperature, with 60-70°C of outlet temperature, and aspirator value of about 60 m 3/h.
Saturated solubility studies
As discussed by Higuchi and Connors (1965), the saturated Solubility estimations were made for the prepared formulations. An excess quantity of prepared formulation (till saturation) was included in 10 ml distilled water. The saturated formulations were then sonicated for 1-2 h at normal temperature followed by shaking at 25°C for 24 h. The suspensions were sifted through Whatmann filter paper (no.41) furthermore; the separated solutions were analyzed by UV-spectrophotometer at 288 nm.
Differential scanning calorimetry (DSC)
The possibility of any interaction between Cefixime and polymer in inclusion complex (IC) or solid dispersion formation (SD) was evaluated by means of DSC examination and comparing it with pure Cefixime. DSC assessment was carried by differential scanning calorimeter (Lab Mettler Star SW 10). The formulations were heated individually in an unsealed aluminium pan with heating a rate of 10°C/ min over a temperature from 25 to 300°C by means of a nitrogen rate flow at 50 ml/min.
X-ray diffraction (XRD)
XRD spectrum of pure Cefixime and formulations were taken at normal temperature with the help of Bruker’s AXS D8-advance X-ray diffractometer. Irradiation of products was carried out with mono-chromatized Cu KA2 emission at wavelength 1.5406 A°. The test formulations were mounted on zero-foundation test holder and subjected to a ceaseless scanning over angles of 3° to 80° 2θ at a stage size of 0.020. The individual diffraction spectrums were generated by voltage of 40 kV, current of 35 mA and with examining rate of 20 min-1.
Scanning electron microscope (SEM)
The topography of pure Cefixime and spray dried formulations were looked under a scanning electron microscope (SEM, JEOL model JSM -6390LV) operating at an excitation voltage of 15 kV.
Attenuated Total Reflectance Spectroscopy (ATR): The IR spectra were generated to check the compatibility of Cefixime and polymers used in the study. Attenuated total reflectance spectra of the drug samples were obtained on a Bruker Eco-ATR machine. For getting IR spectra, scanning was done over the wave number from 3600 to 400 cm-1.
Dissolution studies
The dissolution of formulations was studied by USP 8-station dissolution test assembly (Lab India) with USP type II apparatus.
Dissolution medium of 900 ml (pH 7.2 buffer, 37 ± 0.5°C, 75 rpm) was used for the study. The 5 ml tests were withdrawn from dissolution medium at time interims of 5, 10, 15, 20, 30, 45 min. The volume of dissolution medium was conformed to 900 ml to keep up sink conditions by putting every 5 ml aliquot removed with 5 ml of fresh buffer. The Cefixime’s concentration in tests was assessed by measuring absorbance at 288 nm. The percent drug release was ascertained at each time point in dissolution.
Ex-vivo permeability study
Ex-vivo permeation study of Cefixime and formulations was performed by means of non-everted intestinal sac technique. Two male Wister rats (overnight fasting) were used by sacrificing through spinal dislocation. The small intestines were separated by cutting transversely the upper end of duodenum to the lower end of ilium. The small intestine was cleaned cautiously using saline solution to remove any blood contents in it. The cleaned intestinal tract was immediately cut into long sacs (5 cm). Each sac was filled with 1ml of Cefixime formulation (equivalent to 2 mg Cefixime) by means of blunt needle followed immediately by tying of two sides with thread. Each non everted sac was placed in beaker containing 10 ml mixture of tyroid solution and Isopropyl alcohol (IPA) in the ratio of 7:3. Entire system was maintained at 37 ± 5°C in water bath and aerated (10-15 bubble/min) using laboratory aerator. From outside 2 ml sample was withdrawn after every 30 min for 4 h and replaced with 2 ml of fresh medium. Samples were analysed at λmax 288 nm using UV-Visible spectrophotometer [42-44].
In-vivo study
Wistar-strain rats, of either sex (190-220 g) of animal house (College of Pharmacy, Nashik, India) were housed under normal laboratory conditions. Rats were maintained on regular rodent chow and tap water. The Institutional Animal Ethical Committee (IAEC/ February 2 016/08) sanctioned the experimental methods.
The animals were kept on overnight fasting with clear reach to water 24 h prior to experiment. The 3 rodent groups were utilized in the study with random assignment of 6 rats to each treatment group. The formulation (equivalent to Cefixime 8 mg) along with pure Cefixime was dispersed separately in water for 30s prior to oral dosing to rats groups utilizing oral gavage. Blood samples were collected through tail vein at 30, 60, 120, 240 and 360 min after oral administration directly into EDTA micro centrifuge tubes (eppendorf). Centrifugation of samples was made at 10,000 rpm at 100C for 20 min for separating plasma samples, followed by addition of cold acetonitrile to the plasma sample for precipitating the protein. The processed samples’ centrifugation was again made at 10,000 rpm at 100C for 20 min where, resultant supernatant liquid was stored in refrigerator until analyzed by HPLC [43].
HPLC analysis of plasma samples
The plasma samples are analyzed by using high performance liquid chromatography (HPLC) with binary HPLC pump provided with a UV visible 2000 detector, Waters 1525, Singapore. The sorting was performed using a Cosmosil C18 packed column (250 mm × 4.6 mm dimension, 5 μm) at 288 nm. The mobile phase consisted of a mixing of phosphate buffer (pH 4) with methyl alcohol in equal proportions operating at a flow rate of 1 ml/min [45].
Standard solutions of Cefixime (2, 4, 6, 8, and 10 μl) were prepared in methanol for analyzing by HPLC for plotting calibration curve.
The 20 μl of plasma sample was put into HPLC injector for analysis. Concentration of Cefixime in plasma was determined by calculating area under curve of chromatogram.
Results and Discussion
Saturated solubility studies
Saturation of drug’s solubility in water was conducted to assess the consequence of using different polymers on water solubility of Cefixime. The prepared formulations indicated increment in apparent Cefixime water solubility over pure CFX.
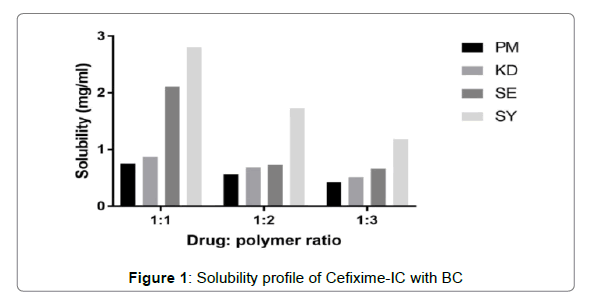
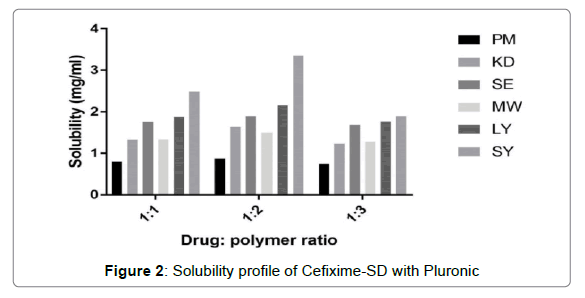
The solubility profiles of inclusion complexes and solid dispersions are illustrated in Figures 1 and 2 respectively. Plain Cefixime was slightly soluble in water. Prepared formulations showed enhancement of Cefixime’s water solubility as compared to plain drug.
The inclusion complex with β-CD prepared by spray drying method was found to show highest water solubility, nearly almost 6.5 fold increment as compared to plain Cefixime. The complex with β-CD prepared by other methods such as PM, KD, SE, found to show 1.6, 1.85, 4.49 fold increase respectively.
The augment in solubility may be due to existence of hydrophilic element i.e. BCD as well as creation of inclusion complexation [46]. The apparent solubility enhancement may be due to these mechanisms like increased wetting and hydrophilicity by complexing agent, amorphous form generation, and inclusion formation [28,47].
Solubility profile for SD with Pluronic is depicted in Figure 2 where it showed that 1:2 drug: polymer ratio showed good solubility results. The solid dispersion prepared by spray drying method exhibited highest apparent solubility increment of 7.2 fold in distilled water. The reason behind the solubility enhancement by Pluronic and spray drying may be due to decrease in particle size due to spray drying which presents increased surface area coming in contact with water. Also as displayed by DSC and XRD results, the high energetic, highly soluble amorphous form generation causing the solubility enhancement of Cefixime, which is consistent with the results by Chutimaworapan on Nifedipene binary system [48]. Study by Shah on Rofecoxib using Poloxamer 188 also demonstrsted same outcomes [45].
The solid dispersions formed by other solubility enhancing methods like PM, KD, SE, LYO, MW showed up nearly 1.87, 3.4, 4.03, 4.59, 3.2 fold increment respectively. The Pluronic (F127) was found to be more efficacious for solubility enhancement of Cefixime than Pluronic (F 68) might be because of its high molecular weight along with high ratio of a hydrophobic segment i.e. polyoxypropylene [49].
For development of solution of solid, drug’s concentration in solid dispersion is having little value than the actual stoichiometry of the mixture. The composition’s stoichiometry actually determines the solid dispersion’s quality. This explains why the solubility of drug is not increasing even the polymer’s ratio is increased after certain ratio in composition. Thus, molecular fundamental interactions are decided by the stoichimetry in the composition [50,51]. The reasons behind the solubility enhancement by dispersion of solid with Pluronic are particle size reduction along with mixing drug and carrier closely [52].
DSC
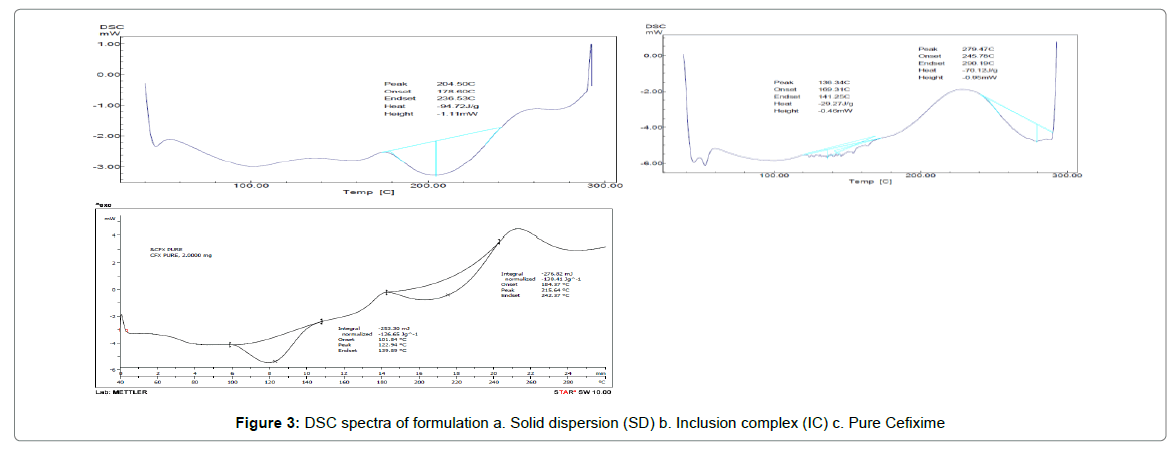
The thermograms of pure CFX and prepared formulations are given away in Figure 3. The DSC thermogram of pure CFX displayed a endotherm at 215.64°C as consistent with its melting point; however, this endotherm was disappeared completely in the thermogram of SD with Pluronic (F127) indicating the formation of solid dispersion of drug and polymer. The existence of single endothermic peak for SD is explained by the drug’s complete dissolution in carrier which is melted in the course of investigation or drug being distributed in amorphous state or forming solution of solids [53-55].
Complete disappearance of endotherm indicates change of state of drug from crystalline to amorphous form. Further, it can be said that this amount of Pluronic (F127) is adequate for solubilizing pure CFX. Complete disappearance of endotherm also demonstrates complete dispersion of Cefixime in F127. The oxidative decomposition temperature for F-127-SD was found to be 279.47°C. The DSC thermogram of IC with BCD showed shifting of endotherm to 205.33°C with broadening and lowering of melting temperature. This shifting of endotherm to lowering temperature indicates formation of inclusion complex with conversion of crystal form of drug to amorphous state. For BCD-IC, the oxidative decomposition was observed at 276°C.
The complete dispersion of drug in polymeric phase along with change of state of Cefixime from crystalline to amorphous may be responsible for increased solubility and dissolution of drug [56-58].
X-ray diffraction (XRD)
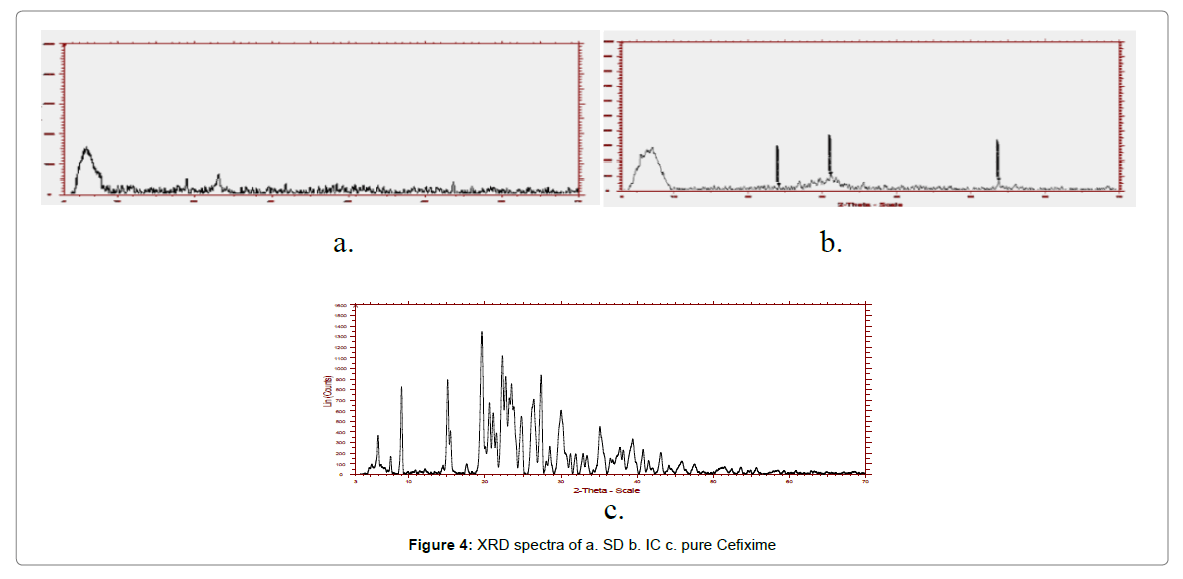
The X-ray powder diffraction of pure CFX and formulations are illustrated in Figure 4. The characteristic Brag’s diffraction peaks given away by pure Cefixime at 2θ angle were 5.89, 8.97, 19.55, 24.745, 26.36 27.34, 31.88 and 35.1120. Pure CFX exhibit acute and strong peaks in the range of 5°–60°, because of its presence in crystalline form. Notwithstanding, for the SD and IC, the peaks of CFX were distinctly lacking and halo form was ascertained for spray dried formulations signifying the lack of crystallinity. Nevertheless, the XRD pattern of the spray dried up complex with Pluronic (F127) was instituted to be distributed and diverse kind as compare to plain Cefixime and thus, substantiating the constitution of other solid form.
The XRD spectra of prepared formulations were altogether diffused and different than plain Cefixime, informing about its amorphous quality. Therefore, modification in solubility and rate of dissolution for solid dispersions might be the consequence of form change i.e. amorphous structure substituting crystalline structure.
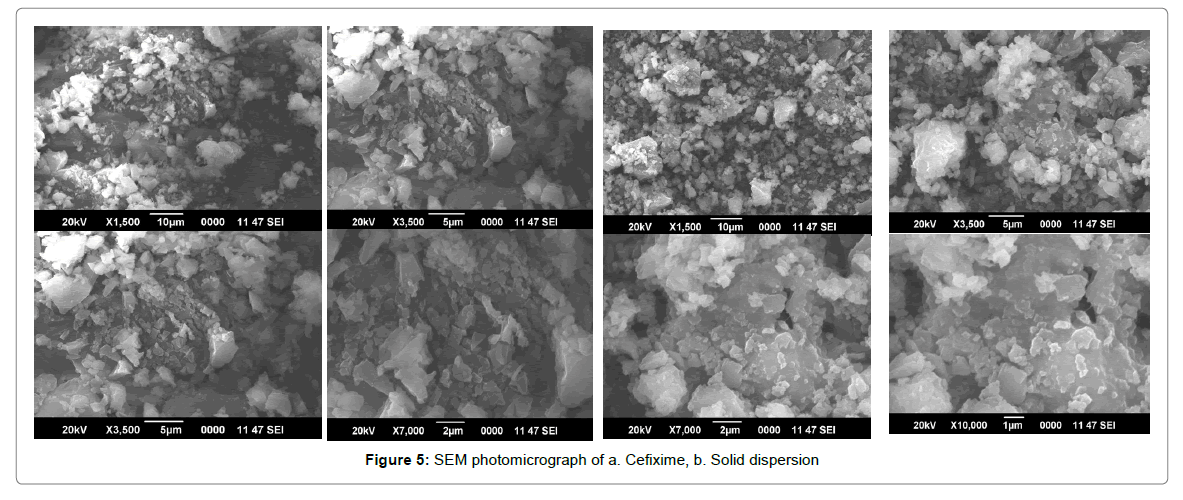
SEM
Scanning electron micrograph for Pure Cefixime and spray dried SD are presented in Figure 5. SEM image of pure Cefixime appear as planar shaped pointed or sharp bordered crystals of dissimilar sizes with well-formedside. The exterior was varied after solid dispersion formation with Pluronic. CFX-Poloxamer SD was appeared as irregular fashioned particles of more dissimilar sizes with smooth surface and blunt edges. There was no crystal structure found of CFX in SDs, pointing towards the alteration of CFX-crystal into an amorphous form, which harmonizes with the resultant of the DSC experiment.
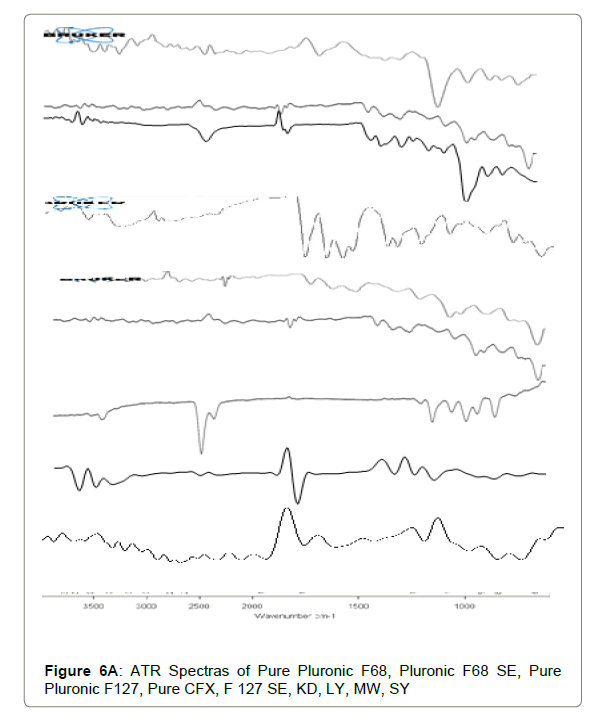
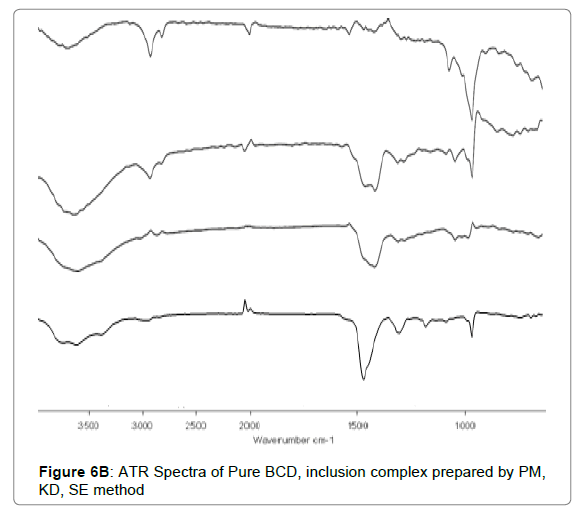
ATR
The ATR spectra disclosed that no chemical interaction present among Cefixime and polymers. The functional groups are unaltered during combination with carriers. The ATR spectra of plain Cefixime and prepared formulations were demonstrated in Figures 6A and 6B. Pure Cefixime displayed vibration peaks of –N-H (primary amine) at wave number 3287.89 cm-1, N-H stretch at 1661.43 cm-1, C-H at 1545.7 cm-1, C-N at 1591.41 cm-1, N-O stretching at 1382 cm-1, Carbonyl stretching at 1761.16 cm-1.
For SD prepared with Pluronic, shifting of the NH stretching vibrations from 3287 cm−1 to 3009.07 cm−1 occurred and at the same time disappearance of NH bending vibrations. These changes in ATR spectra are most probably the result of formation of hydrogen bonds between carbonyl function of Cefixime and hydroxyl group of poloxamers. However, the SDs spectrum prepared by other solubility enhancing method also did not show any additional peak indicating the absence of any chemical reaction between CFX and poloxamers.
The formation of hydrogen bonding between the dispersed drug and the carrier is important in the stabilisation of solid dispersions [59]. The vibrational peaks for Pluronic were observed at 2858 cm−1 for stretching vibrations of the OH and for CO group at1080 cm−1.
The spectrum of Pluronic-SD prepared through spray drying technique showed O-H stretch vibration at 3410.01 cm−1, while Carbonyl stretching of drug at 1761.16 cm-1 was lacking with just the C=O peak at 1634.23 cm−1 was present.
Due to presence of 2 terminal OH molecules in F127, it is capable of forming hydrogen bonds with Cefixime as reported by Ozeki [60]. This change in spectra in ATR of SD demonstrates the formation of H-bond between Pluronic (F127) and Cefixime. It was also noted that no new peak was appeared in spectra suggesting that no chemical interaction has happened between Cefixime and F127. The peaks observed were of reduced intensities representing the dispersion of drug in polymer which may be reason for increased solubility and dissolution of Cefixime in SD [61,62].
For inclusion complexation (IC), with BCD prepared by solvent evaporation method showed O-H stretching vibration at 3386.77 cm-1 and C=O stretching at 1751.24, 1620.09 cm-1 was noted. For inclusion complexes prepared by kneading and physical mix also, new peak was absent which is suggestive of the compatibility among Cefixime and BCD.
Dissolution study
The dissolution studies are in vitro simulation of in vivo absorption of drug; hence, it is essential to perform this study during formulation development.
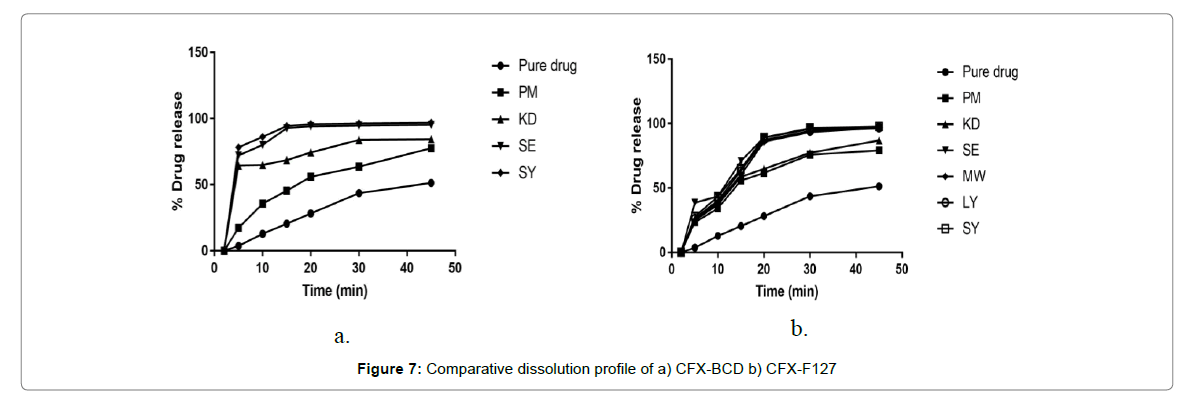
The percentage cumulative drug release profiles of the prepared SD and IC of CFX in buffer pH 7.2 along with pure Cefixime are depicted in Figure 7. Overall, the SDs and ICs were capable of increasing the dissolution rate of CFX. This is in accord with the increment in Cefixime’s saturated solubility studies of respective formulations.
Plain Cefixime displayed dissolution upto 44% release, in 30 min and it reached just 52% following 45 min. As drug is inadequately, water soluble so even physical mixing with polymer is not increasing its close contact and hence, little advancement in dissolution rate was observed.
Binary complex formed via spray drying method with β-CD exhibit a cumulative Cefixime release of 96.3% in pH 7.2 buffer in 45 min. The dissolution of complex formed by SE method with β-CD, in 7.2 po4 buffer showed 95. 2% of Cefixime release in 20 min. The formulation of β-CD prepared by KD method released 74% of Cefixime in 20 min that showed 3-fold increment in dissolution at some point. On the other hand, inclusion complex prepared by PM method showed the drug release of 56% with β-CD in 20 min.
The in vitro dissolution studies of solid dispersion by spray drying with Pluronic F-127 in 7.2 po4 buffer with 89.17% drug being released within 20 min the solid dispersion of Cefixime using Pluronic (F127) formed by solubility enhancing technique like PM, KD SE, LY, MW exhibit a release of 61.73%, 64.8%, 89.24%, 87.32% and 85.79%, respectively in 20 min while 97.8% drug was released by formulation by spray drying method in 45 min. Within 20 min, all the Sds prepared with Pluronic showed max dissolution but the SD’s max dissolution is preceded by the initial lag time i.e. slow dissolution [63].
Cefixime SD exhibited faster dissolution could be due to translation as of the Cefixime crystalline to the unstructured amorphous form (as represented by XRD and DSC results).
As reported by many studies for Sds prepared with Pluronic the dissolution has improved by decrease in drug’s crystal size [64]. For dissolution, the mechanical contact between drug and hydrophile like Pluronic is increased leading to improved wetting of drug by carrier thereby the enhanced drug’s dissolution [65,66].
Several factors such as improved wet ability, rapid dispersion in dissolving medium, decreased particle size, the solubilising effect of the hydrophilic carrier, surface tension reduction between solid insoluble drug and dissolution medium were responsible for these solubility and dissolution rate improvement [67-69].
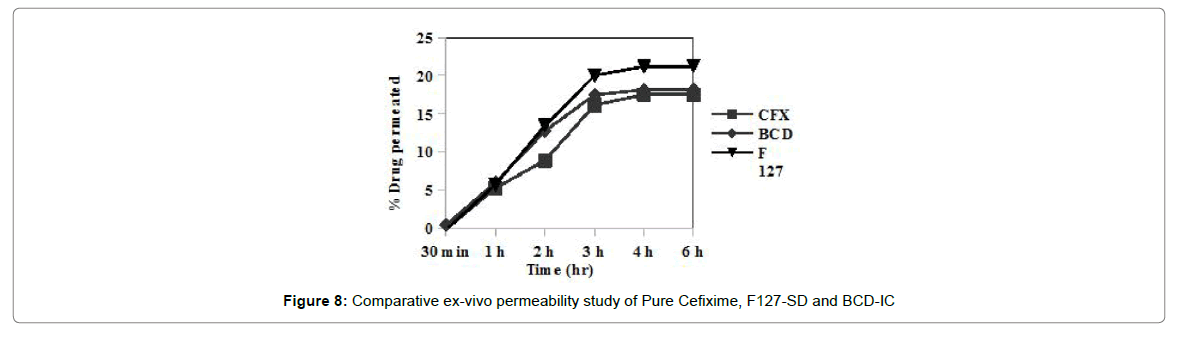
Ex-vivo permeability
Ex-vivo permeability of plain Cefixime, Cefixime-SD and Cefixime-IC was assessed by Non-everted rat intestinal sac model. Figure 8 shows amount of Cefixime which was permeated through rat intestine. Permeability of Cefixime in presence of BCD was low while Cefixime SDs using Pluronic as solubilizer showed better permeability. According to solubility studies Pluronic was better solubilizer than BCD for Cefixime and the magnitude of difference between Pluronic and BCD were same as solubility.
The magnitude of difference between two approaches was not due to permeation enhancing ability of Pluronic but only due to its solubility improving ability. Poloxamer was not found to be much effective in permeability enhancement. Non-everted rat intestinal sac model was found to be useful tool for comparison of permeability of two different formulation approaches.
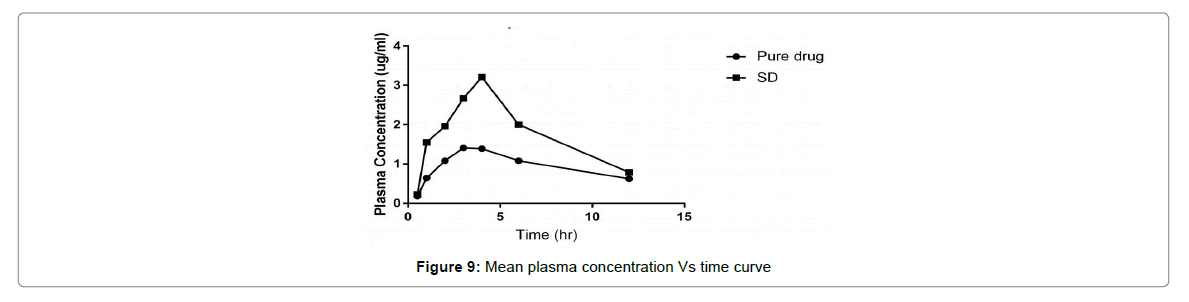
In-vivo study
The average plasma concentration verses time outline for Cefixime after oral administration of pure CFX and solid dispersion are denoted in Figure 9. The difference in the outline of the pharmacokinetic profile among the studied treatments was of elevated Cmax and different tmax for solid dispersion (4 h).
The plain Cefixime ends in the accustomed Cefixime plasma concentrations. Nevertheless, the AUC of Cefixime- SD was 2 times greater than of plain Cefixime suspension. The studied treatment showed statistically significant differences for Cmax (p=0.001) and tmax (p=0.05). These outcome disclose that preparation of Cefixime as SD with Pluronic consequences in a significant (p=0.0 5) increment in absorption of Cefixime, than from the plain Cefixime suspensions.
Thus, better oral assimilation of Cefixime obtained as of the Pluronic-SD, with elevated Cmax and different tmax, may possibly be owing to better dissolution consequential from diminished particle size, greater than before surface area, the close contact between the hydrophilic carrier and the drug, and enhanced wettability.
Conclusion
This work was done to compare the two different solubility enhancing approaches for poorly soluble cefixime as model drug; solid dispersion by using Pluronic and inclusion complexation by BCD. Aforementioned two approaches were found to increase the solubility and thereby the dissolution of drug. The best suited Pluronic was further explored with other solubility enhancing methods like spray drying, microwave irradiation and freeze drying. It was concluded that spray dried SD demonstrated better solubility as well as dissolutions after comparing it with other methods and pure drug. Thus, particle size reduction along with conversion to amorphous form leads to increased dissolution and subsequently increasing oral bioavailability as well as therapeutic action of poorly soluble Cefixime.
Acknowledgment
The authors would like to thank the Sophisticated Analytical Instrumentation Facility (SAIF) at Cochin University, Kochi for the XRD, SEM studies. I am also thankful to the Glenmark pharmaceuticals, Sinnar, Nashik for providing gift sample required for the research work.
References
- Dahan A, Hoffman A (2008) Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release 129: 1-10.
- Chakraborty S, Shukla D, Mishra B, Singh S (2009) Lipid–an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm 73: 1-15.
- Kansy M, Senner F, Gubernator K (1998) Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem 41: 1007-1010.
- Schanke LS, Tocco DJ, Brodie BB, Hogben CAM (1958) Absorption of drugs from the rat small intestine. J Pharm Exp Ther 123: 81-88.
- Dobson PD, Kell DB (2008) Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov 7: 205-220.
- Xiang TX, Anderson BD (1994) The relationship between permeant size and permeability in lipid bilayer membranes. J Membr Biol 140: 111-122.
- Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175: 880-885.
- Avdeef A, Tam KY (2010) How Well Can the Caco-2/Madin− Darby Canine Kidney Models Predict Effective Human Jejunal Permeability? J Med Chem 53: 3566-3584.
- Pade V, Stavchansky S (1998) Link between drug absorption solubility and permeability measurements in Cacoâ€2 cells. J Pharm Sci 87: 1604-1607
- Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, et al. (1999) MDCK (Madin–Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci 88: 28-33.
- Kerns EH, Di L, Petusky S, Farris M, Ley R, et al. (2004) Combined application of parallel artificial membrane permeability assay and Cacoâ€2 permeability assays in drug discovery. J Pharm Sci 93: 1440-1453.
- Youdim KA, Avdeef A, Abbott NJ (2003) In vitro trans-monolayer permeability calculations: often forgotten assumptions. Drug Discov Today 8: 997-1003.
- Avdeef A, Strafford M, Block E, Balogh MP, Chambliss W, et al. (2001) Drug absorption in vitro model: Filter-immobilized artificial membranes: 2. Studies of the permeability properties of lactones in Piper methysticum Forst. Eur J Pharm Sci 14: 271-280.
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, et al. (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflügers Arch 447: 465-468.
- Tucker TG, Milne AM, Fournel GigleuxS , Fenner KS, Coughtrie MW (2012) Absolute immunoquantification of the expression of ABC transporters P-glycoprotein, breast cancer resistance protein and multidrug resistance-associated protein 2 in human liver and duodenum. Biochem Pharmacol 83: 279-285.
- Giacomini KM, Huang SM,Tweedie DJ, Benet LZ, Brouwer KL, et al. (2010) Membrane transporters in drug development. Nat Rev Drug Dis 9: 215-236.
- Estudante M, Morais JG, Soveral G, Benet LZ (2013) Intestinal drug transporters: an overview. Adv Drug Deliv Rev 65: 1340-1356.
- Shugarts S, Benet LZ (2009) The role of transporters in the pharmacokinetics of orally administered drugs. Pharma Res 26: 2039-2054.
- Shaikh MS, Derle ND, Bhamber R (2012) Permeability enhancement techniques for poorly permeable drugs: A review.
- Pande S, Biyani K. Formulation methods of oral drug delivery to enhance bioavailability of BCS class 4 drugs: a review. World J Pharm Sci 2:1641-1657.
- Dahan A, Hoffman A (2008) Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release 129: 1-10.
- Modi A, Tayade P (2007) A comparative solubility enhancement profile of valdecoxib with different solubilization approaches. Indian J Pharm Sci 69: 274.
- Khajuria A, Thusu N, Zutshi U (2002) Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 9: 224-231.
- Maheshwari M (2010) Comparative bioavailability of curcumin, turmeric and Biocurcumaxâ„¢ in traditional vehicles using non-everted rat intestinal sac model. J Funct Foods 2: 60-65.
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R (2010) Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm 76: 475-485.
- Ruan LP, Chen S, Yu BY, Zhu DN, Cordell GA, et al. (2006) Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur J Med Chem 41: 605-610.
- Cui J, Yu B, Zhao Y, Zhu W, Li H, et al. (2009) Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm 371: 148-155.
- Aleem O, Kuchekar B, Pore Y, Late S (2008) Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J Pharm Biomed Anal 47: 535-540.
- Zur M, Hanson AS, Dahan A (2014) The complexity of intestinal permeability: Assigning the correct BCS classification through careful data interpretation. Eur J Pharm Sci 61: 11-17.
- Estudante M, Morais JG, Soveral G, Benet L Z (2013) Intestinal drug transporters: an overview. Adv Drug Deliv Rev 65: 1340-1356.
- Banerjee SK, Jagannath C, Hunter RL, Dasgupta A (2000) Bioavailability of tobramycin after oral delivery in FVB mice using CRL-1605 copolymer, an inhibitor of P-glycoprotein. Life Sci 67: 2011-2016.
- Miller DW, Batrakova EV, Waltner TO, Alakhov VY, Kabanov AV (1997) Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem 8: 649-657.
- Amin ML (2013) P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 7: 27.
- Shaik N, Pan G, Elmquist WF (2008) Interactions of pluronic block copolymers on Pâ€gp efflux activity:Experience with HIVâ€1 protease inhibitors. J Pharm Sci 97: 5421-5433.
- A World Health Organization resource (2006) Proposal to waive in vivo bioequivalence requirements for the WHO model list of essential medicines immediate release, solid oral dosage forms. WHO-Working document. WHO Technical Report Series, No. 937, Annex 8.
- Zhang Y, Wang QS, Cui YL, Meng FC, Lin KM (2012) Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int J Nanomed 7: 4239.
- Nama S, Baburao C (2013) Enhancement ofdissolution rate of cefixime trihydrate by using various solid dispersion techniques.
- Kumar N, Jain AK, Singh C, Kumar R (2014) Development, characterization and solubility study of solid dispersion of terbinafine hydrochloride by solvent evaporation method. Asian J Pharmacol (AJP): Free full text articles from Asian J Pharm 2.
- Aleti SR, Rangaraju D, Kant A, Shankraiah MM, Venkatesh JS, et al. (2011) Solubility and dissolution enhancement of cefixime using natural polymer by solid dispersion technique. Int J Res Pharm Chem 1: 283-288.
- Saindane DS, Kulkarni AS, Sagri AN, Khade TS, Patil MD (2010) Dissolution improvement of poorly water soluble drug by freeze drying with PVP K-30. Int J Pharm Technol 1: 149-162.
- Varia URK (2010) Effect of cyclodextrin inclusion complex on dissolution behaviour of cefixime (Doctoral dissertation, RGUHS).
- Maheshwari M (2010) Comparative bioavailability of curcumin, turmeric and Biocurcumaxâ„¢ in traditional vehicles using non-everted rat intestinal sac model. J Funct Foods 2: 60-65.
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R (2010) Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm 76: 475-485.
- Zhang Y, Wang QS, Cui YL, Meng FC, Lin KM (2012) Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int J Nanomedicine 7: 4239
- Jain N, Jain H, Jain P (2015) Validated Stability Indicating RP-HPLC Method for Simultaneous Estimation of Ofloxacin and Cefixime in their Combined Dosage Form. Sci Tech Art Res J 3: 93-98.
- Craig DQ (2002) The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231: 131-144.
- Derle D, Boddu SHS, Magar M (2006) Studies on the Preparation, Characterization and Solubility of beta-Cyclodextrin-Satranidazole Inclusion Complexes. Ind J Pharma Edu Res 40: 232.
- Chutimaworapan S, Ritthidej GC, Yonemochi E, Oguchi T, Yamamoto K (2000) Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm 26: 1141-1150.
- Dai WG, Dong LC, Li S, Deng Z (2008) Combination of Pluronic/Vitamin E TPGS as a potential inhibitor of drug precipitation. Int J Pharm 355: 31-37.
- Passerini N, Albertini B, González-RodrıÌguez ML, Cavallari C, Rodriguez L (2002) Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci 15: 71-78.
- Ali W, Williams AC, Rawlinson CF (2010) Stochiometrically governed molecular interactions in drug: poloxamer solid dispersions. Int J Pharm 391: 162-168.
- Lee KR, Kim EJ, Seo SW, Choi HK (2008) Effect of poloxamer on the dissolution of felodipine and preparation of controlled release matrix tablets containing felodipine. Arch Pharm Res 31: 1023-1028.
- Shah TJ, Amin AF, Parikh JR, Parikh RH (2007) Process optimization and characterization of poloxamer solid dispersions of a poorly water-soluble drug. AAPS Pharm Sci Tech 8: E18-E24.
- Liu D, Fei X, Wang S, Jiang T, Su D (2006) Increasing solubility and dissolution rate of drugs via eutectic mixtures: itraconazole–poloxamer188 system. Asian J Pharm Sci 1: 213-221.
- Ahuja N, Katare OP, Singh B (2007) Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm 65: 26-38
- Nepal PR, Han HK, Choi HK (2010) Enhancement of solubility and dissolution of Coenzyme Q 10 using solid dispersion formulation. Int J Pharmaceut 383: 147-153.
- Chen Y, Zhang GGZ, Neilly J, Marsh K, Mawhinney D, et al. (2004) Enhancing the bioavailability of ABT-963 using solid dispersion containing Pluronic F-68. Int J Pharm 286: 69-80.
- Rouchotas C, Cassidy OE, Rowley G (2000) Comparison of surface modification and solid dispersion techniques for drug dissolution. Int J Pharm 195: 1-6.
- Janssens S, Van den Mooter G (2009) Review: physical chemistry of solid dispersions. J Pharm Pharmacol 61: 1571-1586.
- Ozeki T, Yuasa H, Kanaya Y (1997) Application of the solid dispersion method to the controlled release of medicine. IX. Difference in the release of flurbiprofen from solid dispersions with poly (ethylene oxide) and hydroxypropylcellulose and the interaction between medicine and polymers. Int J Pharm 155: 209-217.
- Alladi S, Nalini S (2010) Preparation, physico chemical characterization of solid dispersions of tenoxicam with poloxamer. J Pharm Sci Technol 2: 308-311.
- KolaÅ¡inac N, Kachrimanis K, HomÅ¡ek I, Grujić B, Äurić Z, et al. (2012) Solubility enhancement of desloratadine by solid dispersion in poloxamers.Int J Pharm 436: 161-170.
- Nepal PR, Han HK, Choi HK (2010) Enhancement of solubility and dissolution of Coenzyme Q 10 using solid dispersion formulation. Int J Pharm 383: 147-153.
- Passerini N, Albertini B, González-RodrıÌguez ML, Cavallari C, Rodriguez L (2002) Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci 15: 71-78.
- Sekiguchi K, Obi N (1961) Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem Pharm Bull 9: 866-872.
- Serajuddin A, Sheen PC, Mufson D, Bernstein DF, Augustine MA (1988) Effect of vehicle amphiphilicity on the dissolution and bioavailability of a poorly waterâ€soluble drug from solid dispersions. J Pharm Sci 77: 414-417.
- Khan KA (1975) The concept of dissolution efficiency. J Pharm Pharmacol 27: 48-49.
- Alâ€Obaidi H, Brocchini S, Buckton G (2009) Anomalous properties of spray dried solid dispersions. J Pharm Sci 98: 4724-4737.
- Greenhalgh DJ, Cook W (2000) Relationship between drug and carrier solubility parameters and dissolution of solid dispersion systems. J Pharm Pharmacol 52: 299-299.
Citation: Mogal P, Derle D (2017) Cefixime, in General, Class 4 Drug but Individually Class 2 Drug. J Med Physiol Ther 1:103.
Copyright: © 2017 Mogal P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 10731
- [From(publication date): 0-2017 - Jul 14, 2025]
- Breakdown by view type
- HTML page views: 9524
- PDF downloads: 1207