Cervicofacial Actinomycosis of the Maxilla and HIV Infection: A Case Report.
Received: 19-Jan-2017 / Accepted Date: 04-Feb-2017 / Published Date: 11-Feb-2017 DOI: 10.4172/2161-119X.1000288
Abstract
Cervicofacial actinomycosis (CA) is a rare infection caused by Actinomyces spp. and it is infrequently found in the maxilla. The factors associated with the host in the evolution of CA have not been clarified in the literature, and the relationship between actinomycosis and immunodepletion remains surrounded by controversy. CA diagnosis is not usually considered in maxilla injuries, and the potential aggressiveness of these lesions makes it more difficult to reach early diagnosis. This article reports a case of a 31 year old man with a history of drug abuse. The patient was HIV-positive and developed an extensive, destructive lesion in the maxilla. The diagnosis of CA was based on imaging examination and partial biopsy. The patient was treated with crystalline IV penicillin G and amoxicillin orally and preceded under follow-up. CA should be considered in the differential diagnosis of lesions in the maxilla, and health professionals have to pay particular attention when other systemic factors such as HIV infection and drugs abuse are present. CA should be included in the differential diagnosis of maxillary lesions, and healthcare professionals have to dedicate special attention to patients with systemic conditions such as HIV infection and drug abuse, which may intensify the development of more aggressive CA forms.
Keywords: HIV/AIDS; Cervicofacial actinomycosis; Oroantral fistula
253874Abbreviations
CA: Cervicofacial Actinomycosis; HIV: Human Immunodeficiency Virus; HCPA: Hospital De Clínicas De Porto Alegre; CT: Computed Tomography; NUP: Necrotising Ulcerative Periodontitis
Introduction
Actinomycosis is an infrequent, suppurative bacterial disease caused by Actinomyces spp. [1]. The main species identified in these lesions is A. israelii, though strains of A. bovis, A. naeslundii, A. viscous and A. odontolyticus may also be detected. This group of little virulent, grampositive anaerobic bacteria is present in the oral microbiota, including saliva, dental plaque, gingival crevice and periodontal sacs [2-6]. The literature indicates that cervicofacial actinomycosis (CA) manifests after a traumatic event that affects the integrity of the barrier afforded by the oral mucosa, such as periodontal disease, tooth extraction, or any other form of surgical intervention. The occurrence of microtrauma enables the invasion of tissues by bacteria, since the anaerobic environment favors the growth of these microorganisms and the establishment of an infectious process [1,4]. Most CA cases are reputedly caused by A. israelii and A. gerencseriae [7]. However, the disease has a multibacterial character, with different microorganisms triggering the inception and contributing to the evolution of the infectious process in favorable microenvironments [7,8].
Cervicofacial actinomycosis takes on a variety of clinical manifestations, whether chronic or acute, and affects mainly the mandible. Commonly characterized by a hardened, slow-growing, painful mass, CA also produces abscesses, multiple fistulous pathways, and suppuration discharge containing typical ‘sulfur granules’. In addition, the mandibular angle is the site most commonly affected by CA [3,6,9]. The factors associated with the host in the evolution of CA have not been clarified. However, systemic aspects such as immunosuppression by HIV (human immunodeficiency virus) [2,5] and diabetes [1,2,6] have been associated with CA progression. This article describes CA of the maxilla associated with drug abuse and HIV infection. A brief review of the specialized literature on the etiology, clinical manifestations, differential diagnosis, and treatment of CA is also presented.
Case Presentation
A 31 year old Caucasian homosexual male was referred to the Stomatology Unit, Hospital de Clínicas de Porto Alegre (SU-HCPA) of the Federal University of Rio Grande do Sul, Brazil complaining about a lesion on the maxilla that first appeared two months before. The patient’s medical record included an HIV-positive diagnosis six years back, and he was using an antiretroviral regimen of lamivudine 150 mg, ritonavir 100 mg, tenofovir 300 mg and atazanavir 300 mg. The patient revealed having discontinued the antiretroviral therapy for 10 months, resuming treatment in the month prior to the appointment. During the interruption of treatment the patient had been diagnosed with secondary syphilis, pneumocystis pneumonia, and retinitis caused by cytomegalovirus. The patient reported cocaine and cannabis abuse, and imputed the emergence of the maxilla lesion to the extraction of the upper right premolar tooth.
Clinical examination revealed a far-reaching destructive lesion on the right maxilla. The lesion affected both the bone tissue and the oral mucosa on the alveolar ridge (teeth 14, 15 and 16), along the lingual and vestibular aspects (Figure 1). Necrotic gingiva and alveolar mucosa exposed bone and roots of teeth 13 and 17. The patient also presented pseudomembranous white plaque susceptible to mechanical removal on the hard-soft palate transition region, to the left of the medial line, which was compatible with pseudomembranous candidiasis. Pseudomembranous candidiasis was treated with nystatin 100,000 UI/ mL four times a day for 30 days. Pantomography and plain computed tomography (CT) were requested to investigate the extension of the lesion. An appointment to perform the biopsy of the destructive lesion was scheduled, but the patient did not show up.
Figure 1: Intraoral photograph of the patient at the first appointment. Physical examination revealed extensive, destructive lesion that had emerged two months before. The lesion affected the right maxilla, exposing the alveolar bone corresponding to teeth 14, 15 and 16 and affecting the lingual and vestibule oral mucosa. In addition, pseudomembranous candidiasis was observed in the transition between the hard and the soft palate to the left of the median line.
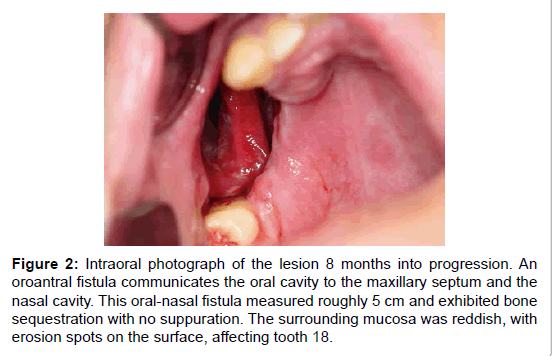
Figure 2:Intraoral photograph of the lesion 8 months into progression. An oroantral fistula communicates the oral cavity to the maxillary septum and the nasal cavity. This oral-nasal fistula measured roughly 5 cm and exhibited bone sequestration with no suppuration. The surrounding mucosa was reddish, with erosion spots on the surface, affecting tooth 18.
Six months later the patient returned to the Stomatology Unit. Intraoral examination revealed progression of the maxillary destructive lesion, which communicated the oral cavity to the maxillary septum and with the nasal cavity (Figure 2). This oral-nasal fistula measured roughly 5 cm and exhibited bone sequestration with no suppuration. The surrounding mucosa was reddish, with erosion spots. The lesion was asymptomatic, and tooth 18, which remained in place, was involved in the bone destruction process. The patient suffered with halitosis, nasal speech and exhibited poor oral health conditions. Based on the patient’s medical record and on the clinical aspect of the lesion, the provisional diagnosis pointed to necrotising ulcerative periodontitis (NUP), deep mycosis and lymphoma.
The most recent blood tests at the time carried out to monitor the HIV infection indicated CD4 count of 367 cells/μL (9% of total lymphocytes), CD4/CD8 ratio of 0.14, and HIV-1 viral load of 491 copies/mL (log 2.69). Anemia (3.81 million erythrocytes/μL; 11.8 mg/ dL hemoglobin and 34.4% hematocrit), glycemia (88 mg/dL), and nonreactive VDRL were observed. In addition, minimally reactive FTAAbs IgG and negative Mantoux test results were also obtained.
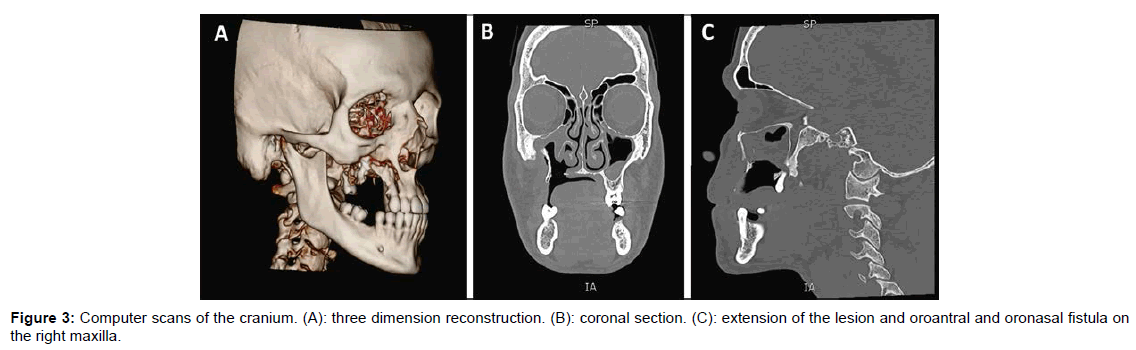
Bone CT scan of the face indicated oroantral and oronasal fistula, revealing the extension of the lesion on the zygomatic bone, starting at the lateral wall of the right maxillary septum. Mucosae of the maxillary septa were thicker than expected, especially on the right side (Figure 3).
A surgical intervention was performed to remove the maxillary bone sequestration and to obtain a sample of the soft tissue adjacent to the oroantral fistula. Tooth 18 was involved in the lesion and therefore was extracted on the occasion.
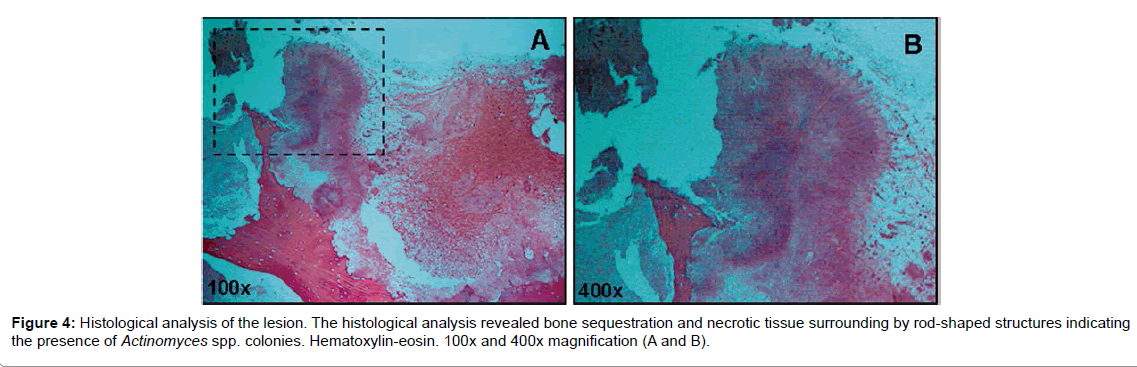
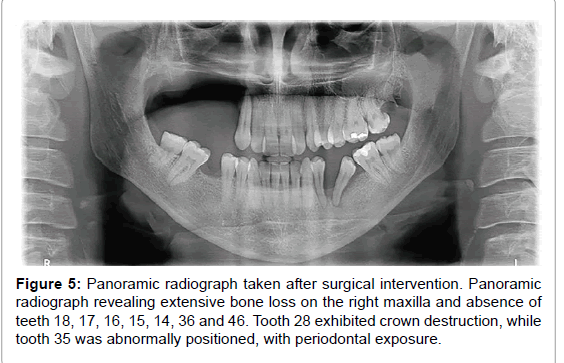
Microscopic examination pointed to bone sequestration, necrotic area, and colonies of Actinomyces spp. (Figure 4). Fungal culture was negative and staining assay for fungal presence revealed but a few fungal elements. Panoramic radiography carried out after surgery showed extensive bone loss on the right maxilla, and the absence of teeth 18, 17, 16, 15, 14, 36 and 46. Tooth 28 exhibited crown destruction. Moreover, tooth 35 was abnormally positioned, with periodontal exposure (Figure 5).
Taken together, these findings led to the diagnosis of CA. The patient remained hospitalized for 22 days. The treatment included 5,000,000 IU of penicillin G crystalline IV for three weeks supplemented with amoxicillin 500 mg orally administered every 8 h for six months.
Remission of lesion was observed at the follow-up appointment, with no bone tissue exposed. However, the oroantral fistula remained in the same place, asymptomatic and covered with a clinically healthy mucosa. The patient was refereed to dental treatment and prosthetic rehabilitation of the palate and is currently awaiting the evaluation of the possibility of reconstructive cosmetic surgery to close the oronasal and oroantral fistula. Ten months after treatment the patient continues to be followed up by the Infectology Service and SU-HCPA healthcare teams, with no recurrence of the lesion.
Discussion
Actinomycosis is classified according to its anatomical position as cervicofacial, thoracic or abdominal, the first of which is the most common manifestation of the disease [3,9]. However, CA is an infrequent disease, which affects mainly young male subjects [4], as observed in this case report.
In the head and neck region, CA manifests mainly in the mandibular angle and its evolution is normally rather slow [10]. However, the disease is rare in the maxilla, with few cases reported in the literature [1-4,9,10].
The establishment of CA is associated with a series of situations that promote the penetration of a microorganism in the mucosa, such as a disease (caries, periodontal disease), or a surgical procedure (tooth extraction, orthognathic surgery) [4,9,11,12]. Periodontal disease and poor oral hygiene are considered important factors in the establishment and evolution of CA [1,6,7], which is compatible with the case described.
The relationship between actinomycosis and immunodepletion is object of consistent discussion in the literature. So far, no consensus about the role of immune status in the inception and evolution of the disease has been reached. Yet, some authors suggest that HIV patients are more susceptible to the disease [2,5]. Apart from this, diabetes [1,2,6,9], alcohol abuse [1,4] and use of immunosuppressant drugs have been implicated in CA in the oral cavity. In the present case, the patient had a CD4 count of 367 cells/μL, resembling a previous CA case report in a HIV patient found in the literature, who presented 480 cells/μL [5]. Considering the patient’s discontinuation of antiretroviral therapy (when his immune status was not monitored) and the expression of past opportunistic infections, the diagnosis carried out in the present case report point to a immunodepletion scenario that may have induced CA, coupled to periodontal disease and a previous trauma in the region.
The different manifestations of CA may turn a proper diagnosis into a difficult challenge. Macroscopic granules (named ‘sulfur granules’) are a significant clinical sign4, though they provide no confirmation of the disease and, oppositely to what was observed in the present study, may not even be present at all [5]. In this sense, CA diagnosis should be made based on the results of histopathology and microbiology assays. Moreover, radiography, CT scans, and magnetic resonance are important resources in the evaluation of the extension of lesions and of the extent to which adjacent structures are affected [1,3,4,6].
Due to the different clinical manifestations, differential diagnosis of CA includes a wide variety of diseases, such as benign and malignant neoplasias, as well as infectious diseases [4-6,9]. In the present case report, due to the location of the lesion and the patient’s HIV infection status, the hypothesis of lymphoma could not be initially ruled out. This is explained in light of the fact that the maxilla is one of the main structures where lymphoma manifests in the oral cavity, though the lesion described did not exhibit the usual clinical characteristics of the disease [10]. The clinical condition and the medical record of the patient supported the hypotheses of NUP and deep fungal infections. The appearance (tissue loss, ulceration, necrosis, and harm to bone structure) as well as the location (the gingival mucosa) of the lesion indicated that the condition could be NUP. In addition, the HIV infection, poor oral hygiene and halitosis indicated to the stomatology team that NUP was a reasonable diagnosis hypothesis. Nevertheless, the evolution of the condition, the far-reaching effect on tissues and the absence of any pain were not compatible with NUP [13]. Fungal diseases such as histoplasmosis and mucormycosis were also included in the differential diagnosis. Such conditions are commonly diagnosed in HIV patients and, apart from manifesting similarly to the expression detected in the present case report, they may even evolve into extensive bone destruction [14,15]. Thus, considering that CA may be have a series of differential diagnoses, even mimicking malignant lesions, the accurate diagnosis of the disease is extremely important in the definition of proper treatment strategies [3-6,9].
The antibiotic regimen prescribed to the patient in this case report is the therapy of choice in these situations, as observed in the literature [1,4-6]. When large lesions are present, this treatment should be prolonged in order to prevent recurrence of infection [1,3-5]. In most cases treatment with antibiotics is enough to control these lesions [1,6,9]. Yet, at times surgical interventions are required in order to drain abscesses, in addition to the removal of necrotic tissue [3-5,7]. In the present case, the removal of sequestered and infected bone tissue, combined with a prescription of systemic antibiotics, sufficed to heal the infection.
Conclusion
Although CA is rarely observed in dentistry, the disease should be appropriately diagnosed, in light of the local aggressiveness of the lesions involved. These may leave severe sequelae, affecting the patient’s quality of life. When a treatment is suitably conducted, prognosis is good. In the present case, lesion extension and the consequent mutilation of the oral cavity required surgical intervention and prosthetic rehabilitation. The patient had history of drugs exposure and was HIV-positive, which could contribute to the disease incidence and its progression. This case report highlights the importance of including CA in the differential diagnosis of lesions on the maxilla, especially in patients with some kind of systemic exposure. Health conditions and patient’s medical history should be fully investigated by health professionals to ensure early diagnosis of CA and prevent the establishment of destructive consequences.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. The ethics committees of the Hospital de Clínicas de Porto Alegre approved this work and the reference number is 14-0467.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
MK and MCM have made the clinical examination and the analysis and interpretation of hematological and radiographic exams. MK and VCC performed the histological examination of the lesion and its diagnosis. MK, VCC, and MCM wrote the draft of the manuscript and revised it critically. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgement
We would like to acknowledge the patient whose condition we described in this case report.
References
- Fonseca VAO, Reis G, Alves C, Simões MJ, Camacho E, et al. (2009) Caso raro de coinfecção tuberculose pulmonar e actinomicose oronasal (A rare case of pulmonary tuberculosis coinfection and oronasal actinomycosis). J Bras Pneumol 35:1152-1155.
- Ablanedo-Terrazas Y, Ormsby CE, Reyes-Terán G (2012) Palatal actinomycosis and kaposi sarcoma in an HIV-infected subject with disseminated Mycobacterium avium-intracellulare infection. Case Rep Med 1: 1-3.
- Crossman T, Herold J (2009) Actinomycosis of the maxilla–A case report of a rare oral infection presenting in general dental practice. Br Dent J 206: 201-202.
- Schmidt T, Caselli G, DÃaz R (2003) Actinomicosis del hueso maxilar superior. Rev Otorrinolaringol Cir Cabeza Cuello 63: 127-131.
- Vazquez AM, Marti C, Reñaga I, Salavert A (1997) Actinomycosis of the tongue associated with human lmmunodeficiency virus infection: Case report. J Oral Maxillofac Surg 55: 879-881.
- Volpi L, Ferreli F, Bignami M, Pistochini A, Meloni F, et al. (2013) A rare localization of actinomycosis mimicking ulcerative malignancy. Case Rep Otolaryngol 2013: 323210.
- Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, et al. (2014) Actinomycosis: Etiology, clinical features, diagnosis, treatment and management. Infect Drug Resist 7: 183-197.
- Moghimi M, Salentijn E, Debets-Ossenkop Y, Karagozoglu KH, Forouzanfar T (2013) Treatment of cervicofacial actinomycosis: A report of 19 cases and review of literature. Med Oral Patol Oral Cir Bucal 18: e627-e632.
- de Andrade AL, Novaes MM, Germano AR, Luz KG, de Almeida Freitas R, et al. (2014) Acute primary actinomycosis involving the hard palate of a diabetic patient. J Oral Maxillofac Surg 72: 537-541.
- Herman WW, Whitaker SB, Williams MF, Sangueza OP (1998) Acute actinomycosis presenting as an ulcerated palatal mass. J Oral Maxillofac Surg 56: 1098-1101.
- Schwartz HC, Wilson MC (2001) Cervicofacial actinomycosis following orthognathic surgery: Report of 2 cases. J Oral Maxillofac Surg 59: 447-449.
- Kolm I, Aceto L, Hombach M, Kamarshev J, Hafner J, et al. (2014) Cervicofacial actinomycosis: A long forgotten infectious complication of immunosuppression-Report of a case and review of the literature. Dermatol Online J 20: 22640.
- Patton LL (2003) Oral lesions associated with human immunodeficiency virus disease. Dent Clin North Am 57: 673-698.
- Iatta R, Napoli C, Borghi E, Montagna MT (2009) Rare mycoses of the oral cavity: A literature epidemiologic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 647-655.
- White J, Khammissa R, Wood NH, Meyerov R, Lemmer J, et al. (2007) Oral histoplasmosis as the initial indication of HIV infection: A case report. SADJ 62: 454-455.
Citation: Klein M, Carrard VC, Munerato MC (2017) Cervicofacial Actinomycosis of the Maxilla and HIV Infection: A Case Report. Otolaryngol (Sunnyvale) 7:288. DOI: 10.4172/2161-119X.1000288
Copyright: © 2017 Klein M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6557
- [From(publication date): 0-2017 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 5602
- PDF downloads: 955