Cognitive Training Using Percussion Instruments for Persons with Mild Cognitive Impairment: A Feasibility Study
Received: 05-Jan-2019 / Accepted Date: 21-Jan-2019 / Published Date: 24-Jan-2019
Abstract
Research question: Can a cognitive training method using musical percussion instruments improve attention, working- and episodic memory in persons with amnestic mild cognitive impairment (a-MCI)? Background: The positive effect of cognitive training for persons with dementia has been demonstrated in several studies. Music, in contrast, has largely failed to improve cognition in persons with dementia, but has shown a positive impact on improving emotional, social and behavioural aspects. The combination of music and cognitive training could therefore be interesting. However, evidence for combining music and cognitive training is missing. Aim- To test the feasibility of a cognitive training intervention, using percussion instruments to improve attention, working- and episodic memory function in persons with a-MCI. Methods: A feasibility study lasting 6 weeks, with sessions twice per week, for 60 minutes. Study included persons (n=3) clinically diagnosed with a-MCI. The Brief Cognition Rating Scale was administered as baseline screening. 11 exercises were used for the training sessions. Outcome measures: The Kokmen Short Test of Mental Status (STMS), The Quality of Life – AD questionnaire (QOL-AD) and the Non-Pharmacological Therapy Experience Scale (NPT-ES) Results: STMS scores improved for all participants when measured after 6 weeks of intervention and slightly fell when re-measured at one month after the final intervention at follow up. The QOL-AD measure improved for two participants from baseline to 6 weeks, but declined at follow up. NPT-ES scores remained high throughout the entire study period. The STMS was not sensitive enough to detect and assess working memory deficits or measure improvements. Conclusion: Cognitive training with percussion instruments showed efficacy in improving attention (attention span and immediate recall) as well as episodic memory (delayed recall) in persons with a-MCI. This training method should be tested for efficacy in a randomized controlled trial.
Keywords: Dementia; Mild cognitive impairment; Attention; Memory; Cognitive training; Music
Introduction
Dementia is a serious public health problem and challenge of our time, with disease prevalence growing exponentially as societies around the world are rapidly growing older. According to the World Health Organization (WHO), 58% of people with dementia are currently living in low to middle income countries; this number is expected to grow to 71% by 2050. Moreover, there are currently 35.6 million people suffering from dementia world-wide and this will grow to 65.7 million by 2030 and to 115.4 million by 2050 [1]. Alzheimer’s disease (AD) is the leading form of dementia at 60%, with Mild Cognitive Impairment (MCI) representing a transitional state between healthy aging and AD [2]. Furthermore, MCI can be subdivided into amnestic and nonamnestic MCI, with 60-85% of persons with amnestic MCI (a-MCI) converting to AD [3,4] with a conversion rate of approximately 10-15% per year [5]. The core cognitive deficit in a-MCI is memory and it has been reported that from the various deficits found in MCI, attentional processing, working memory (WM) and episodic memory (EM) are the most prevalent [2,6-10].
Pharmacological therapies, which have dominated the scope of dementia treatment, have shown to be of limited value and far less research has been aimed at non-pharmacological therapies to treat deterioration of cognitive functioning [11]. A systematic review [12] concluded that cognitive training (CT) is promising for enhancement of cognitive function in MCI and may slow decline in at-risk individuals. All interventions included in the review, except for one are computerbased the remaining intervention employs pen and paper cognitive drills. Certain aspects are discussed regarding cognitive training’s transferability to real world activities of daily living (ADL) and the unclear effect on quality of life (QoL), since none of the ten included studies in the review included QoL measures. Another systematic review [13] evaluating the efficacy of non-pharmacological therapies for AD found evidence for the improvement of cognition in favour of CT and Cognitive Stimulation Therapy (CST), which were also mainly computer-based programs, but also included reminiscence, and reality orientation. CST has been described as labour-intensive and in need of further evaluation regarding its cost-effectiveness [11]. From the 10 included CT interventions in the review, only one measured QoL, and in this study, the improvement of cognition did not generalize to QoL.
The use of music for persons with dementia is a non-invasive and cost-effective intervention [14,15] and its positive effect on QoL has been demonstrated [15,16]. However, in contrast to CST and CT interventions, it has failed to demonstrate efficacy in improving cognition in dementia, showing to have greater effects on behavioural, emotional and social domains [17-22].
That the use of music to improve cognition in dementia has not shown efficacy, in contrast to cognitive training interventions, leads to the question if there is a difference in how music is incorporated i.e. can music lead to improvements in cognitive parameters and still maintain its efficacy for qualitative measures if used in a structured, systematic and stimulating form to target particular deficits in a specific sample? This particular question remains unanswered, as music in dementia care has not yet been constructed and tailored to target specific cognitive deficits in, for example, the MCI population. The implication of a positive answer to this question may be significant, as cognitive training can potentially extend the realm of music into a serious cognitive training regimen.
The aim of this study is to test the feasibility of a cognitive training intervention, using percussion instruments to improve attention, working- and episodic memory function in persons with a-MCI. The study will also address the question if this form of cognitive training can also lead positive changes on qualitative measurements, such as Quality of Life.
Background
Some of the most prevalent deficits found in MCI involve workingand episodic memory systems; however, it is essentially attention which is a prerequisite for any form of memory encoding [2]. Thus, impaired attention will directly affect these memory systems as well as other neuropsychological functions. Attention, defined as the ability to effectively process stimuli, depends heavily on a properly functioning cholinergic system, as cholinergic modulation supports attentional processing and plays a defining role for the function of working- and episodic memory [23]. Research has demonstrated the particular role played by the nucleus basalis of Meynert (NB) as an essential component of the basal forebrain cholinergic system, innervating many sites in the neocortex as well as limbic cortices, such as the hippocampus and entorhinal cortex. When attentive and focused on a task, the NB is activated, leading to increased cholinergic activity [24-31] and this plays a crucial role in enabling plastic change [24,25,26-37].
According to a meta-analysis, the most successful forms of CT use restorative strategies, including engaging in creative activities [37]. Following this rationale, this study aims to integrate music in the form of percussion instruments with a creative cognitive training program to target the most impaired cognitive functions found in a-MCI, such as attention and memory. This intervention has the potential to compliment a cognitive training program for persons with MCI as a novel and “attractive” form of CT.
ICF codes
b144 (Memory functions), b160 (Thought functions), b164 (Higher-level cognitive functions), d160 (Focusing attention), d163 (Thinking).
Methods
Design
This is a feasibility study lasting a total of 6 weeks, with sessions twice per week, each for 60 minutes, with a follow-up evaluation at 6 weeks after the last intervention. Ethical approval was obtained prior to the start of the study. A “no objection” statement vgl. Art. 51 Abs.2 Human for schungsgesetz was received by the ethical commission Ethikkommission Nordwest- und Zentralschweiz (EKNZ) to conduct the study in Basel, Switzerland.
Study inclusion
Persons (N=3) clinically diagnosed with having a-MCI were recruited with assistance from the Alzheimer’s disease Organisation Alzheimervereinigung beider Basel in Switzerland. Specific criteria for the diagnosis of a-MCI, as defined by Petersen [38] were employed. Participants were excluded if they had any other form of dementia, including persons with non-amnestic MCI, other neuropsychiatric impairments interfering with attentional processing, or persons with depression. The Brief Cognition Rating Scale (BCRS) was administered to serve as a baseline screening evaluation. Demographic and baseline variables are described in Table 1. Using the corresponding global deterioration scale (GDS), developed for the assessment of primary degenerative dementia, the screening revealed that all participants’ scores correspond to the pre-stage of dementia, called mild cognitive decline, which is defined as stages 2-3, with stage 2 representing a subjective cognitive deficit and stage 3, MCI. Participant 2, who scored 3.4, is near the midpoint between pre-dementia and dementia.
| Name | Gender | Age | GDS* score | Education level (in years) |
|---|---|---|---|---|
| Participant 1 | M | 72 | 2,6 | 13 |
| Participant 3 | M | 75 | 3 | 18 |
| Participant 2 | M | 81 | 3,4 | 15 |
Table 1: Demographic and baseline variables. *GDS indicates Global Deterioration Scale scores for participants 1, 2 and 3
Intervention
To guarantee the standardization of the exercises, a detailed exercise protocol, including a thorough description of the exercise format, materials, all exercises and difficulty adjustment is included in the appendix.
The room used for the study period was private, comfortable and well lit, with plenty of room to move around. Important was the limitation of any surrounding distractions, such as open windows, which could have allowed nearby traffic noise to interfere with the participant’s attention. The therapist and all participants sat primarily in chairs facing each other to practice the exercises, but on occasion an exercise required participants to walk from one side of the room to the other. In addition, in order to choose or change an instrument, which happened often, participants were required to get out of their chair and walk to one of the surrounding tables to choose out an instrument of their liking for an exercise.
11 standardized exercises, described in detail in the study’s exercise protocol, were used for the exercise sessions. Some exercises focused on training mainly one cognitive function whereas several exercises trained several functions. As can be expected, there was a naturallyoccurring overlap with certain cognitive areas of focus. Table 2 shows the 6-week exercise schedule that was used for the study and provides an overview in which combination the exercises were given for each training session.
| EXERCISE PRACTICED * | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| session 1 | x | x | x | |||||||||
| session 2 | x | x | x | |||||||||
| session 3 | x | x | x | x | ||||||||
| session 4 | x | x | x | x | ||||||||
| session 5 | x | x | x | x | x | |||||||
| session 6 | x | x | x | x | ||||||||
| session 7 | x | x | x | x | x | |||||||
| session 8 | x | x | x | x | x | |||||||
| session 9 | x | x | x | x | x | |||||||
| session 10 | x | x | x | x | x | x | ||||||
| session 11 | x | x | x | x | x | x | ||||||
| session 12 | x | x | x | x | x | x |
Table 2: Training schedule. *Key exercises practiced, including names of the exercises: # 1: “Forwards and backwards”, # 2: “Spin the bottle”, # 3: “Copycat”, # 4: “Change it up”, # 5: “Copycat 2”, # 6: “Fast, soft, loud, stop”, # 7: “Pay attention”, # 8: “Rhythms,” # 9: “Double it,” # 10: “Only ears allowed,” # 11: “Beats”
Throughout each individual exercise session, special attention was placed on each participant’s performance in relation to several factors, such as: Do they give the impression that they understand the exercise? Are they performing as instructed? Do they seem to be attentive and challenged, or bored and disinterested? Do they seem overwhelmed? If a participant performed an exercise incorrectly, it was explained again slowly and clearly and illustrated with an example. In the case that a person seemed to be overly-challenged during an exercise, a break for relaxation was recommended, so as to reach a good level of arousal without sacrificing performance.
Feedback was of significant importance throughout the exercise sessions. After completing an exercise, positive feedback on the performance was given, and it was attempted to end each exercise round with the participant feeling good about their performance.
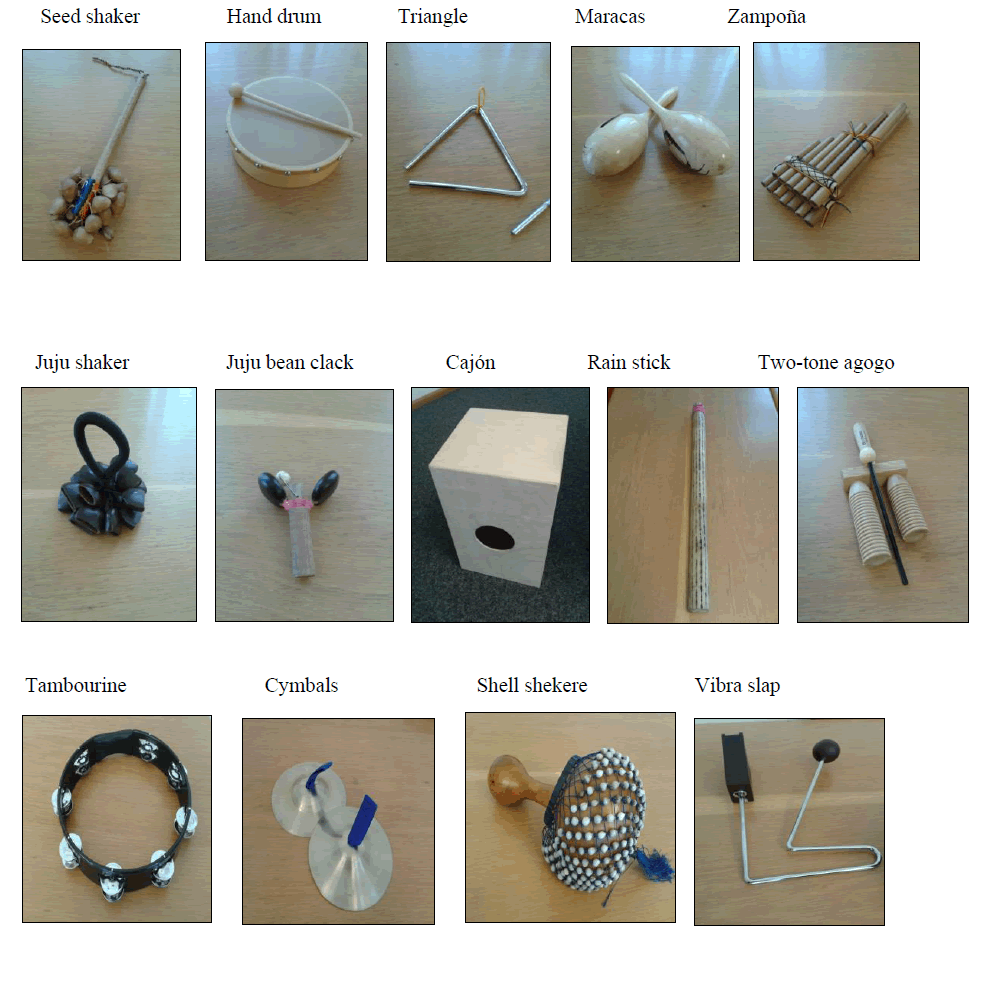
A wide variety of relatively simple percussion instruments to be chosen by the participants were used throughout each exercise session. An exact description of the percussion instruments used, including a picture is included in the appendix.
Assessment
The Kokmen Short Test of Mental Status (STMS) [39], with a score range between 0 and 38 was the primary measurement outcome for cognition and was carried out at baseline (prior to the first exercise session), on the 12th session (before the intervention) and one month after the 12th intervention, as a follow up assessment. The STMS was developed and validated as a screening bedside mental status test specifically for use in mild dementia. Moreover, the construction of the recall task in the STMS was intended to make it more sensitive to the problems of learning and recall in MCI, in that the delayed recall task is longer (approximately 3 minutes) than in the Mini Mental State Examination (MMSE) [40]. The Quality of Life – AD questionnaire (QOL-AD) [41] and the “Nicht Pharmakologische Therapie Erfahrungskala” (NPT-ES) [42] or non-pharmacological therapy experience scale served as the intervention’s secondary- and qualitative outcome measures. The QOL-AD questionnaire was given at baseline, post-intervention and at follow up. This measure also includes the exact same questions for a family member or caregiver to answer and is meant to increase the validity of the questionnaire. Thus, there are two questionnaire results. The NPT-ES was used to assess all participant experiences for each exercise session.
An independent, external clinician carried out testing throughout the entire study period, except for the scoring of the NPT-ES, which was graded by the therapist after each individual exercise session.
Analysis
The effectiveness of the applied training intervention was evaluated by statistically analysing post-training changes at 6 weeks. This was carried out with a two-tailed, paired samples student t-test. The effect size (Cohen’s d) was calculated using mean t (0) and t (1) and standard deviation (SD1 and SD2).
Results
Primary outcome measurement
Table 3a and b shows the baseline and 6-week STMS measurements for all participants. The t-test analysis resulted in a P value of 0.053, with a Cohen’s d effect size of 1.54.
| Participant 1 | Participant 2 | Participant 3 | |
|---|---|---|---|
| Baseline | 34 | 22 | 27 |
| 6 weeks | 38 | 32 | 35 |
| Follow up | 37 | 30 | 34 |
Table 3a: STMS scores.
| Mean t(0) | 27.67 |
| SD(1) | 6.03 |
| Mean t(1) | 35 |
| SD(2) | 3 |
| P | 0.053 |
| Cohen’s | 1.54 d |
Table 3b: STMS scores (t-test).
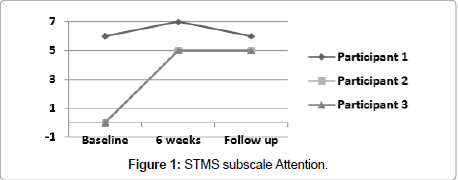
Figure 1 illustrates scores for subscale item Attention for participants 1, 2 and 3, which were measured at baseline, 6 weeks and follow up. Test score range is a minimum of 0 to a maximum of 7 points (actual attainable points are 0, 5, 6 or 7).
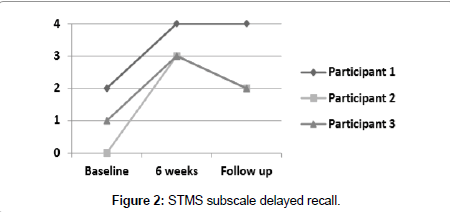
Figure 2 illustrates subscale item Delayed Recall for participants 1, 2 and 3, which were measured at baseline, 6 weeks and follow up. Test score range is a minimum of 0 to a maximum of 4 points.
Participant 1 showed an improvement at 6 weeks of 4 points from his initial score of 34. He improved one point on subscale item attention, 2 points on subscale item delayed recall, one point on subscale item immediate recall. At follow up his score of 37 was one point lower from his 6-week score because of his lower score on the attention span.
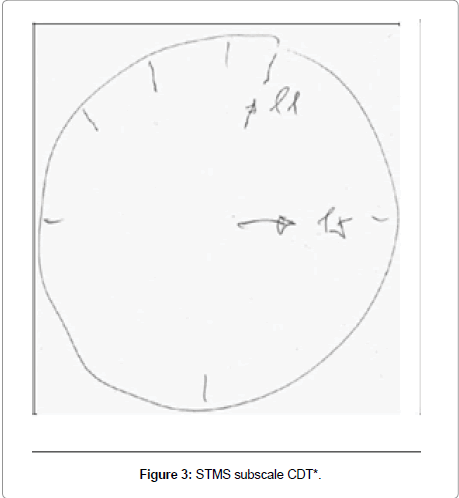
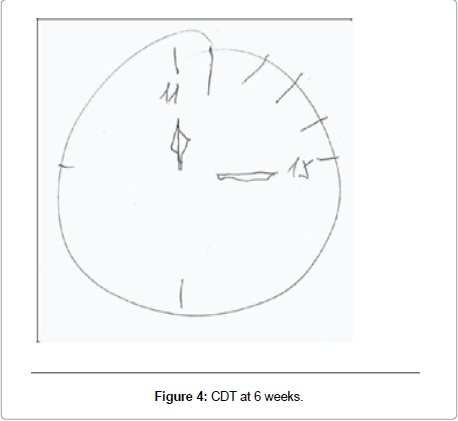
Participant 2 showed the most improvement of 10 points from 22 at baseline to 32 at 6 weeks, unable at baseline to score any points, neither for attention span nor for delayed recall; in addition, he lost one point on immediate recall, one point on information and one point on subscale item construction (Figure 3), which is tested with the Clock Drawing Test (CDT). At 6 weeks he improved 5 points on attention, 3 points on delayed recall, one point on immediate recall, and one point for construction (Figure 4). At follow up, his score fell 2 points from his 6-week score, losing one point on immediate and one point for delayed recall.
Participant 3 improved 8 points from 27 at baseline to 35 at 6 weeks. At baseline he did not score any points on attention and only one point on delayed recall, and he lost one point on subscale item orientation, and at 6 weeks scored 5 points on attention and 3 points on delayed recall and full points on orientation. His follow up score fell one point due to scoring only 2 points on the delayed recall task.
Immediate recall was not illustrated as a figure due to the simplicity of this measure. This subscale item improved for Participant 1 and Participant 2 from requiring two tries at baseline to remember four words on the word list, to being able to remember the same words on the first try at the 6-week measurement.
Secondary outcome measurements
Table 4a-d shows the baseline and 6-week QoL-AD measurements for all participants. The t-test resulted in a P value of 0.31, with a Cohen’s d effect size of 0.61. The QoL-AD measure for caregivers resulted in a P value of 0.199 and a Cohen’s d effect size of 0.29. A raw improvement was recorded for all participants, with the exception of Participant 1, whose baseline score of 42 remained the same for the 6 week and follow up measurement.
| Baseline | 6 Weeks | Follow up | |
| Participant 1 | 42 | 42 | 42 |
| Participant 2 | 33 | 35 | 33 |
| Participant 3 | 27 | 36 | 34 |
Table 4a: QOL-AD scores.
| t(0) | 34 |
| SD(1) | 7.55 |
| t(1) | 37.67 |
| SD(2) | 3.79 |
| P | 0.311 |
| Cohen’s d | 0.61 |
Table 4b: QOL-AD scores (t-test).
| (QoL-AD CG)* | Baseline | 6 Weeks | Follow up |
|---|---|---|---|
| Participant 1 | 41 | 41 | 41 |
| Participant 2 | 29 | 31 | 30 |
| Participant 3 | 32 | 35 | 35 |
Table 4c: QOL-AD scores.
| t(0) | 34 |
| SD(1) | 6.24 |
| t(1) | 35.67 |
| SD(2) | 5.03 |
| P | 0.199 |
| Cohen’s d | 0.29 |
Table 4d: QOL-AD scores (t-test).
Participant 2 increased 2 points from 33 at baseline to 35 at the 6-week measurement, grading his energy level at baseline as poor, then good at 6 weeks. At follow up his score fell two points, due to the subscale item energy level falling back to poor.
Participant 3 had the most improvement from baseline to follow up, respectively from 27 to 36 points. At baseline, he graded items on physical health, energy level and mood as poor, and good at 6 weeks; and items self as whole and life as whole as fair at baseline and as good at 6 weeks. The item living situation improved from good to excellent and item financial situation from fair to good. The item ability to do things for fun fell from good to fair. At follow up his score fell two points, as items energy level and mood decreased to fair. Caregiver scores improved slightly except for Participant 1 (CG), whose score remained at 41 throughout the study period, including at follow up.
Participant 2 (CG) increased 2 points from 29 at baseline to 31 at 6 weeks. He gained one point for item mood and one point for self as a whole, from poor to fair, respectively. At follow up, item mood fell back to poor, but self as a whole remained at fair.
Participant 3 (CG) showed an improvement of 3 points from baseline to 6 weeks, and this remained at follow up. Item physical health went from poor to good and life as a whole increased from fair to good.
The NPT-ES scores (Table 5) were high throughout the study period. Participant 1 had an average score of 13.8, Participant 2: 12.4 and Participant 3: 14. The collective study average was 13.4 from a total of 15 possible points.
| Participant 1 | Participant 2 | Participant 3 | |
|---|---|---|---|
| Session 1 | 13 | 12 | 12 |
| Session 2 | 13 | 12 | 15 |
| Session 3 | 14 | 13 | 14 |
| Session 4 | 14 | 13 | 15 |
| Session 5 | 14 | 12 | 15 |
| Session 6 | 14 | 12 | 15 |
| Session 7 | 14 | 13 | 12 |
| Session 8 | 14 | 12 | 13 |
| Session 9 | 14 | 13 | 14 |
| Session 10 | 14 | 12 | 15 |
| Session 11 | 14 | 12 | 14 |
| Session 12 | 14 | 13 | 14 |
Table 5: NPT-ES scores.
Discussion
Although the calculated effect sizes for several of the study’s outcomes showed small to large changes at the 6-week measurement, none reached statistical significance. This was to be expected as the sample size was very small, which limits generalizability and highlights the study’s main limitation. However, being that the purpose of this study was to determine if this intervention is feasible, it does provide insight regarding practical, clinical significance and a potentially new future direction to test it on a larger randomized sample.
The STMS was unable to show neither deficits nor improvements in WM, as all of the participants earned a full score on the calculation task when measured at baseline. However, this “serial 7” calculation has been reported to be biased, as persons with higher education do better on this item [43]. Since all three participants have relatively high levels of education, this could have been a factor. Furthermore, studies have pointed out that this bias has considerably large implications, as it may result in a false positive diagnosis in less educated subjects; conversely, having high education may mask MCI and result in a false negative diagnosis [44]. With the absence of additional testing e.g. more rigorous WM and EM assessment, the participants’ perfect scores on the calculation task would be an example where such a false negative diagnosis result could occur. As can be noted from Table 1, this would be a serious mistake, because all of the study’s participants fall within the pre-stage of dementia, based on the GDS.
A possible solution to increase the sensitivity could be for future studies testing persons with MCI and wishing to use the STMS (or MMSE, which has the same serial 7 task, as in the STMS) to add an additional working memory assessment tool. The Wechsler’s Working Memory Index (WMI) might be a good test to compliment the STMS in that it has digit span (forward and backward) and letter-number sequencing; the arithmetic subtest for the WMI is supplemental, which greatly diminishes the influence of mathematical skills [45].
STMS subscale items attention, based on attention span, and immediate recall, were parameters of attention and short-term memory that demonstrated a positive change from baseline to the 6-week measurement. This is not surprising when considering that the majority of the study’s exercises intended to target participant’s attentional systems. All of the study’s 11 exercises required from the participants a considerable “investment” of attention to task for practically 60 minutes per session.
The improvement on the subscale item attention from baseline to 6 weeks remained up to the follow up evaluation. The delayed recall improvement, however, did not remain as high at follow up. This could imply that the parameter attention is more resistant to decaying over time than measures of delayed recall, which could reflect a principle of specificity and the intensity of time spent training the measure of attention over the period of 6 weeks.
It has been reported that the test requirements to draw a clock and set the hands to a pre-specified time, demands persons’ visuospatial- constructive skills, auditory comprehension, verbal and visual memory, motor programming, numerical knowledge, spatial attention, concentration, frustration tolerance, and executive functioning including organization, planning, abstract reasoning, and parallel processing [46]. Several study exercises, such as exercise #9, focused especially on spatial attention, or directing and maintaining attention to the exercise and the sounds and visual cues. It must be considered, however, that there are several factors, which could have influenced the initial poor performance on the CDT, such as mood [47].
This study shows that although the use of CT can be used to positively influence cognition, the qualitative aspects for the use of music can also be achieved. These positive changes were demonstrated through the QOL-AD questionnaire (subject and caregiver values). This result is meaningful because CT interventions’ effect on QoL largely remains unknown. Through the NPT-ES scores it was evident that this creative form of cognitive training was enjoyable, which makes it perhaps potentially more attractive than for example, pen and paper drills.
Interestingly, despite improvements in the cognitive parameters of the STMS examination, the item memory of the QOL-AD questionnaire remained the same for all participants i.e. there was no subjective improvement of memory. The explanation for this can be attributed to evaluation of memory as a whole and more importantly, in relation to activities of daily living. This raises the very important question how transferable the present study’s intervention is to daily living. All of the study’s exercises targeted the most prevalent cognitive deficits encountered in amnestic MCI, however, the larger context in which these deficits are manifested throughout the day was not simulated, which reflects a potentially considerable limitation of the intervention regarding its transferability to real world activities of daily living.
Conclusion
Cognitive training with musical percussion instruments showed efficacy in improving attention (attention span and immediate recall) as well as episodic memory (delayed recall) in persons with a-MCI.
This training method should now be tested for efficacy in a randomized controlled trial.
The study intervention can be considered a form of secondary dementia prevention, meant to slow the progression of a pre-clinical disease to a clinical disease, or the conversion from MCI to AD [48]. For classification purposes, it can be further considered an appropriate form of restorative and repetitive cognitive training, in which the goal is structured practice of mental activity to enhance cognitive function. Through the integration of music to the field of cognitive training for persons with pre-clinical to clinical dementia, impaired cognitive measures found in this disease continuum may be effectively addressed in a creative and enjoyable way.
References
- Yanhong O, Chandra M, Venkatesh (2013) Mild cognitive impairment in adult: A neuropsychological review. Ann Indian Acad Neurol 16: 310-318.
- Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B (2006) Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord 22: 312-9.
- Maioli F, Coveri M, Pagni P, Chiandetti C, Marchetti C, et al. (2007) Conversion of mild cognitive impairment to dementia in elderly subjects: A preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr 44: 233-41.
- Petersen R, Thomas R, Grundman M, Bennett D, Doody, R et al. (2005) Alzheimer's Disease Cooperative Study Group N Engl J Med 352: 2379-88.
- Belleville S, Chertkow H, Gauthier S (2007) Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychol 21: 458-69.
- Irish M, Lawlor B, Coen R, O'Mara SM (2011) Everyday episodic memory in amnestic mild cognitive impairment: A preliminary investigation. BMC Neurosci 12: 80.
- Saunders N, Summers M (2010) Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol 32: 350-7.
- Baddeley A, Bressi S, Della Sala S, Logie R, Spinnler H (1991) The decline of working memory in Alzheimer's disease. A longitudinal study. Brain 114: 2521-42.
- Belleville S, Peretz I, Malenfant D (1996) Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychology 34: 195-207.
- Ballard C, Khan Z, Clack H, Corbett A (2011) Nonpharmacological Treatment of Alzheimer Disease. Can J Psychiatry 56: 589-595.
- Gates N, Sachdev P, Fiatarone S, Valenzuela, M (2011) Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr 11: 55.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, et al. (2010) Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 30: 161-78.
- Vasionyte I, Madison G (2013) Musical intervention for patients with dementia: A meta-analysis. J Clin Nurs 22: 1203-1216.
- Witzke J, Rhone R, Backhaus D, Shaver N (2008) How sweet the sound: research evidence for the use of music in Alzheimer's dementia. J Gerontol Nurs 34: 45-52.
- Cooke M, Moyle W, Shum D, Harrison S, Murfield J (2010) A randomized controlled trial exploring the effect of music on quality of life and depression in older people with dementia. J Health Psychol 15: 765-776.
- Groene R (1993) Effectiveness of music therapy 1:1 intervention with individuals having senile dementia of the alzheimer’s type. J Music Ther 30: 138-157.
- Lord T, Garner J (1993) Effects of music on Alzheimer patients. Perceptual and Motor Skills 76: 451-5.
- Brotons M, Koger S (2000) The impact of music therapy on language functioning in dementia. J Music Therapy 37: 183-95.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, et al. (2008) The efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord 22: 158-162.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio M, et al. (2010) Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial. Aging Ment Health 14: 900-904.
- Guétin S, Portet F, Picot M, Pommie C, Messaoudi M, et al. (2009) Effect of music therapy on anxiety and depression in patients with Alzheimer’s type dementia: Randomised, controlled study. Dement Geriatr Cogn Disord 28: 36-46.
- Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME (2012) Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci 6: 24.
- Gluck M, Mercado E, Myers C (2008) Learning and memory: From brain to behavior. Worth New York.
- Wright B, Sabin A, Zhang Y, Marrone N, Fitzgerald M (2010) Enhancing perceptual learning by combining practice with periods of additional sensory stimulation. J Neurosci 30: 12868-12877.
- Kametani H, Kawamura H (1990) Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci 47: 421-426.
- Lee MG, Hassani OK, Alonso A, Jones BE (2005) Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci 25: 4365-4369.
- Hasselmo ME, McGaughy J (2004) High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res 145: 207-231.
- Keuroghlian AS, Knudsen E (2007) Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol 82: 109-121.
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, et al. (2012) Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA. 109: E2832-2841.
- Stefan K, Wycislo M, Classen J (2004) Modulation of associative human motor cortical plasticity by attention. J Neurophysiol 92: 66-72.
- Recanzone G, Schreiner C, Merzenich M (1993) Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13: 87-103.
- Xerri C, Merzenich M, Jenkins W, Santucci S (1999) Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex 9: 264-276.
- Keuroghlian AS, Knudsen E (2007) Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol 82: 109-121.
- Sur M, Nagakura I, Chen N, Sugihara H (2013) Mechanisms of plasticity in the developing and adult visual cortex. Prog Brain Res 207: 243-254.
- Sitzer DI, Twamley EW, Jeste DV (2006) Cognitive training in Alzheimer's disease: a meta-analysis of the literature. Acta Psychiatr Scand 114: 75-90.
- Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183-194.
- Kokmen E, Naessens JM, Offord KP (1987) A short test of mental status: description and preliminary results. Mayo Clin Proc 62: 281-288.
- Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC (1991) The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 48: 725-728.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, et al. (2003) Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer's Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 17: 201-8.
- Muñiz R, Olazarán J, Poveda S, Lago P, Peña-Casanova J (2011) NPT-ES: A Measure of the Experience of People with Dementia during Non-pharmacological Interventions. Nonpharmacol Ther Dement 1: 239-250.
- Teresi JA, Golden RR, Cross P, Gurland B, Kleinman M (1995) Item bias in cognitive screening measures: comparisons of elderly white, Afro-American, Hispanic and high and low education subgroups. J Clin Epidemiol 48: 473-483.
- Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the Mini-Mental State Examination by age and educational level. J Am Medical Association 269: 2386-91.
- Flanagan DP, Kaufman AS (2009) Essentials of WISC-IV assessment, 2nd Edition.
- Ueda H, Kitabayashi Y, Narumoto J, Nakamura K, Kita H, et al. (2002) Relationship between clock drawing test performance and regional cerebral blood flow in Alzheimer's disease: a single photon emission computed tomography study. Psychiatry Clin Neurosci 56: 25-29.
- Ainslie NK, Murden RA (1993) Effect on education on the clock drawing dementia screen in non-demented elderly persons. J Am Geriatr Soc 41: 249-252.
- Thal LJ (2006) Prevention of Alzheimer disease. Alzheimer Dis Assoc Disord 20: S97-S99.
Appendix
Exercise protocol
The exercise manual is intended to provide an accurate description of how the intervention was carried out, including the recommended setting, format for the exercise sessions, required materials and percussion instruments. For each exercise, detailed instruction is provided as to what the therapist is required to do, how the participants can be engaged for the exercises and how the individual exercise difficulty can be adjusted.
Setting
The room should be private and comfortable, with plenty of room to move around. Limit any surrounding distractions, such as open windows, which could allow nearby traffic noise to interfere with the participant’s attention.
Furniture
Use comfortable chairs for the therapist and participants to sit on. Set up at least two tables around the room on which to place the instruments.
Instruments
The instruments used for this study (pictures provided below) were purchased from online music stores and so-called “world culture stores”, which offer a large variety of hand-made products including percussions and instruments from Asia, South America and Africa. Between five and 15 Euros were spent on each instrument used for the study, with exception of the cajón (40 euros) and the djembe (30 euros).
Additional material
Standard or digital metronome, bottle, paper to write on, pen or pencil, stopwatch
How to begin
Once the therapist and all participants have seated themselves, the therapist will make sure that everyone is comfortable and that the room conditions are appropriate to begin. It is recommended to provide refreshments, as sustained focus and concentration may be tiring. Introduce all available instruments and have each participant observe, feel and play the instruments for familiarization.
Some of the exercises have been listed as good introductory exercise; an exercise session may begin with one of these. Introduce the exercise, explain clearly and slowly and illustrate with an example. A “practice round” for better understanding is highly recommended prior to the exercise.
Therapists should pay special attention to participant performance. Do they give the impression that they understand the exercise? Are they performing as instructed? Do they seem to be attentive and challenged, or bored and disinterested? Do they seem overwhelmed? If a participant performs an exercise incorrectly, explain again slowly and clearly and illustrate once again with an example. In the case that a person seems to be overly-challenged during an exercise, recommend a break for relaxation.
After each exercise, a break for several minutes is recommended before a new exercise is introduced and practiced.
Feedback
Feedback is important, however allow time for individual reflection after each exercise. After completing an exercise, give positive feedback on the performance. For example, look into ways how someone used an instrument in a creative, perhaps non-conventional way and yet still maintained the exercise structure. Attempt to end each exercise round with the participant feeling good about their performance.
Exercises
1. "Forwards and backwards"
The therapist begins the exercise by playing their instrument once loud and clear, and subsequently counting out loud "one". The person sitting next to the therapist then plays their chosen instrument and will count out loud "one", then play their instrument a second time, followed by counting out loud "two". The third person will play their instrument three times, counting “three” and so forth. When the last person is finished playing and counting, the exercise continues backwards until the therapist plays and says “1”.
More difficult: Playing in two’s: The exercise round can begin with the first person saying “two”, then playing their instrument twice, their neighbor saying “four”, and playing four times and so on. Playing in three’s raises the difficulty level more.
2. "Spin the bottle"
A bottle is placed on the floor between all participants and is spun by the therapist or a participant. The bottle mouth and base will choose the two participants who will play their instruments once the bottle has rested. The two “chosen” participants will decide spontaneously who will "lead" the session. The participant that leads the session begins by setting a rhythm of their choosing and the second participant joins in with their instrument as they desire. Playing sessions will last 30 seconds. Good introductory exercise
3. "Copycat"
A participant begins the session by playing a rhythm of their choosing for several seconds (max three seconds) The participant may choose to play soft, hard, fast, slow, or as complex as they desire. Their neighbor will try to play the same exact rhythm on their instrument; they are, thus, the “copycat.” The exercise can be done either clockwise or counterclockwise. There is no grading of any sort, however, participants are told to try their best to copy the sounds they hear. When the couple has completed their set, it’s the copycat's turn to play a new rhythm for their own neighbor (a different participant). When the last person is reached, the round works backwards until the first person plays once again.
More difficult: The exercise can be made more difficult by extending the playing time to, for example, 4 or 5 seconds or/and by a person choosing another person (a copycat) according to, for example, their indicated color placed in front of their seats, instead of working clockwise or counter-clockwise.
4. “Change it up”
Participants choose two instruments. The instrument is played once and the number “one” is said, then the second instrument is played once and the number “two” is said. The neighbor then plays their instrument and says “three”, then plays their other instrument and says “four” and so forth until the last participant is reached, then they work backwards with the instruments until reaching “one”
More difficult: Have the different instruments further apart from each other (for example one on a chair, the other on the other side of the room) so that the person must remember the numbers for a longer period of time between playing their instruments.
5. “Copycat” 2
The therapist begins the exercise by playing a simple rhythm for three seconds, slowly walking simultaneously to another part of the room (approximately three meters away). Then the therapist plays a DIFFERENT simple rhythm for three seconds as they walk back to the starting point. The “copycat,” must try to remember and reproduce the first and the second sounds that were played in the exact order while walking the three meters forwards and walking back.
More difficult: If the two sounds are mastered, then the participants can be told to additionally imitate or copy the pattern of walking or, for example, if a person squats once during the first walk then this can be imitated. Also, a different instrument can be used for the walk back. Increasing the level of difficulty beyond this can be done by including a third or fourth round.
6. "Fast, loud, soft, stop"
The therapist demonstrates that certain movements of the therapist’s body pertain to styles of playing: loud, soft, fast, or stop. When the therapist’s arms are abducted and rise with the palms facing upwards, this indicates LOUD. When the hands are turned with the palms facing downward and the arms are slowly moving towards the ground from the abducted position, this means “play softer”. A rhythmic tapping movement of the right foot indicates “play faster.” When both feet are raised, with the heels remaining on the floor, this means “STOP playing”. To begin the round, the therapist plays a basic rhythm and all participants play the same rhythm at approximately the same rate (ca. 90 BPM). The therapist challenges the participants by choosing different extremity movements and at times may combine the movements. For example, simultaneously raising the arms upwards and tapping the right foot, indicating “Loud” and “Fast”
7. “Pay attention”
A metronome is played, indicating that participants play their instruments in tact with the metronome (90 BPM). The metronome is hidden from the participants to remove any visual cue. In the case that a metronome is not available, the therapist can provide metronome sounds through different instruments. As soon as the metronome ceases, participants must also stop playing. Attention to the metronome sound is essential.
More difficult: Difficulty is controlled by having a metronome playing loud at the beginning and then getting quieter and quieter. This is done by lowering the sound of the metronome or placing a cloth over it. Whoever continues to play when the metronome is off is “out” of the round until the new round begins. This is played until there is only one person left. As mentioned above, the metronome can be played through instruments by the therapist. An example of the difficulty set is the therapist initially using the triangle (a loud and clear sound), then the small drum (less obvious than the triangle), then the shekere, then the “frog”, then tapping the tambourine, then scraping the top of an instrument softly. The participants are not allowed to see the therapist playing, thus, the therapist will turn their back to the participants to remove the visual cue.
8. “Rhythms”
- Heart beat, Woodpecker, Old clock, Knock on the door, Footsteps, Storm, Car driving by, Sewing machine, Beach waves, Hammering a long nail The participant will try to use a variety of instruments to create the sounds from the list above (10 all together), while the other participants will also have a copy of the list and will try to guess which sound is being played. After the 10 sounds are played, the other participants try the same and the other participants guess the sounds.
9. “Double it”
Every beat that’s played has to be doubled by the challenger. If four beats are played, for example, on the djembe, then eight sounds are played, for example on the frog. The therapist may initially provide the beats to be doubled.
More difficult: Increase the number of beats to be doubled and/or use multiple instruments. For example playing two beats on the tambourine, three movements of the rainstick and one ring on the triangle, equaling 6 sounds, which require 12 sounds to be played back by the challenger.
10. “Only ears allowed”
A participant turns their back to the instruments and other participants. Two instruments are set up at the end of the room on the other side. The therapist plays each instrument loud and clear for two seconds. The participant with his back turned away from the instruments must remember which instruments were played, the order in which they were played and replay the correct instruments and in the exact order.
More difficult: Increase the numbers of instruments played. For example, instead of two, use three or four and/or play the instruments for a longer period of time.
11. “Beats”
The therapist begins a “one beat rhythm” of approximately 90 beats per minute (BPM). All
participants play their percussion instrument with the same rhythm; this rhythm is maintained for a minute or two, with the goal that all instruments synchronise with each other. Following this, a “two beat rhythm” is played and this is repeated for several minutes. The same is repeated for a “three beat rhythm”.
Good introductory exercise
Instruments

Citation: Blum CG (2019) Cognitive Training Using Percussion Instruments for Persons with Mild Cognitive Impairment: A Feasibility Study. J Dement 2: 108.
Copyright: © 2019 Blum CG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3930
- [From(publication date): 0-2018 - Jul 05, 2025]
- Breakdown by view type
- HTML page views: 3090
- PDF downloads: 840