Review Article Open Access
Combined Effect of Platelets and Anidulafungin against Aspergillus fumigatus Infections
Schiefermeier-Mach N1, Bellmann R2, Frealle E3 and Perkhofer S4*
1Educational Center West for Allied Health Professions, Innsbruck, Austria
2Clinical Pharmacokinetics Unit, Division of Intensive Care and Emergency Medicine, Department of Internal Medicine I, Medical University of Innsbruck, Austria
3Laboratoire de Parasitologie-Mycologie, Centre Hospitalier Universitaire de Lille, Université de Lille, Lille, France
4University of Applied Sciences Tyrol, Austria
- *Corresponding Author:
- Susanne Perkhofer
Head of Research, University of Applied Sciences Tyrol
Innrain 98, 6020 Innsbruck, Tyrol, Austria
Tel: +43 512 5322-76725
E-mail: susanne.perkhofer@fhg-tirol.ac.at
Received date: August 11, 2017; Accepted date: September 18, 2017; Published date: September 22, 2017
Citation: Schiefermeier-Mach N, Bellmann R, Frealle E, Perkhofer S (2017) Combined Effect of Platelets and Anidulafungin against Aspergillus fumigatus Infections. J Infect Dis Ther 5:334. doi: 10.4172/2332-0877.1000334
Copyright: © 2017 Schiefermeier-Mach, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Invasive aspergillosis (IA) is one of the major causes of morbidity and mortality in severely immunocompromised patients. Despite the existence of antifungal treatment IA mortality rate remains extremely high and may reach up to 80%. Previous studies have suggested important role of platelets in antifungal host defence. In vitro data show that platelets are able to attenuate germination, hyphal elongation and viability of Aspergillus fumigatus. Interaction of platelets with Aspergillus fumigatus induces differential expressions of fungal genes associated with stress response regulation, cellular transport and metabolism.
A new class of antifungals, echinocandins (caspofungin, micafungin and anidulafungin), have entered the market. Anidulafungin displays strong antifungal activity against Candida and Aspergillus species and has very few side effects due to its specific inhibiting effect on the fungal cell wall synthesis. Anidulafungin is currently licensed for the treatment of adult patients with invasive candidiasis. Clinical significance of anidulafungin for IA treatment has to be further evaluated.
Recently published studies have shown that efficiency of antifungal substances can be enhanced when combined with platelets. In this review we discuss the literature on the potential combined effect of platelets and anidulafungin against Aspergillus fumigatus infections.
Keywords
Aspergillus; Platelets; Anidulafungin; Infection
Introduction
Invasive aspergillosis (IA) is one of the major causes of morbidity and mortality in severely immunocompromised patients such as cancer patients with chemotherapy-induced neutropenia, hematopoietic stem cell and transplant recipients [1,2]. The number of immunocompromised patients is constantly increasing due to improved survival of cancer patients, intensive cytotoxic therapy and higher number of organ transplantation recipients. These factors render the patients at risk of acquiring opportunistic fungal infections [3].
The frequency and relative importance of these infections are on the rise in all developed countries, which is possibly related to increased numbers of immunocompromised patients, owing to improved survival from AIDS, malignancies and more intensive cytotoxic therapy, more transplantation (with immunosuppression) for organ dysfunctions, and better therapy and prophylaxis for candidal infections.
Despite the existence of antifungal treatment IA mortality rate may reach up to 80% [4]. Reported reasons for the inefficiency of the antifungal medication include late IA diagnosis, developed drug resistance and infection of sites such as central nervous system that cannot be effectively treated with existing drugs [5]. IA has also strong impact on hospital costs. While aspergillosis is found in 0.03% off all US hospitalizations, the pharmacy costs account for 30% of total expenditures. Analyses of the hematopoietic stem cell transplant recipients sub-group displayed mean costs of $ 442 233 per patient per year [6].
A new class of antifungals, echinocandins (caspofungin, micafungin and anidulafungin), have entered the market. These compounds are associated with fewer side effects as compared to antifungal drugs of other classes due to their exclusive activity on the fungal cell wall, a structure which is absent in mammalian cells. Currently, caspofungin is the only echinocandin licensed for the second line therapy of IA [7].
Recent publications from our group and others have shown surprising interaction of platelets with Aspergillus that resulted in fungal cell damage and supressed fungal germination. Moreover, we have shown that platelets may enhance antifungal effect when combined with anidulafungin. The exact molecular mechanisms of platelet interaction with Aspergillus as well as the role of platelets in extended antifungal effect of anidulafungin remain obscure.
Pharmacokinetics and pharmacodynamics of Anidulafungin
Chemical structure and mechanism of action. Anidulafungin (Ecalta®, Pfizer Limited, Sandwich, Kent, UK) belongs to echinocandin class of antifungal drugs. Similar to other echinocandins, anidulafungin is a cyclic hexa-lipo-peptide; its N-aryl side chain comprises three phenyl groups. The common mechanism of action of echinocandins is a non-competitive inhibition of 1, 3-β-D-glucan synthase. 1, 3-β-D-glucan is a polysaccharide which is essential for the stability of the inner layer of the fungal cell wall [7–9]. Lack of the β-(1, 3)-D-glucan causes abnormal morphology of the fungal cell with thinning of the cellular wall, abnormal swelling and aberrant budding [10].
Pharmacodynamics and clinical use. Anidulafungin displays strong antifungal activity against Candida and Aspergillus species. It is not active against Cryptococcus neoformans, Fusarium species, and Zygomycetes. Quantification of the activity of anidulafungin against Aspergilli is based on its effect on fungal morphology which is expressed by the minimal effective concentration (MEC), in contrast to minimal inhibitory concentration (MIC).
Anidulafungin and other echinocandins display a pharmacodynamic particularity which is termed “paradoxical pharmacodynamic effect”. Exposure of a susceptible fungus to increasing drug concentrations results in growth inhibition when the sub-inhibitory concentration is exceeded. However, when the concentration is further increased, a decline of the antifungal activity takes place. Finally, at highest concentrations, fungal growth is inhibited again [11–13]. This phenomenon was observed at therapeutic concentrations in vitro and in vivo [13], although clinical relevance and underlying cellular mechanisms remain unclear. A variety of effects has been hypothesized to contribute, such as increased synthesis of cell wall chitin and altered activity of protein kinase C, and calcineurin [12]. The pharmacokinetic/pharmacodynamic indices correlating best with antifungal activity of anidulafungin are the ratio betweenpeak level and the MIC of pathogen (Cmax/MIC) as well as the ratio between the area under the concentration-time curve and the MIC (AUC/MIC) [14,15]. This is based on a relevant post-antifungal effect of echinocandins [16].
Anidulafungin is licensed for treatment of adult patients with invasive candidiasis. Together with other echinocandins, anidulafungin has been recommended for treatment of candidaemia by current guidelines [17–19]. High dose echinocandin treatment is mentioned as an alternative therapeutic option for Candida endocarditis, but not for the Candida infections of the central nervous system, because of the poor penetration into brain tissue [19]. Although anidulafungin is active against Aspergilli in vitro, it is currently not used for the treatment of IA. Efficacy of anidulafungin in combination with voriconazole has been recently investigated in 277 haematological patients. This double-blind randomized trial did not reveal a significant difference in mortality between the voriconazole-anidulafungin combination and voriconazole monotherapy [20]. Therefore, the routine use of this combination for IA is discouraged by current guidelines but it can be considered in selected patients under particular clinical conditions [21]. The adverse effects of anidulafungin are similar to caspofungin and micafungin and include nausea, diarrhoea, headache, phlebitis and pruritus, leukopenia, neutropenia, anaemia, hypokalaemia and hepatotoxicity. The risk of hepatic adverse effects, however, appears to be lower for anidulafungin when compared to other antifungal medications such as micafungin [22–24].
Pharmacokinetics: Similarly to other currently licensed echinocandins, anidulafungin displays insufficient enteral absorption and has therefore to be administered by intravenous infusion [25]. The standard dose of anidulafungin recommend by the manufacturer comprises a 200-mg-loading-dose applied over 3 h once on day 1, and a maintenance dose of 100 mg once daily (infusion time [Tinf]=90 min). In plasma, anidulafungin undergoes spontaneous ring opening. The respective product which is further degraded by hydrolysis and N-acetylation independently from phase I and phase II metabolism is eliminated subsequently via biliary excretion [24,26]. The degradants of anidulafungin are eliminated mainly by the faeces. Only 10% of the administered radioactivity was recovered as intact drug, 90% as degradants. Plasma pharmacokinetics of anidulafungin has been investigated under various conditions [27]. In a small study of healthy volunteers, the mean peak level was 4.1 µg/mL, the AUC was 102 µg•h/mL and the half-life was 28 h after administration of 90 mg of 14C-labeled anidulafungin. By population pharmacokinetics similar data were obtained. The mean half-life was 26 h, the volume of distribution at steady state was 33 L, and the anidulafungin clearance amounted to 0.9 L/h.
So far drug-drug interactions involving anidulafungin appear to be rare. Its potential for causing interactions with concomitant medications has been shown to be even lower than that of other echinocandins, as anidulafungin does not inhibit cytochrome-P-450 isoenzymes. Recently, however, anidulafungin has been reported to inhibit the ATP-binding cassette transporter breast cancer resistance protein (BCRP) in vitro [28]. A few clinical studies have assessed drug-drug interactions involving anidulafungin. Simultaneous administration with voriconazole did not cause an altered exposure to anidulafungin or voriconazole [29]. Administration of cyclosporine A together with anidulafungin resulted in a slight increase in anidulafungin exposure (22% increases in AUC0-∞). No relevant drug-drug interaction was observed between tacrolimus and anidulafungin [30].
As anidulafungin is degraded spontaneously, renal impairment has no influence on its elimination [24]. In patients with impaired liver function, a slightly decreased anidulafungin exposure was observed which, however, does not require increased doses [31]. This holds also true for critically ill patients, where the median AUC and the median peak concentration were slightly below those in healthy subjects or in patients in a more stable condition [32]. Extracorporeal organ support, e.g. renal replacement therapy, had no relevant effect on anidulafungin pharmacokinetics [33–37].
Data on tissue penetration of anidulafungin has been limited so far. In human pulmonary epithelial lining fluid of healthy volunteers, relatively low concentrations (mean, 0.9 µg/mL) were achieved, whereas the drug accumulated in pulmonary alveolar macrophages (mean concentration ~100 µg/mL) and in peripheral blood mononuclear cells and in polymorphonuclear leukocytes [38,39]. In rabbits and in rats, the highest concentrations were measured in lung and liver. The lowest amounts were recovered from the brain, the vitreous humour, the aqueous humour, and the choroid [40,41].
Antifungal activity of platelets
Function of neutrophils, dendritic cells and alveolar macrophages during innate lung defence against Aspergillus species is extensively studied and discussed in the literature [42-45]. Interestingly, an increasing number of publications suggests that internal communication and interactions between immune cells result in a stronger cell activation and increase response to fungal pathogens [46,47]. Moreover, a recent publication by Savers et al has demonstrated that exposure of mice deficient in T-, B- and NK-cells to a nonlethal A. fumigatus inoculum consequently protected mice against a lethal exposure [48]. In pre-exposed mice neutrophils and macrophages have been recruited in higher numbers and also secreted higher amounts of specific cytokines [48]. Further intriguing aspects of “trained immunity” concept may arise in future studies and involve other cellular and molecular players.
In the raw of recent publications the surprising function of platelets in Aspergillus species defence has been recognized. Beyond long-established function in haemostasis regulation and thrombus formation, platelets are highly active in the host defence against pathogens [42,43]. Platelet interaction with other cell types relays on variety of surface receptors that include integrins, selectins, leucine-rich repeats receptors, tyrosine kinase receptors and others. Platelet granules and lysosomes contain extensive collection of inflammatory mediators; their rapid exocytosis is a unique feature of platelets. Moreover, platelets contain functional protein translation machinery and may also synthesize microvesicles (reviewed in [42]).
It has been previously observed that low platelet counts associate with poor outcome of IA [49]. Thrombocytopenia was shown to precede fungal infections in liver transplant recipients [50]. An early study by Christin et al. demonstrated that in the presence of A. fumigatus platelets express glycoprotein CD63 and supplement polymorphic neutrophils [51]. In the immunocompromised patient, inhaled Aspergillus conidia germinate into hyphae, the growing and invading structures of filamentous fungi. Consequently, blocking fungal germination and delaying hyphal growth is crucial in preventing invasive disease. The fungal cell wall plays an important role during growth and helps Aspergillus spp. to invade host tissue [52]. The fungal cell wall also protects fungi against a hostile environment and represents a target for the host immune system and antifungal substances. Hence, the maintenance of cell wall integrity and functionality is crucial for A. fumigatus.
We have shown a direct binding of platelets to Aspergillus conidia and hyphae; this interaction induced platelet activation and resulted in the fungal cell damage. The interaction with platelets also reduced hyphal germination and elongation of Aspergillus whereas serotonin was immediately released from dense granules of platelets [53,54]. Furthermore, the platelet treatment decreased metabolic activity of A. fumigatus and caused reduction of polysaccharide galactomannan [55], which is released by growing and vital hyphae [1]. The presence of platelets that were pre- activated by fungal supernatant nearly completely suppressed any germination of A. fumigatus, thus pointing out that platelets can be stimulated by the direct contact with Aspergillus and may also sense the presence of fungus-derived soluble factors [56]. We have also found a specific transcriptional response of A. fumigatus disposed to platelets. Fungal detoxification mechanisms were induced at early time points, while respiration and associated processes were down-regulated over the course of time. Fungal Apoptosis- and cell death-related genes were also found to be induced due to platelets contact in vitro [57].
Only few studies have investigated molecular mechanisms of platelet-Aspergillus interaction. Results of Rambach et al. [58] suggest that platelets activation involves interaction with fungal polysaccharide galactosaminogalactan (GAG). GAG is localized to fungal cell wall and can also be secreted [58]. Previous studies in two murine models of IA identified GAG as a fungal virulence factor that mediates A.fumigatus adhesion and supresses inflammatory responses in vivo [59]. GAG was also proposed to shield hyphal β-glucan from the immune system [59]. In recent publication, Lee et al has hypothesised that protective effect of the cell wall-associated GAG is direct: GAG contributes to resistance to NADPH oxidase-dependent neutrophil damage during fungal infection [60]. Other cell wall components, including chitin, galactomannan and β-1,3-glucan were not affected by the platelet stimulation of Aspergillus [58].
Recent compelling publication by Lefrancias et al. showed that production of platelets from mouse megakaryocytes takes place in lung microcirculation where more than 10 milllion platelets are produced per hour, thus making lung to a primary site of platelet biogenesis [61]. Furthermore, results of Lefrancias et al. suggest that the lung may contain specific signalling partners for megakaryocytes that promote platelet release and may play a role in pathogen defence mechanisms [61,62].
A variety of physiological functions taken by platelets makes it unlikely that every platelet will contribute to all these processes. The concept of platelet heterogeneity has been discussed previously [63]. However, the emerged question whether platelets in circulation represent different populations, each prone to a certain response, or whether it is the local activating environment that governs their ultimate fate remains unanswered. Up until now platelet heterogeneity was studied only in thrombus formation: even within a single thrombus platelets were shown to differ in morphology and in surface characteristics. Inter-platelet variations in expression of receptors and adhesive surface proteins were linked to response variation [64,65]. Diverse environmental factors including adherence surface and local differences in rheology were suggested to contribute to platelet heterogeneity and response variation. On the other hand, in vitro experiments using isolated platelets showed evidence for non-environmental factors contributed to platelet response heterogeneity [66].
Platelets enhance effect of antifungals
Interaction of platelets and fungal substances is of high interest as it might be beneficial in combating fungal infections. Our in vitro studies have shown that platelets in combination with polyenes and azoles antifungals exert additive effects in reducing the germination rate and hyphal elongation of A. fumigatus. Among tested antimycotic substances, amphotericin B in combination with human platelets revealed the best results [54]. Interestingly, exposure of A. fumigatus to platelets caused downregulation of several genes associated with cell wall integrity [63]. Similarly, cell wall integrity/maintenance-related genes were repressed by antifungals e.g. Afu3g08110 by amphotericin B or Ags2 by voriconazole.
Platelets and anidulafungin
In our previous studies we have investigated whether platelets and anidulafungin in combination have an added antifungal effect. We have found that the antifungal in vitro activity of anidulafungin against A. fumigatus is enhanced when combined with anidulafungin. Using two clinical isolates of A. fumigatus we have shown that germination rate and hyphal elongation of A. fumigatus was significantly stronger inhibited when treated with platelets and anidulafungin as compared to exclusively platelet-treated or anidulafungin-treated fungi. In addition, the glucan synthase FKS gene, that encodes 1,3-β-D-glucan synthase, was downregulated.
Interestingly, mechanism of A. fumigatus resistance to echinocandin has been shown to involve amino acid changes in hot-spot regions of Fks subunits, which results in decreased sensitivity of the enzyme to this antimycoticum class.
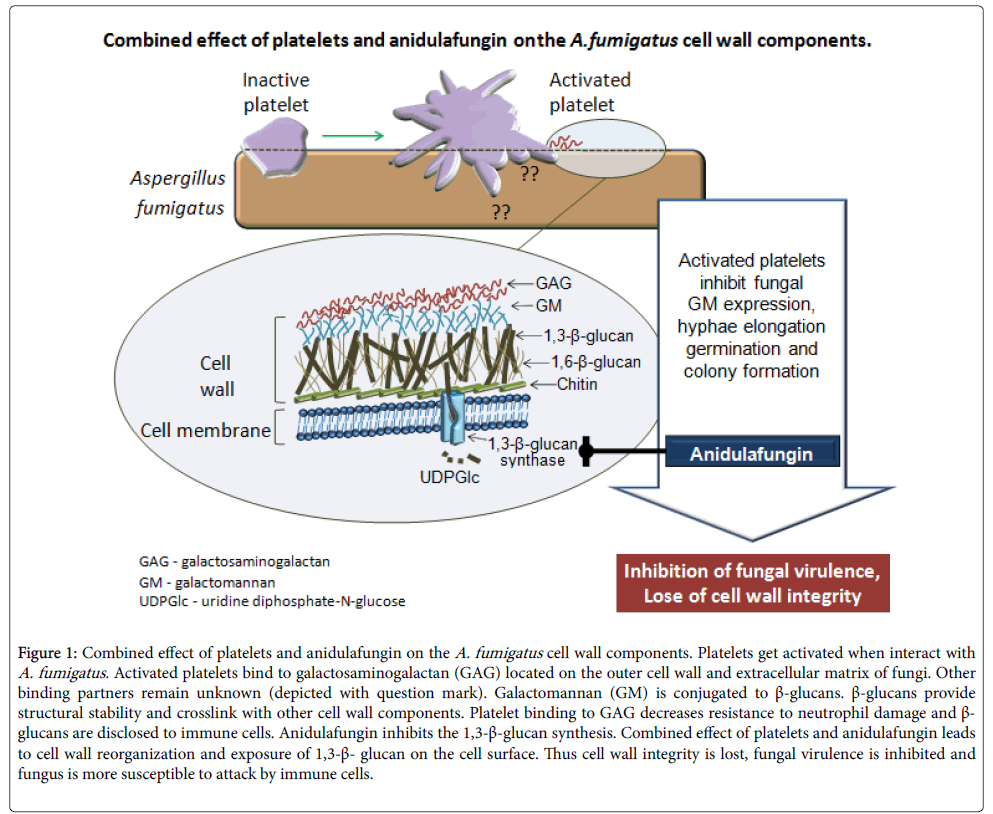
Figure 1 displays a model of combined effect of platelets and anidulafungin on the A. fumigatus. Activation of platelet by A. fumigatus causes direct effect of platelet on fungal wall component(s), one of which was identified as GAG. Anidulafungin on the other hand inhibits the 1,3-β-D-glucan synthesis. As a consequence of platelet/anidulafungin treatment fungal cell wall integrity is lost, fungal virulence is inhibited and fungus becomes more susceptible to further attack by immune cells. Exact molecular mechanisms underlying platelet activation as well as binding further partners remain to be identified.
Figure 1: Combined effect of platelets and anidulafungin on the A. fumigatus cell wall components. Platelets get activated when interact with A. fumigatus. Activated platelets bind to galactosaminogalactan (GAG) located on the outer cell wall and extracellular matrix of fungi. Other binding partners remain unknown (depicted with question mark). Galactomannan (GM) is conjugated to β-glucans. β-glucans provide structural stability and crosslink with other cell wall components. Platelet binding to GAG decreases resistance to neutrophil damage and β- glucans are disclosed to immune cells. Anidulafungin inhibits the 1,3-β-glucan synthesis. Combined effect of platelets and anidulafungin leads to cell wall reorganization and exposure of 1,3-β- glucan on the cell surface. Thus cell wall integrity is lost, fungal virulence is inhibited and fungus is more susceptible to attack by immune cells.
Concluding Remarks and Outlook
Growing experimental evidence underlines important role of platelets in anti-fungal defence. In terms of platelet-Aspergillus interaction it is intriguing to speculate that release of fungus-specific platelets may take place in lung at the primary contact with conidia or/and with not yet identified fungus-derived soluble factors. This particular platelet population may directly bind and attack conidia to prevent hyphae growth locally, release soluble factors (e.g. serotonin) and possibly express microvesicles that will message to other immune cells thus supporting antifungal defence. Since contact of a healthy individual with Aspergillus conidia occurs on a daily basis one can suggest existence of fungi-specific platelet population in blood circulation. How these platelets are activated and regulated in immunocompromised patient and whether immunosuppression setting changes platelet biogenesis in lungs remains to be investigated.
The concept of antimycotic consolidation with host immune cells has been rising over last years. Existing research data point on the platelets/anidulafungin synergetic effect in targeting fungal cell wall. Interestingly, anidulafungin accumulates in pulmonary alveolar macrophages, peripheral blood mononuclear cells and in polymorphonuclear leukocytes [38,39]. Therefore it is compelling to investigate whether anidulafungin also accumulates in platelets and whether it influences the potential production of platelets in the lung as a primary site of platelet biogenesis.
More studies are needed to undercover underling molecular mechanisms and to improve the clinical effectiveness of anidulafungin.
References
- Gregg KS, Kauffman CA (2015) Invasive Aspergillosis: Epidemiology, Clinical Aspects, and Treatment. Semin Respir Crit Care Med 36: 662–672.
- Hope WW, Denning DW (2004) Invasive aspergillosis: current and future challenges in diagnosis and therapy. Clin Microbiol Infect 10: 2–4.
- Stevens DA, Kan VL, Judson MA, Morrison VA, Dummer S, et al. (2000) Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis 30: 696–709.
- Denning DW, Bromley MJ (2015) How to bolster the antifungal pipeline. Science 347: 1414–1416.
- Lass-Flörl C, Mayr A, Perkhofer S, Hinterberger G, Hausdorfer J, et al. (2008) Activities of antifungal agents against yeasts and filamentous fungi: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother 52: 3637–3641.
- Tong KB, Lau CJ, Murtagh K, Layton AJ, Seifeldin R (2009) The economic impact of aspergillosis: analysis of hospital expenditures across patient subgroups. Int J Infect Dis 13: 24–36.
- Denning DW (2003) Echinocandin antifungal drugs. Lancet 362: 1142–1151.
- Chen SC-A, Slavin MA, Sorrell TC (2011) Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 71: 11–41.
- Cappelletty D, Eiselstein-McKitrick K (2007) The echinocandins. Pharmacotherapy 27: 369-388.
- Bowman JC, Hicks PS, Kurtz MB, Rosen H, Schmatz DM, et al. (2002) The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 46: 3001–3012.
- Lewis RE, Albert ND, Kontoyiannis DP (2008) Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J Antimicrob Chemother 61: 1140-1144.
- Wiederhold NP (2007) Attenuation of echinocandin activity at elevated concentrations: A review of the paradoxical effect. Curr Opin Infect Dis 20: 574–578.
- Vanstraelen K, Lagrou K, Maertens J, Wauters J, Willems L, et al. (2013) The Eagle-like effect of echinocandins: What's in a name? Expert Rev Anti Infect Ther 11: 1179-1191.
- Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, et al. (2012) Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother 56: 2435–2442.
- Pea F (2013) Current pharmacological concepts for wise use of echinocandins in the treatment of Candida infections in septic critically ill patients. Expert Rev Anti Infect Ther 11: 989–997.
- Tsai D, Lipman J, Roberts JA (2015) Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care 21: 412–420.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, et al. (2016) Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62: e1-50.
- Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, et al. (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18 Suppl 7: 53–67.
- Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, et al. (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin Microbiol Infect 18 Suppl 7: 19–37.
- Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, et al. (2015) Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann Intern Med 162: 81–89.
- Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, et al. (2016) Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 63: e1-e60.
- European Medicines Agency C (2016) EPAR-Product Information.
- European Medicines Agency M (2011) EPAR-Product Information.
- European Medicines Agency E (2014) EPAR-Product Information.
- Wiederhold NP, Lewis RE (2003) The echinocandin antifungals: An overview of the pharmacology, spectrum and clinical efficacy. Expert Opin Investig Drugs 12: 1313–1333.
- Damle BD, Dowell JA, Walsky RL, Weber GL, Stogniew M, et al. (2009) In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin. Antimicrob Agents Chemother 53: 1149–1156.
- Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, et al. (2004) Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol 44: 590–598.
- Lempers VJ, van den Heuvel JJ, Russel FG, Aarnoutse RE, Burger DM, et al. (2016) Inhibitory Potential of Antifungal Drugs on ATP-Binding Cassette Transporters P-Glycoprotein, MRP1 to MRP5, BCRP, and BSEP. Antimicrob Agents Chemother 60: 3372-3379.
- Dowell JA, Schranz J, Baruch A, Foster G (2005) Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J Clin Pharmacol 45: 1373–1382.
- Dowell JA, Stogniew M, Krause D, Henkel T, Damle B (2007) Lack of pharmacokinetic interaction between anidulafungin and tacrolimus. J Clin Pharmacol 47: 305–314.
- Dowell JA, Stogniew M, Krause D, Damle B (2007) Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J Clin Pharmacol 47: 461–470.
- Bruggemann RJM, Middel-Baars V, Lange DW de, Colbers A, Girbes ARJ, et al. (2017) Pharmacokinetics of Anidulafungin in Critically Ill Intensive Care Unit Patients with Suspected or Proven Invasive Fungal Infections. Antimicrob Agents Chemother 24: 61.
- Aguilar G, Ferriols R, Carbonell JA, Ezquer C, Alonso JM, et al. (2016) Pharmacokinetics of anidulafungin during venovenous extracorporeal membrane oxygenation. Crit Care 20: 325
- Aguilar G, Azanza JR, Carbonell JA, Ferrando C, Badenes R, et al. (2014) Anidulafungin dosing in critically ill patients with continuous venovenous haemodiafiltration. J Antimicrob Chemother 69: 1620–1623.
- Rosa FG de, Corcione S, Baietto L, Pasero D, Di Perri G, et al. (2013) Pharmacokinetics of anidulafungin in two critically ill patients with septic shock undergoing CVVH. J Chemother 25: 376–378.
- Leitner JM, Meyer B, Fuhrmann V, Saria K, Zuba C, et al. (2011) Multiple-dose pharmacokinetics of anidulafungin during continuous venovenous haemofiltration. J Antimicrob Chemother 66: 880–884.
- Aguilar G, Azanza JR, Sádaba B, Badenes R, Ferrando C, et al. (2014) Pharmacokinetics of anidulafungin during albumin dialysis. Crit Care 18: 422.
- Crandon JL, Banevicius MA, Fang AF, Crownover PH, Knauft RF, et al. (2009) Bronchopulmonary disposition of intravenous voriconazole and anidulafungin given in combination to healthy adults. Antimicrob Agents Chemother 53: 5102–5107.
- Farowski F, Cornely OA, Vehreschild JJ, Wiesen M, Steinbach A, et al. (2013) Intracellular concentrations of anidulafungin in different compartments of the peripheral blood. Int J Antimicrob Agents 41: 379–382.
- Damle B, Stogniew M, Dowell J (2008) Pharmacokinetics and tissue distribution of anidulafungin in rats. Antimicrob Agents Chemother 52: 2673–2676.
- Groll AH, Mickiene D, Petraitiene R, Petraitis V, Lyman CA, et al. (2001) Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): Reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother 45: 2845–2855.
- Duerschmied D, Bode C, Ahrens I (2014) Immune functions of platelets. Thromb Haemost 112: 678–691.
- Garraud O, Hamzeh-Cognasse H, Pozzetto B, Cavaillon J-M, Cognasse F (2013) Bench-to-bedside review: Platelets and active immune functions - new clues for immunopathology? Crit Care 17: 236.
- Garth JM, Steele C (2017) Innate Lung Defense during Invasive Aspergillosis: New Mechanisms. J Innate Immun 9: 271–280.
- Shah A, Kannambath S, Herbst S, Rogers A, Soresi S, et al. (2016) Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death. Am J Respir Crit Care Med 194: 1127–1139.
- Park SJ, Burdick MD, Mehrad B (2012) Neutrophils mediate maturation and efflux of lung dendritic cells in response to Aspergillus fumigatus germ tubes. Infect Immun 80: 1759–1765.
- Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, et al. (2010) Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 185: 6190–6197.
- Savers A, Rasid O, Parlato M, Brock M, Jouvion G, et al. (2016) Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge. PLoS One 11: e0153829.
- Nouer SA, Nucci M, Kumar NS, Grazziutti M, Restrepo A, et al. (2012) Baseline platelet count and creatinine clearance rate predict the outcome of neutropenia-related invasive aspergillosis. Clin Infect Dis 54: e173-183.
- Christin L, Wysong DR, Meshulam T, Hastey R, Simons ER, et al. (1998) Human platelets damage Aspergillus fumigatus hyphae and may supplement killing by neutrophils. Infect Immun 66: 1181–1189.
- McDonagh A, Fedorova ND, Crabtree J, Yu Y, Kim S, et al. (2008) Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 4: e1000154.
- Perkhofer S, Trappl K, Nussbaumer W, Dierich MP, Lass-Florl C (2010) Potential synergistic activity of antimycotic substances in combination with human platelets against Aspergillus fumigatus. J Antimicrob Chemother 65: 1309–1311.
- Speth C, Hagleitner M, Ott HW, Würzner R, Lass-Flörl C, et al. (2013) Aspergillus fumigatus activates thrombocytes by secretion of soluble compounds. J Infect Dis 207: 823–833.
- Chang FY, Singh N, Gayowski T, Wagener MM, Mietzner SM, et al. (2000) Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation 69: 70–75.
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, et al. (2017) The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544: 105–109.
- Cauwenberghs S, Feijge, Marion A H, Harper, Alan G S, Sage SO, Curvers J, et al. (2006) Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett 580: 5313–5320.
- Munnix, Imke C A, Cosemans, Judith M E M, Auger JM, et al. (2009) Platelet response heterogeneity in thrombus formation. Thromb Haemost 102: 1149–1156.
- Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, et al. (2005) P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 105: 3552–3560.
- den Dekker E, van Abel M, van der Vuurst, Hans, van Eys, Guillaume J J M, et al. (2003) Cell-to-cell variability in the differentiation program of human megakaryocytes. Biochim Biophys Acta 1643: 85–94.
- Heemskerk JW, Willems GM, Rook MB, Sage SO (2001) Ragged spiking of free calcium in ADP-stimulated human platelets: regulation of puff-like calcium signals in vitro and ex vivo. J Physiol (Lond) 535: 625–635.
- Perkhofer S, Striessnig B, Sartori B, Hausott B, Ott HW, et al. (2013) Interaction of platelets and anidulafungin against Aspergillus fumigatus. Antimicrob Agents Chemother 57: 626–628.
- Latge J-P, Beauvais A, Chamilos G (2017) The Cell Wall of the Human Fungal Pathogen Aspergillus fumigatus: Biosynthesis, Organization, Immune Response, and Virulence. Annu Rev Microbiol 71: 99-116.
- Perkhofer S, Zenzmaier C, Frealle E, Blatzer M, Hackl H, et al. (2015) Differential gene expression in Aspergillus fumigatus induced by human platelets in vitro. Int J Med Microbiol 305: 327–338.
- Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, et al. (2010) What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27: 155–182.
- Gautam P, Shankar J, Madan T, Sirdeshmukh R, Sundaram CS, et al. (2008) Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother 52: 4220–4227.
- Ingham CJ, Schneeberger PM (2012) Microcolony Imaging of Aspergillus fumigatus Treated with Echinocandins Reveals Both Fungistatic and Fungicidal Activities. PLoS One 7.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 5088
- [From(publication date):
October-2017 - Aug 12, 2025] - Breakdown by view type
- HTML page views : 4219
- PDF downloads : 869