Research Article Open Access
Chemical Synthesis and Structural Properties of Nd, Gd and Dy Doped BiFeO3 Lead Free Ceramics
1Materials Science Laboratory, School of Physics, Vigyan Bhawan, Devi Ahilya University, Khandwa Road Campus, Indore-452001, India
2Department of Physics, Southeast University, Nanjing, 211189-People’s Republic of China, China
- *Corresponding Author:
- Varshney D
Materials Science Laboratory, School of Physics
Vigyan Bhawan, Devi Ahilya University
Khandwa Road Campus, Indore-452001, India
Tel: +91-731-2467028
E-mail: zhg1200@sina.com
Received Date: January 04, 2017; Accepted Date: January 26, 2017; Published Date: February 14, 2017
Citation: Kumar A, Varshney D (2017) Chemical Synthesis and Structural Properties of Nd, Gd and Dy Doped BiFeO3 Lead Free Ceramics. J Powder Metall Min 6: 154. doi:10.4172/2168-9806.1000154
Copyright: © 2017 Kumar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
The present study reports the structural aspects of BiFeO3, Bi0.9Ba0.1Fe0.9M0.1O3, (M=Co, Mn) and Bi0.80RE0.2FeO3 (RE=Nd, Gd and Dy) powders as prepared by solid state reaction route while Bi0.80Sr0.2FeO3 ceramic has been prepared using citrate sol-gel process. X-ray diffraction along with the Rietveld-refinement reveals the rhombohedral (R3c) structure for BiFeO3 and Bi0.9Ba0.1Fe0.9M0.13, (M=Co, Mn), whereas, tetragonal (P4/mmm) for Bi0.80Sr0.2FeO3 ceramic. In case of rare earth substitution there is an abrupt change in the crystal structure. Bi0.8Nd0.2FeO3 ceramic crystallizes in triclinic structure (P1), Bi0.8Gd0.2FeO3 compound shows a major contribution is related to orthorhombic (Pna21) symmetry and minor contributions are attributed to Pnma and R3c phase, whereas the X-ray diffraction of Bi0.8Dy0.2FeO3 confirms the biphasic (Pnma+R3c) nature of the compound. All the properties of the ceramics reflect their structure so, structural evolution is important for enhancing the physical properties at room temperature.
Keywords
Ceramics; BiFeO3; Phase transformations; X-ray diffraction; Rietveld refinement
Introduction
Due to their multiferroic properties, BiFeO3 (BFO) plays an important role in research and technology because of their wide range of potential applications, including information-storage device, spintronics, and magnetoelectric sensor devices [1,2]. The BFO is specifically widely studied due to coupling between the ferroelectric and magnetic order at room temperature and causing possibility of room temperature multifarious devices. BFO has a rhombohedrally distorted perovskite structure (R3c) [3] with high Curie temperature (TC~1100 K) and antiferromagnetic Neel temperature (TN~675 K) with a spatially modulated spiral spin structure [4]. From the existing literature, it has been observed that partial substitution of rare-earth and metal ion elements of a Bi site in BFO helps in eliminating the impurity phase along with a structural phase transformation and improving the ferroelectric and ferromagnetic properties. It has been reported a structural phase transition from rhombohedral to orthorhombic phase for 30% La substituted BiFeO3 and enhances the magneto-electric interaction. A structural transformation from rhombohedral structure for BiFeO3 to triclinic structure for Bi1-xNdxFeO3 (x=0.05–0.15) and the magneto-electric coupling was clear in Bi1-xNdxFeO3 (x=0.15–0.175) near the Néel temperature of (653 K whereas, for further Nd doped samples Bi1-xNdxFeO3 (x=0.175–0.2) a pseudotetragonal structure has been reported [5]. In the La and Smmodified BFO ceramics (i.e. Bi1-x-ySmxLayFeO3), the ceramic with 0≤ x≤0.1 belongs to the triclinic structure, the ceramics with 0.1≤ x≤0.3 have a mixed phase (rhombohedral+orthorhombic) and a triclinic phase was observed for 0≤y≤0.15 ceramics. A low tanδ (˜ 0.43%) and a large d33 (~50 pC/N) were observed for x=0.025, y=0.05 ceramic so this is promising candidate for high temperature applications [6]. For Gd substituted Bi1-xGdxFeO3 a compositional driven structural phase transition R3c → Pn21a occur at x=0.1 and Pn21a → Pnma occur in the range of 0.2<x<0.3 and was proved to be more effective in improving the multiferroic property of antiferromagnetic BiFeO3 [7]. Bi1-xDyxFeO3 ceramic at concentration x=0.15 crystallizes in biphasic structure with R3c+Pnma model. On the other hand 20% Dy substitution confirms the orthorhombic structure with Pnma structural model [8]. The partial substitution of Sr2+ at Bi3+ in BiFeO3 changes the symmetry from rhombohedral to pseudotetragonal or cubic and it remains in the cubic phase with further Sr substitution in Bi1-x SrxFeO3 [9,10]. It has also been reported that, the Bi0.7A0.3FeO3 (A=Ca, Sr and Pb) ceramics were described within the rhombohedral (space group R3c) phase [11,12]. These improved properties obtained by rare-earth and metal ion doping demonstrate the possibility of enhancing the multiferroic applicability of BFO which depends on crystal structure. On the other hand, Zn and Mn-doped BFO thin films as deposited on the SrRuO3-buffered silicon substrate was able to reduce leakage current density and enhance ferroelectric behaviour of 2Pr~235 μC/cm2 and 2EC~612 kV/cm [13,14]. Because of the technological importance and interesting physical properties behind the structure, it is necessary to study and analyze the crystal structure of compounds. With the above motivation, we prepare some rare earth and alkaline earth metals substituted BiFeO3 multiferroic Bi0.8RE0.2FeO3 (RE=Nd, Gd and Dy) samples via solid-state reaction route and Sol-gel route. A detailed structural analysis using the Rietveld refinement method has been reported. It is noticed that the specific divalent doping does not create any change in the crystal structure while particular substitution of rare earth ions changes the crystal symmetry of BFO ceramics.
Experimental Details
The polycrystalline samples are prepared by both solid-state reaction route and citrate Sol-gel route. The polycrystalline samples of BiFeO3, Bi0.9Ba0.1Fe0.9M0.1O3 (M=Co, Mn), Bi0.80Sr0.20FeO3, Bi0.8Nd0.2FeO3, Bi0.8Gd0.2FeO3, Bi0.8Dy0.2FeO3, designated as BFO, BBFCO, BBFMO BSFO, BNFO, BGFO and BDFO. The ceramics BFO, BBFCO, BBFMO, BNFO, BGFO, BDFO, was prepared by conventional solid-state reaction route while Bi0.80Sr0.2FeO3 by citrate sol-gel method.
Solid state reaction route
For solid state reaction route starting materials of oxides and carbonates such as Bi2O3, Fe2O3, BaCo3, Co3O4, MnO2, Gd2O3, and Dy2O3 were weighed in stoichiometric ratio, mixed, and grounded thoroughly in an agate mortar for homogeneous mixture and calcined for 6 hours at 650°C for the desired composition of ceramics. All the calcined compositions were uniaxially dye-pressed into pellets of size 10 mm in diameter and 1-2 mm in thickness. Finally, the sintering process was performed at 820°C for 3 hours.
Citrate sol-gel process
For citrate Sol-gel method, equimolar amount of Bi(NO3)2.6H2O, Fe(NO3)2.9H2O and Sr(NO3) were dissolved in deionized water and then calculated amount of citric acid were added (with molar amount of citric acid equal to total molar amount of nitrates in the solution). Then the homogenous solution was gently evaporated at 80°C on hot plate to obtain gel. The dried powder was then calcined at 650°C for 4 hours to get the resultant substituted BiFeO3 crystalline compounds. The obtained calcined powder was finally pressed into thin pallets of 10 mm diameter and 1-2 mm thickness and then sintered at high temperature 820°C for 1.3 hours in air. Sol-gel method involves exothermic and self-sustaining thermally-induced anionic redox reaction of xerogel, which is obtained from aqueous solution containing desired metal salts (oxidizer) and organic complexant (reductant). The nitrate salts are favoured as precursors, because they serve as water-soluble low temperature NO-3 oxidant source for synthesis of samples. This method is easy, cheep and less time taking process. Through this process, we can get phase pure ceramic samples.
Characterization
These compounds are characterized for structural understanding. X-ray powder diffraction was carried out with CuKα1 (1.5406 Å) radiation using Bruker D8 Advance X-ray diffractometer over the angular range 20-70° (with a scanning rate of 2° min-1 at room temperature working at 40 kV voltage and 40 mA current. The lattice parameters and other detailed structural information were obtained by the Rietveld refinement FullPROF program.
Results and Discussion
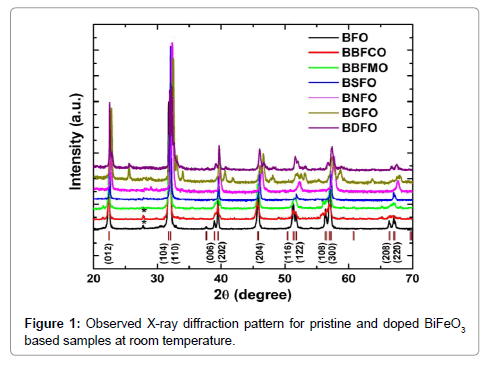
X-ray powder diffraction patterns of the BiFeO3, Bi0.9Ba0.1Fe0.9M0.1O3 (M=Co, Mn), Bi0.80Sr0.20FeO3, Bi0.8Nd0.2FeO3, Bi0.8Gd0.2FeO3, Bi0.8Dy0.2FeO3 samples (abbreviated as BFO, BBFCO, BBFMO BSFO, BNFO, BGFO and BDFO, respectively) are shown in Figure 1. XRD data reveal that the BFO ceramic powder has a rhombohedral distorted perovskite structure with a=0.55798 nm and c=1.3867 nm. A value of 8.549 g cm-3 is obtained for the theoretical density of BFO, which is calculated from the lattice constants by X-ray diffraction measurement data. All the diffraction peaks matches well with the standard crystal data corresponding to the JCPDS file No. 86-1518, except a minor low-intensity impurity peak at about 2θ=28.42° (marked by *) which is associated with Bi2Fe4O9 (JCPDS file no. 72-1832). The occurrence of Bi2Fe4O9 secondary phase peaks is generally observed in pure BFO due to the kinetics of phase formation and the high volatility of Bi2O3 (Figure 1).
In the BBFCO ceramic compound, a small amount of Bi2Fe4O9 impurity phase was detected (marked by *). XRD data pattern of both BBFCO, BBFMO the samples revealed the formation of nearly singlephase rhombohedral structure that can be described in hexagonal frame of reference with R3c space group. The diffraction peaks of BSFO samples revealed the pseudotetragonal structure (P4/mmm) are good matches with the earlier reported data [15]. Henceforth, the BSFO samples as prepared by the sol-gel method have a single-phase pseudotetragonal structure. The XRD pattern of Bi0.8RE0.2FeO3 (RE=Nd, Gd and Dy) ceramic samples exhibits different crystal structures.
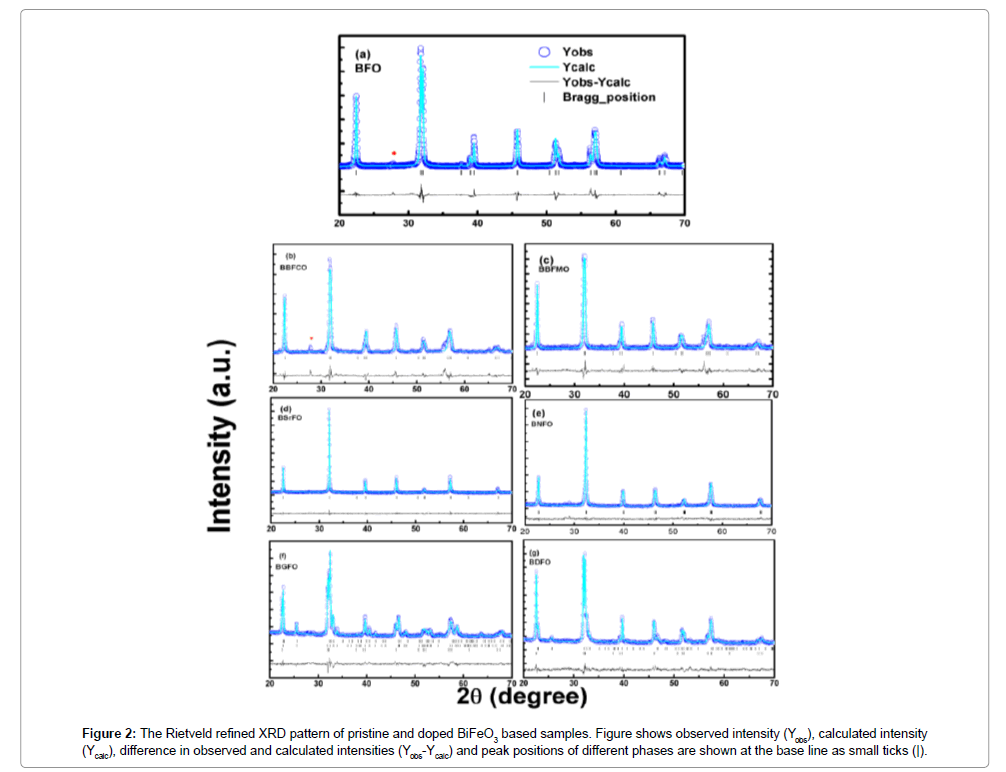
In order to further analyze the structural transformation measured XRD patterns of the samples were simulated based on Rietveld refinement using FullPROF program (Figure 2). The Rietveld refinement result of all the prepared ceramics is documented in Figure 2. For pristine BFO, both the refined crystal axes (a=0.5579 nm and c=1.3865 nm) and crystal axial angles (α=β=90°, γ=120°) are found to coincide with those of the rhombohedral R3c symmetry. It is noticed that the simulated XRD pattern agrees well with the measured data with no structural phase transition from rhombohedral to any other phase as it has been reported earlier that Ba and Mn substitution with (x≤0.4) has not affected the crystalline structure of the parent compound BFO, which is important for the FE properties of the compounds [16] (Table 1).
Figure 2: The Rietveld refined XRD pattern of pristine and doped BiFeO3 based samples. Figure shows observed intensity (Yobs), calculated intensity (Ycalc), difference in observed and calculated intensities (Yobs-Ycalc) and peak positions of different phases are shown at the base line as small ticks (|).
| Structure | Lattice parameters (nm) | Atoms | x | y | z | R-factors (%) |
|---|---|---|---|---|---|---|
| BiFeO3 | ||||||
| R3c | a=0.5579 | Bi | 0 | 0 | 0 | RBragg=4.01 |
| b=0.5579 | Fe | 0 | 0 | 0.2163 | Rp=10.2 | |
| c=1.386 | O | 0.5638 | 0.0197 | 0.9475 | Rwp=14.6 | |
| V=37.38 nm3 | c2=3.76 | |||||
| GOF=1.12 | ||||||
| Bi0.9Ba0.1Fe0.9Co0.1O3 | ||||||
| R3c | a=0.5588 | Bi/Ba | 0 | 0 | 0 | RBragg=10.6 |
| b=0.5588 | Fe/Co | 0 | 0 | 0.263 | Rp=16.3 | |
| c=1.3846 | O | 0.459 | 0.2577 | 0.9612 | Rwp=23.0 | |
| V=37.45 nm3 | c2=4.75 | |||||
| GOF=2.2 | ||||||
| Bi0.9Ba0.1Fe0.9Mn0.1O3 | ||||||
| R3c | a=0.5581 | Bi/Ba | 0 | 0 | 0 | RBragg=8.59 |
| b=0.5581 | Fe/Mn | 0 | 0 | 0.235 | R=18.4 | |
| c=1.3818 | O | 0.4437 | 0.0255 | 0.9617 | Rwp=27.2 | |
| V=37.28 nm3 | χ2=5.88 | |||||
| GOF=2.7 | ||||||
| Bi0.8Sr0.2O3 | ||||||
| P4/mmm | a=0.5401 | Bi/Sr | 0 | 0 | 0 | RBragg=5.94 |
| b=0.7782 | Fe | 0.5 | 0.5 | 0.5 | Rp=6.38 | |
| c=0.5590 | O | 0.5 | 0.5 | 0 | Rwp=8.2 | |
| V=23.49 nm3 | χ2=1.56 | |||||
| GOF=1.2 | ||||||
Table 1: Structural parameter for BiFeO3, Bi0.9Ba0.1Fe0.9M0.1O3 (M=Co, Mn), Bi0.80Sr0.20FeO3 obtained by Rietveld refinement of the XRD patterns at room temperature.
For the Sr2+ ion doped BFO sample, it was found to be almost cubic with a ≈ b ≈ c. Sr2+ ion creates a much distortion in the BFO and even will affect the properties. The calculated parameters of all alkaline earth metal, ceramics after refinement are listed in Table 1. On the other hand, the XRD pattern of rare earth Nd3+-doped BFO ceramic indexed in triclinic structure (P1 space group) with cell parameter a=0.3907 nm, b=0.3911 nm, c=0.3901 nm. According to a recent study, a substitutional induced structural phase transition (R3c (Pnma) was observed in Gd doped Bi1-xGdxFeO3 at x>0.1 [17]. However, using only R3c or Pnma structural model in the fitting of diffraction spectra for present BGFO compound gave no satisfactory result. Fitting undertaken for R3c or Pnma structural models showed that the reflection intensities could not be properly described within any one of them. The fitting was improved with the addition of Pna21 model. The best iteration for the corresponding model yield χ2≈19.03 for only R3c, χ2≈8.96 for Pnma+R3c and χ2≈3.35 for Pna21+R3c. The most excellent refinement showed that the diffraction profile of BGFO compound is a result of superposition of three spectral contributions.
A major contribution is related to orthorhombic phase (94.20%) with Pna21 symmetry (a=0.5610 nm, b=0.7783 nm, c=0.5414 nm). Minor contributions are attributed to orthorhombic phase (2.78%) with Pnma symmetry and rhombohedral phase (3.01%) with R3c symmetry. It has been observed that, the fitting was improved best by undertaking the three structural models (Pnma+Pna21+R3c) for BGFO compound with generally small R values and minimum χ2 ≈ 2.5 as described in Table 2. This indicates that the crystal structure of BGFO compound is characterized by the coexistence of three phases. Similarly, for BDFO compound, the refinement was performed with Pnma+R3c structural model (Table 2).
| Structure | Lattice parameters (nm) | Atoms | x | y | z | R-factors (%) |
|---|---|---|---|---|---|---|
| Bi0.8Nd0.2FeO3 | ||||||
| P1 | a=0.3907 | Bi/Nd | 0 | 0 | 0 | RBragg=10.0 |
| b=0.3911 | Fe | 0.5689 | 0.4362 | 0.5467 | Rp=7.79 | |
| c=0.3900 | O1 | -0.0815 | 0.4542 | 0.6774 | Rwp=11.2 | |
| V=59.60 nm3 | O2 | 0.4538 | -0.072 | 0.6835 | c2=1.85 | |
| O3 | 0.4541 | 0.4756 | 0.0176 | GOF=1.16 | ||
| Bi0.8Gd0.2FeO3 | ||||||
| Pn21a | a=0.5610 | Bi/Gd | 0.0469 | 0.2574 | 0.9926 | RB1=22.8 |
| -94.20% | b=0.7783 | Fe | 0.0065 | 0 | 0.5059 | RB2=6.43 |
| c=0.5414 | O1 | 0.4682 | 0.2762 | 0.1103 | RB3=8.72 | |
| V=23.62 nm3 | O2 | 0.17 | 0.5337 | 0.2061 | Rp=4.77 | |
| O3 | 0.2234 | 0.543 | 0.7937 | Rwp=6.14 | ||
| Pnma | a=0.5404 | Bi/Gd | 0.0498 | 0.25 | 0.9934 | c2=2.35 |
| -2.78% | b=0.7802 | Fe | 0 | 0 | 0 | GOF=1.5 |
| c=0.5547 | O1 | 0.4664 | 0.25 | 0.0818 | ||
| V=23.34 nm3 | O2 | 0.2076 | 0.5448 | 0.2026 | ||
| R3c | a=0.5404 nm | Bi/Gd | 0 | 0 | 0.2676 | |
| -3.01% | b=0.7802 nm | Fe | 0 | 0 | 0 | |
| c=0.5547 nm | O | 0.6794 | 0.7801 | 0.5544 | ||
| V=36.65 nm3 | ||||||
| Bi0.8Dy0.2FeO3 | ||||||
| Pnma | a=0.5401 | Bi/Dy | 0.0472 | 0.25 | 0.9933 | RB1=10.7 |
| -80.62% | b=0.7782 | Fe | 0 | 0 | 0.5 | RB2=6.96 |
| c=0.5590 | O1 | 0.3832 | 0.25 | 0.0818 | Rp=5.62 | |
| V=23.49 nm3 | O2 | 0.2076 | 0.5414 | 0.2044 | Rwp=7.20 | |
| R3c | a=0.5550 | Bi/Dy | 0 | 0 | 0.2676 | c2=1.85 |
| -19.38% | b=0.5550 | Fe | 0 | 0 | 0 | GOF=1.4 |
| c=1.3788 | O | 0.6794 | 0.7801 | 0.5544 | ||
| V=36.78 nm3 | ||||||
Table 2: Rietveld refined structural parameters of the Bi0.8RE0.2FeO3 (RE=Nd, Gd, Dy) samples simulated based on the measured XRD patterns.
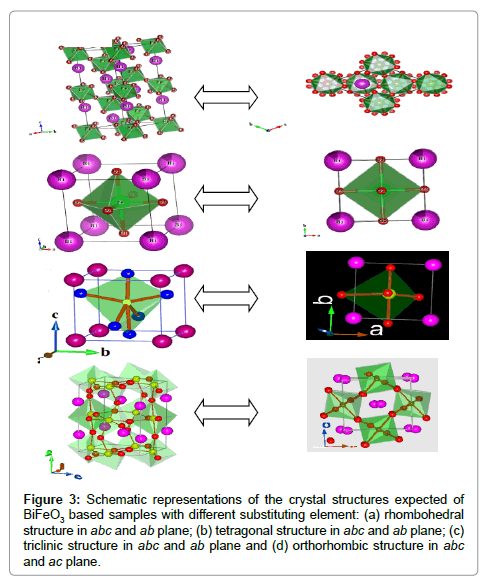
The Rietveld refined XRD pattern of BDFO shown in Figure 1. The dominant contribution is related to orthorhombic phase (Pnma, 80.62%) with lattice parameters a=0.5401 nm, b=0.7784 nm, c=0.5589 nm. Another component is related to rhombohedral phase (R3c, 19.38%). The best iteration gives χ2 ≈ 4.32 for R3c, χ2 ≈ 10.08 for Pnma and χ2≈1.84 for R3c+Pnma model attributing to the fact that the crystal structure of BDFO compound is characterized by coexistence of two phases with a minimum χ2 value (Figure 1). The obtained result is consistent with the earlier reported work [18] (Figure 3). The structure of BiFeO3, Bi0.9Ba0.1Fe0.9M0.1O3, (M=Co, Mn), Bi0.80Sr0.2FeO3, Bi0.8RE0.2FeO3 (RE=Nd, Gd and Dy) samples generated using FullPROF studio program looks like as mentioned in Figure 3. It is eminent that BFO is a rhombohedrally-distorted perovskite with R3c space group. This structure can be derived from the rotations of the oxygen octahedra around [111]c direction relative to the parent cubic cell and displacements of the Bi3+ and Fe3+ cations along the same [111]c direction. Owing to the lone pair effect, the Fe3+ ions in distorted oxygen octahedra, while the Bi3+ ions, in the dodecahedral positions are strongly shifted from the central position towards one of the Fe3+ ions [19,20]. We summarized structural parameters for all prepared ceramics and also identify the residuals for the pattern Rp, weighted pattern Rwp, Braggs factor Bragg, and goodness of fit χ2.
Figure 3: Schematic representations of the crystal structures expected of BiFeO3 based samples with different substituting element: (a) rhombohedral structure in abc and ab plane; (b) tetragonal structure in abc and ab plane; (c) triclinic structure in abc and ab plane and (d) orthorhombic structure in abc and ac plane.
Conclusions
In summary, polycrystalline samples of BiFeO3, Bi0.9 Ba0.1 Fe0.9 M0.1O3, (M=Co, Mn) and Bi0.8RE0.2FeO3 (RE=La, Nd, Gd and Dy) were successfully prepared by solid state reaction route and Bi0.80Sr0.2FeO3 ceramic has been prepared using citrate sol-gel process. These samples were further investigated by powder X-ray diffraction for structural analysis. All the samples fitted with Rietveld refinement using FullPROF program. X-ray diffraction along with the Rietveld-refinement reveals the rhombohedral (R3c) structure for BiFeO3 and Bi0.9Ba0.1Fe0.9M0.1O3, (M=Co, Mn), whereas, tetragonal (P4/mmm) for Bi0.80Sr0.2FeO3 ceramic. In case of rare earth substitution there is an abrupt change in the crystal structure. Bi0.8Nd0.2FeO3 ceramic crystallizes in triclinic structure (P1), Bi0.8Gd0.2FeO3 compound shows a major contribution is related to orthorhombic (Pna21) symmetry and minor contributions are attributed to Pnma and R3c phase, whereas the X-ray diffraction of Bi0.8Dy0.2FeO3 confirms the biphasic (Pnma+R3c) nature of the compound. All the properties of the ceramics reflect their structure so, structural evolution is important for enhancing the physical properties at room temperature.
Acknowledgements
Authors are thankful to UGC-DAE-CSR for financial assistance. UGC-DAECSR, as an institute is acknowledged for extending its facilities. The authors acknowledge Dr. M. Gupta of UGC-DAE-CSR, Indore for useful discussions and the UGC-DAE Consortium for Scientific Research, Indore for XRD measurements and financial support.
References
- Fiebig M (2005) Revival of the Magnetoelectric Effect. J Phys D: Appl Phys 38: R123.
- Catalan G, Scott JF (2009) Physics and Applications of Bismuth Ferrite. Adv Mater 21: 2463-2485.
- Neaton JB, Ederer C, Waghmare UV, Spaldin NA, Rabe KM (2005) First-principles study of spontaneous polarization in multiferroic BiFeO3. Phys Rev B 71: 014113.
- Zhang ST, Zhang Y, Lu MH, Du CL, Chen YF, et al. (2006) Substitution-induced phase transition and enhanced multiferroic properties of Bi1−xLaxFeO3 ceramics. Appl Phys Lett 88: 162901.
- Yuan GL, Or SW, Liu JM, Liu ZG (2006) Appl Phys Lett 89: 052905.
- Zheng T, Wu J (2015) J Mater Chem C 3: 3684-3693.
- Khomchenko VA, Kiselev DA, Bdikin IK, Shvartsman VV, Borisov P, et al. (2008) Appl Phys Lett 93: 262905.
- Khomchenko VA, Karpinsky DV, Kholkin AL, Sobolev NA, Kakazei GN, et al. (2010) J Appl Phys 108: 074109.
- Varshney D, Kumar A (2013) Room temperature structure vibrational and dielectric properties of Ho modified YMnO3. J Mol Struct 1038: 242.
- Li J, Duan Y, He H, Song D (2001) Crystal Structure, Electronic Structure, and Magnetic Properties of Bismuth-Strontium Ferrites. J Alloys Compd 315: 259-264.
- Khomchenko VA, Kiselev DA, Vieira JM, Kholkin AL (2007) Appl Phys Lett 90: 242901.
- Khomchenko VA, Shvartsman VV, Borisov P, Kleemann W, Kiselev DA, et al. (2009) Crystal structure and magnetic properties of Bi0.8(Gd1−xBax)0.2FeO3 (x=0, 0.5, 1) multiferroics. J Phys D: Appl Phys 42:045418.
- Wu J, Qiao S, Wang J, Xiao D, Zhu J (2013) A giant polarization value of Zn and Mn co-modified bismuth ferrite thin films.
- Wu J, Wang J (2010) BiFeO3 thin films of (1 1 1)-orientation deposited on SrRuO3 buffered Pt/TiO 2/SiO2/Si(1 0 0) substrates. Acta Mater 58: 1688-1697.
- Troyanchuk IO, Bushinsky MV, Karpinsky DV, Sirenko V, Sikolenko V, et al. (2010) Structural and magnetic phases of Bi1−xAxFeO3−δ (A=Sr, Pb) perovskites. Eur Phys J B 73: 375.
- Yin LH, Song WH, Jiao XL, Wu WB, Zhu XB, et al. (2009) Multiferroic and magnetoelectric properties of Bi1−xBaxFe1−xMnxO3 system. J Phys D: Appl Phys 42: 205402.
- Lazenka VV, Zhang G, Vanacken J, Makoed II, Ravinski AF, et al. (2012) Structural transformation and magnetoelectric behaviour in Bi1−xGdxFeO3 multiferroics. J Phys D: Appl Phys 45: 125002.
- Khomchenko VA, Karpinsky DV, Kholkin AL, Sobolev NA, Kakazei GN, et al. (2010) J Appl Phys 108: 074109.
- Fischer P, Polomskya M, Sosnowska I, Szymanksi M (1980) J Phys C 13: 29.
- Bucci JD, Robertso BK, James WJ (1972) The precision determination of the lattice parameters and the coefficients of thermal expansion of BiFeO3. J Appl Crystallogr 5: 187-191.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 4769
- [From(publication date):
April-2017 - Aug 10, 2025] - Breakdown by view type
- HTML page views : 3749
- PDF downloads : 1020