Clinicopathological Assessment of Patients with Locally Advanced Breast Cancer with 10 or More Lymph Node Metastases
Received: 01-May-2016 / Accepted Date: 14-May-2016 / Published Date: 30-May-2016 DOI: 10.4172/2572-4118.1000107
Abstract
Background and Objective: Prognosis is generally very poor in patients with breast cancer with 10 or more axillary node metastases, but long-term recurrence-free survival is observed. We assess the clinicopathological features of these patients with and without recurrent disease and review the literature. Patients and Methods: We retrospectively examined the background, clinicopathological features, and prognoses of 29 patients who underwent surgery at our hospital for primary breast cancer with 10 or more axillary lymph node metastases between April 2003 and March 2015 and compared findings between those with and without disease recurrence. Metastases were identified based on hematoxylin and eosin staining. Results: The mean number of lymph node metastases was 19 and of dissected lymph nodes, 26. The cumulative disease-free survival plateaued at 59% 3 years after treatment, and the cumulative overall survival rate was 68.4% at 5 years and plateaued at 61% at 6 years. The mean disease-free survival was significantly shorter in those whose disease recurred (13.6 months) than those without recurrence (62.2 months). The expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) differed significantly between the 2 groups. Conclusion: Prognosis is generally very poor in patients with breast cancer with 10 or more axillary node metastases if tumors recur within 3 years. Furthermore, findings of all five patients with recurrent disease demonstrating a triple-negative subtype and all 14 patients without recurrence demonstrating luminal A intrinsic subtype suggest the use of these subtypes as prognostic factors.
Keywords: Locally advanced breast cancer, Prognostic indicator,Tenor more metastases to axillary nodes
35795Introduction
In the more than 70 years since World War II, the increasing number of Japanese women with breast cancer seems to reflect the substantial transformation that has taken place in the lifestyle habits of the Japanese people. The numbers of cases of cancer of the breast first overtook those of gastric cancer and by 1996 exceeded those of all other organ cancers. An estimated one in 12 Japanese women will develop cancer of the breast during their lifetimes. For those who do, the presence or absence and number of axillary lymph node metastases have been widely recognized as independent prognostic factors [1-6], and recommended treatment for patients with 4 or more involved lymph nodes combines regional nodal irradiation with postoperative adjuvant chemotherapy [7]. We assess the clinicopathological features of patients with 10 or more lymph node metastases, which generally bear a very poor prognosis, and review the literature.
Patients and Methods
From women with breast cancer who underwent surgeryin the Department of Surgery of the Jikei University Kashiwa Hospital between April 1, 2003 and March 31, 2015, we identified 29 cases (approximately 2.5%) with more than 10 histopathologically confirmed axillary lymph node metastases and retrospectively examined the patient background, clinicopathological features, and prognoses associated with these cases. Tumors were classified histopathologically using the criteria of the International Union against Cancer (UICC)-World Health Organization (WHO), and the expression of steroid hormone receptors was determined on the basis of the J-score [8] with the use of immunohistological staining. The overexpression of human epidermal growth factor receptor 2 (HER2) protein was determined using immunohistological staining and the HercepTestTM (Dako Agilent Pathology Solutions, Denmark), and if the score was 2, findings were confirmed using dual-color fluorescence in situ hybridization. We used the Kaplan-Meier method to calculate cumulative survival rates, a generalized Wilcoxon test to determine differences, and t-tests and chi-square tests to analyze statistics, with P ≤0.05 deemed significant.
Results
Table 1summarizes patient characteristics. Three of the 29 declined postoperative chemotherapy because of their advanced age, and trastuzumab was added to the treatment regimen of each patient when indicated. Patients with positive hormone receptors received endocrine therapy and regional nodal and chest wall irradiation.
| Total | |
|---|---|
| No. of patients | 29 |
| median age (years) | 59.4 ± 12.6 (32-83) |
| median duration of follow up (months) | 49.5 ± 31.2 (7-122) |
| median DFI (months) | 41.8 ± 35.3 (0-122) |
| median tumor size (mm) | 51.5 ± 33.5 (11-155) |
| No. of dissected lymph nodes | 25.7 ± 5.3 (16-38) |
| No. of positive lymph nodes | 19.0 ± 7.0 (10-37) |
| No. of positive ER | 16 |
| No. of positive PgR | 13 |
| No. of positive HER2 | 9 |
| No. of intrinsic subtype | |
| luminal A type | 14 |
| luminal B type | 4 |
| HER2 enriched type | 5 |
| (triple negative type) | 6 |
| No. of hitologic type | |
| IDC | 16 |
| ILC | 6 |
| IMPC | 7 |
| adjuvant chemotherapy (±trastuzumab) | 26 |
| adjuvant radiation therapy | 25 |
| adjuvant endocrine therapy | 16 |
Table 1: Characteristics of 29 patients and their tumors.DFI: disease free interval, ER: estrogen receptor, PgR: progesterone receptor, HER2: human epidermal growth factortype2, IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma, IMPC: invasive micropapillary carcinoma.
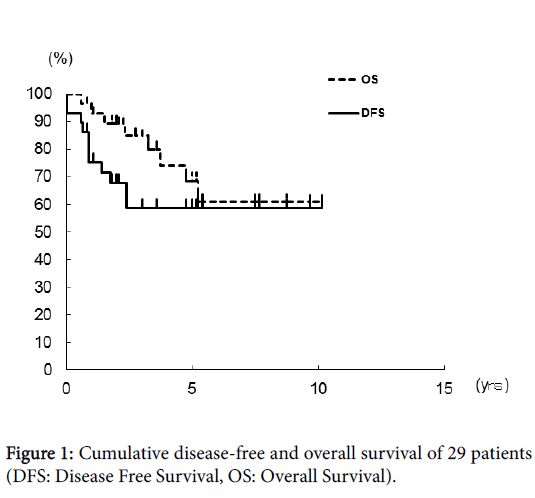
The mean follow-up was 49.5 months, mean age of patients, 59.4 years, mean number of lymph node metastases, 19.0, and mean number of dissected lymph nodes, 25.7. The cumulative disease-free survival was 75.4% at one year after surgery, 67.9% at 2 years, and 58.8% at 3 years, where it remained until 10 years after surgery (Figure 1). The cumulative overall survival was 93.0% at one year after surgery, 84.8% at 3 years, and 68.4% at 5 years and plateaued at 6 years. Survival at 10 years after surgery was 60.8% (Figure 1). The mean survival of the 8 patients who died from confirmed recurrence of their cancers was 23.4 months.
Sixteen patients had invasive ductal carcinoma, six had invasive lobular carcinoma, and seven had invasive micropapillary carcinomas. We excluded one patient from study who died from an accident after a year with no sign of recurrence and divided the remaining 28 patients into 2 groups, 17 who survived without recurrence and 11 who developed recurrent disease. Table 2 summarizes the 2 groups.
| Recurrence-free group | Recurrence group | P | |
|---|---|---|---|
| No. of patients | 17 | 11 | |
| Median age (years) | 59.0±13.3(32-82) | 60.8±11.7(39-83) | NS |
| Median Duration of Follow up (months) | 62.2±32.5(21-121) | 33.5±16.8(7-63) | 0.01 |
| Median DFI (months) | 62.2±32.5(21-121) | 13.6±9.9(0-30) | <0.000 |
| Median Tumor Size (mm) | 47.1±27.5 (11-112) | 60.9±40.1 (24-155) | NS |
| No. of dissected lymph nodes | 24.2±4.7(16-32) | 28.5±5.3(22-38) | 0.03 |
| No. of positive lymph nodes | 18.1±5.1(11-31) | 20.4±9.4(10-37) | NS |
| No. of positive ER | 14 | 2 | 0.001 |
| No. of positive PgR | 12 | 1 | 0.001 |
| No. of positive HER2 | 3 | 6 | 0.04 |
| No. of intrinsic subtype | |||
| luminal A type | 14 | 0 | <0.000 |
| luminal B type | 2 | 2 | NS |
| HER2 enriched type | 1 | 4 | 0.04 |
| (triple negative type) | 0 | 5 | 0.002 |
| No. of hitologic type | |||
| IDC | 10 | 6 | NS |
| ILC | 4 | 1 | NS |
| IMPC | 3 | 4 | NS |
| Adjuvant chemotherapy(±trastuzumab) | 15 | 10 | |
| Adjuvant radiation therapy | 17 | 8 | |
| Adjuvant endocrine therapy | 14 | 2 | |
Table 2: Characteristics of patients and their tumors in the recurrence-free group and recurrence group.
Comparisons of patients without recurrence and those whose disease recurred demonstrated no significant differences in the mean values of age (59.0 years versus 60.8 years) and number of lymph node metastases(18.1 versus 20.4). We did observe significant differences in the mean values of number of dissected lymph nodes (24.2 versus 28.5, P = 0.04) and of numbers of patients with estrogen receptor (ER)-positive (14 versus 2, P = 0.001), progesterone receptor (PgR)-positive (12 versus one, P;=;0.001), HER2-positive (3 versus 6, P = 0.04), and triple-negative (TN) tumor subtypes (0 versus 5, P = 0.002).
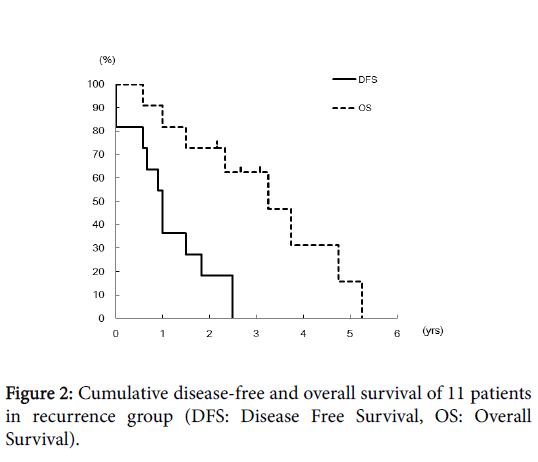
The mean disease-free survival in the group with recurrence, 13.6 months, was significantly shorter than that in the recurrence-free group, 62.2 months (P < 0.000). In the recurrence group, the cumulative disease-free survival was 36.4% at one year after surgery and 18.2% at 2 years; disease of all 11 recurred within 3 years of treatment. The cumulative overall survival was 81.8% at one year, 62.3% at 3 years, and 16.6% at 5 years; all 11 patients died within 6 years of treatment (Figure 2).
Discussions
The status of axillary lymph node metastases is one of the most important prognostic factors in patients with breast cancer [1-6]. When breast surgeons developed the method of modified radical mastectomy, prognoses were discussed according to the number and sites of axillary lymph node metastases [9].
Many studies [1-6] have confirmed the early findings of the National Surgical Adjuvant Breast Project (NSABP) trial [10] that first correlated disease-free and overall survival with the number of axillary lymph node metastases. In general, when the number of positive lymph nodes exceeds 10, 5-year disease-free survival is 20 to 30%, and the prognosis is very poor [4]. Nevertheless, some of these patients live long term without recurrence, and that observation led us to conduct this study.
Seven of our 29 patients (24%) had invasive micropapillary carcinoma, which would generally lead to extended lymph node metastases and follow an aggressive clinical course. This was a higher frequency than that reported by others (about 2%) [11-13].
Despite our small number of cases (n=29), the 3-year and subsequent cumulative disease-free survival rates plateaued at 58.8%, and the 6-year and subsequent cumulative survival rates plateaued at 60.8%. Thus, most patients whose disease recurred within 3 years of surgery died within 5 to 6 years of surgery.
Of our 28 study patients, 17 had no disease recurrence by 10 years after surgery, but only 18.2% of the other 11 were disease-free at 2 years after treatment, and all had developed recurrent disease by 3 years after treatment. Their cumulative overall survival was 15.6% at 5 years after treatment, and all had died by 6 years. The 2 groups did not differ significantly in age, number of lymph node metastases, histopathological type, or postoperative treatment. However, the expression of ER, PgR, and HER2 did differ significantly between the 2 groups.
In recent years, gene analyses using microarray techniques have permitted further classification of breast cancer into such subtypes as luminal A, luminal B, HER2-enriched, basal-like, and claudin-low [14]. From this perspective, the luminal A type was more common in our group without recurrence, the HER2-enriched subtype was more common in the group whose disease recurred, and all 5 patients with TN breast cancer were in the recurrence group. The triple-negative subtype constitutes only 10 to 15% of breast cancers [15] but made up 45% of the cancers in our group with recurrent disease, an exceptionally high number.
We broadly divided patients with breast cancer with more than 10 axillary lymph node metastases into 2 groups according to the biological properties of their tumors and observed that patients whose disease recurred within 3 years of surgery were those who died within the next few years. However, without disease recurrence within 3 years of treatment, long-term recurrence-free survival can be expected. Taking the intrinsic subtypes of their cancers into account, 100% of those whose disease recurred had TN type, and 100% of patients without recurrent disease had tumors of luminal A type. The high percentages of each of these subtypes specific to each group suggest their use as prognostic factors.
Conclusions
Prognosis was very poor in patients who underwent surgery for breast cancer with involvement of more than 10 axillary lymph nodes when cancer recurred within 3 years of treatment, but patients who remained cancer-free at 3 years after treatment could expect a long-term prognosis. Furthermore, preponderance of tumors of TN subtypes in the group who developed recurrent disease and of luminal A subtype in those who remained free of disease suggest the use of these subtypes as prognostic factors.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, et al. (1983) Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52: 1551-1557.
- Fisher ER (1984) The impact of pathology on the biologic, diagnostic, prognostic, and therapeutic considerations in breast cancer.SurgClin North Am 64: 1073-1093.
- Clark GM, McGuire WL (1988) Steroid receptors and other prognostic factors in primary breast cancer. SeminOncol 15: 20-25.
- Sunderland MC, McGuire WL (1990) Prognostic indicators in invasive breast cancer. SurgClin North Am 70: 989-1004.
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, et al. (2000) Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124: 966-978.
- Corben AD (2013) Pathology of invasive breast disease. SurgClin North Am 93: 363-392.
- National Comprehensive Cancer Network Breast Cancer Panel. Breast cancer version 3.
- Umemura S, Kurosumi M, Moriya T, Oyama T, Arihiro K, et al (2006) Immunohistochemical evaluation for hormone receptors in breast cancer: a practically useful evaluation system and handling protocol. Breast Cancer 13: 232-235.
- Auchincloss H (1963) Significance of location and number of axillary metastases in carcinoma of the breast. Ann Surg 158: 37-46.
- Fisher B, Ravdin RG, Ausman RK, Slack NH, Moore GE, et al. (1968) Surgical adjuvant chemotherapy in cancer of the breast: results of a decade of cooperative investigation. Ann Surg 168: 337-356.
- Siriaunkgul S, Tavassoli FA (1993) Invasive micropapillary carcinoma of the breast. Mod Pathol 6: 660-662.
- Luna-Moré S, Gonzalez B, Acedo C, Rodrigo I, Luna C (1994) Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 190: 668-674.
- Umeda T, Ishida M, Murata S, Mori T, Kawai Y, et al. (2015)Immunohistochemical analyses of CD44 variant isoforms in invasive micropapillary carcinoma of the breast: comparison with a concurrent conventional invasive carcinoma of no special type component. Breast Cancer.
- Prat A, Perou CM (2011) Deconstructing the molecular portraits of breast cancer. MolOncol 5: 5-23.
- Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S (2011) Triple negative breast cancer - prognostic factors and survival.RadiolOncol 45: 46-52.
Citation: Kinoshita S, Fukushima N, Miyake R, Ishigaki T, Hirano A, et al. (2016) Clinicopathological Assessment of Patients with Locally Advanced Breast Cancer with 10 or More Lymph Node Metastases. Breast Can Curr Res 1:107. DOI: 10.4172/2572-4118.1000107
Copyright: © 2016 Kinoshita S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12370
- [From(publication date): 6-2016 - Jul 13, 2025]
- Breakdown by view type
- HTML page views: 11417
- PDF downloads: 953