Comparative immunohistochemical expression of TROP2 in brain metastatic and primary tumors
Received: 31-Jul-2025 / Manuscript No. DPO-25-168400 / Editor assigned: 04-Aug-2025 / PreQC No. DPO-25-168400 / Reviewed: 18-Aug-2025 / QC No. DPO-25-168400 / Revised: 25-Aug-2025 / Manuscript No. DPO-25-168400 / Accepted Date: 01-Sep-2025 / Published Date: 01-Sep-2025
Abstract
Background: TROP2 overexpression was reported to predict poor prognosis and increased metastatic potential. This study evaluates TROP2 expression in brain metastases from diverse solid tumors and its consistency with primary tumors, addressing the limited research on TROP2 heterogeneity and its implications for CNS-targeted therapies.
Methods: TROP2 immunohistochemical staining was performed on 61 brain metastatic tumors and 14 corresponding primary tumors. Based on TROP2 expression, cases were categorized into three groups: Diffuse positive (>95%), focal positive (0-95%) and negative (0%).
Results: Among the 61 brain metastatic tumors, TROP2 expression was diffusely positive in 62.3%, focally positive in 16.4% and negative in 21.3%. Diffuse TROP2 positivity was most observed in tumors of lung origin, while focal positivity was predominant in colorectal metastases. In terms of pathologic diagnosis, adenocarcinoma was the most common type, with 55.6% showing diffuse positivity, 33.3% showing focal positivity and 11.1% showing negativity. Notably, all cases of Invasive Breast Carcinoma of No Special Type (IBC-NST) exhibited diffuse TROP2
positivity. No significant association was found between TROP2 expression levels and post-CNS metastasis survival. In the paired analysis of 14 cases with both primary and metastatic tumors, TROP2 expression was consistent between primary and metastatic sites in 78.6% of cases. However, 21.4% of cases, including colorectal adenocarcinoma and kidney clear cell carcinoma, showed discordant expression patterns between the primary and metastatic lesions.
Conclusion: This study demonstrated variable TROP2 expression in brain metastasis samples and confirmed high consistency of TROP2 expression between primary and brain metastatic lesions.
Keywords: TROP2, Brain metastasis, Immunohistochemistry, Cancer, Primary tumor
Abbreviations
TROP2: Trophoblast Cell Surface Antigen 2; CNS: Central Nervous System; ADCs: Antibody-Drug Conjugates; SG: Sacituzumab Govitecan; OS: Overall Survival; TNBC: Triple-Negative Breast Cancer; BBB: Blood-Brain Barrier; IBC-NST: Invasive Breast Carcinoma of No Special Type.
Introduction
The human trophoblast cell surface antigen 2 (TROP2) is a transmembrane glycoprotein that acts as an intracellular calcium signal transducer, driving self-renewal, cell proliferation, invasion and survival [1,2]. It is overexpressed in various solid tumors, including breast, Colorectal, lung, gastric, skin and pancreatic cancers, with expression reported in 55%-75% of cases [3-9]. Overexpression of TROP2 is generally associated to poor prognosis, increased tumor aggressiveness and higher metastatic potential [10,11].
Metastatic solid tumors remain a significant unmet medical challenge, particularly Central Nervous System (CNS) metastases, which are associated with extremely poor outcomes. While targeted therapies, such as monoclonal antibodies and Antibody-Drug Conjugates (ADCs), have improved outcomes in advanced cancer, their efficacy against CNS metastases is limited. Sacituzumab Govitecan (SG), a TROP2-directed ADC, has demonstrated significant Overall Survival (OS) benefits in patients with Triple-Negative Breast Cancer (TNBC) but showed no OS improvement for those with CNS metastases [12,13]. The prevailing explanation attributes this limitation to the inability of these therapies to effectively penetrate the Blood-Brain Barrier (BBB) [14]. However, the distinct molecular and genomic features of CNS metastases, which often diverge from those of the primary tumor, must also be considered [15]. Research on TROP2 heterogeneity between primary and metastatic lesions, particularly in the CNS, remains scarce. Based on this context, this study aims to evaluate TROP2 expression in brain metastases from various solid tumors and compare it with expression in primary tumors.
Materials And Methods
Case selection
After the approval from the institutional review board (approval number: 2024AN0580), A total of 61 surgically resected brain metastasis specimen between 2015 to 2023 were retrieved for the pathology database of the Department of Pathology at the Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea. When available, corresponding primary tumor tissues were also collected. Clinical data, including the patient’s age, sex and origin of tumor was reviewed in the electronic medical records.
Tissue microarray construction
Tissue Microarrays (TMAs) were constructed from formalin-fixed, paraffin-embedded tissue blocks by using a manual tissue microarrayer (UNITIMA, Seoul, South Korea). Areas occupied for >75% of tumor cells without accompanying tumor necrosis were selected and two representative cores were punched out from a donor block and transferred into a new recipient block with a punch of a 3mm diameter.
Immunohistochemical staining
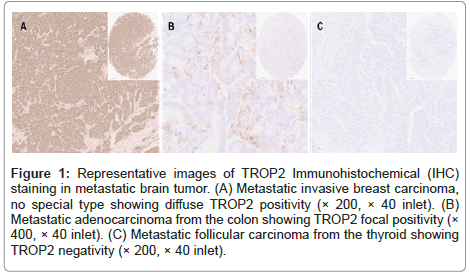
The Immunoshistochemical (IHC) staining was performed as follows. Briefly, 4-μm tissue sections from TMAs were subjected to IHC using a Ventana auto-stainer and an ultra-Vies DAB Detection Kit (Ventana, Tucson, Arizona), according to the manufacturer’s instruction. Primary antibodies for TROP2 (EPR20043, catalog No. ab214488, rabbit monoclonal, 1:2000, Abcam, Cambridge, UK) were used. The TROP2 IHC staining was evaluated by two pathologists (YNS and YSK) and classified into 3 categories (Figure1): Diffuse positive (>95%), focal positive (>0% and <95%) and negative.
Figure 1: Representative images of TROP2 Immunohistochemical (IHC) staining in metastatic brain tumor. (A) Metastatic invasive breast carcinoma, no special type showing diffuse TROP2 positivity (× 200, × 40 inlet). (B) Metastatic adenocarcinoma from the colon showing TROP2 focal positivity (× 400, × 40 inlet). (C) Metastatic follicular carcinoma from the thyroid showing TROP2 negativity (× 200, × 40 inlet).
Statistical methods
The R software (version 4.02, Vienna, Austria) was used. The association between TROP2 expression pattern and tumor origin, as well as between TROP2 expression pattern and pathologic diagnosis, was evaluated using the χ2 and/or fisher’s exact tests. Survival analysis based on expression levels was performed using log rank test and Kaplan-Meier analysis. P-values less than 0.05 were considered statistically significant.
Results
Clinicopathological characteristics of cases
A total of 61 surgically resected brain metastasis specimens were available for analysis. Table 1 summarizes the characteristics of cases. The mean age of the patients was 61.5 ± 10.0 years (range, 37 to 82 years) with a male-to-female ratio of 1.1. Metastatic brain tumors originating from various organs were included. The most common primary site was lung (23 cases, 37.7%), followed by the breast (11 cases, 18.0%) and the colorectum (10 cases, 16.4%). Other primary sites included the bladder and skin (3 cases each, 4.9%), kidney and ovary (2 cases each, 3.3%) and well as less common sites such as the ear, gallbladder, hypopharynx, thymus and thyroid, each contributing 1 case (1.6%). In terms of pathologic diagnosis, adenocarcinoma was the most frequently observed type, with 27 cases (44.3%), followed by invasive ductal carcinoma with 11 cases (18.0%), Squamous cell carcinoma with 6 cases (9.8%) and undifferentiated carcinoma in 4 cases (6.6%). Melanoma and urothelial carcinoma were found in 3 cases each (4.9%). Less common diagnoses included clear cell carcinoma and high-grade serous carcinoma (2 cases each, 3.3%), along with adenoid cystic carcinoma, follicular carcinoma and thymoma each present in 1 case (1.6%) as shown in Table 1.
| Characteristics | No. of patients | % of patients |
|---|---|---|
| Age (years) | 61.5 ± 10.0 | |
| Sex | ||
| Male | 32 | 52.5 |
| Female | 29 | 47.5 |
| Primary site | ||
| Lung | 23 | 37.7 |
| Breast | 11 | 18 |
| Colorectal | 10 | 16.4 |
| Bladder | 3 | 4.9 |
| Skin | 3 | 4.9 |
| Kidney | 2 | 3.3 |
| Ovary | 2 | 3.3 |
| Unknown | 2 | 3.3 |
| Ear | 1 | 1.6 |
| Gallbladder | 1 | 1.6 |
| Hypopharynx | 1 | 1.6 |
| Thymus | 1 | 1.6 |
| Thyroid | 1 | 1.6 |
| Diagnosis | ||
| Adenocarcinoma | 27 | 44.3 |
| Invasive ductal carcinoma | 11 | 18 |
| Squamous cell carcinoma | 6 | 9.8 |
| Carcinoma, undifferentiated | 4 | 6.6 |
| Melanoma | 3 | 4.9 |
| Urothelial carcinoma | 3 | 4.9 |
| Clear cell carcinoma | 2 | 3.3 |
| High-grade serous carcinoma | 2 | 3.3 |
| Adenoid cystic carcinoma | 1 | 1.6 |
| Follicular carcinoma | 1 | 1.6 |
| Thymoma | 1 | 1.6 |
| Median time to brain metastasis (months, range) | 23.5 (0-137) | |
| Median overall survival (months, range) | 40.3 (5-98) | |
| Median survival post-CNS metastasis (months, range) | 9.9 (0-76) | |
| CNS metastasis number | ||
| Single | 37 | 60.7 |
| Multiple | 24 | 39.3 |
| CNS metastasis treatment | ||
| Surgery | 61 | 100 |
| Radiotherapy | 31 | 50.8 |
Table 1: Clinicopathological characteristics of the analyzed cases.
TROP2 expression in metastatic and primary tumor
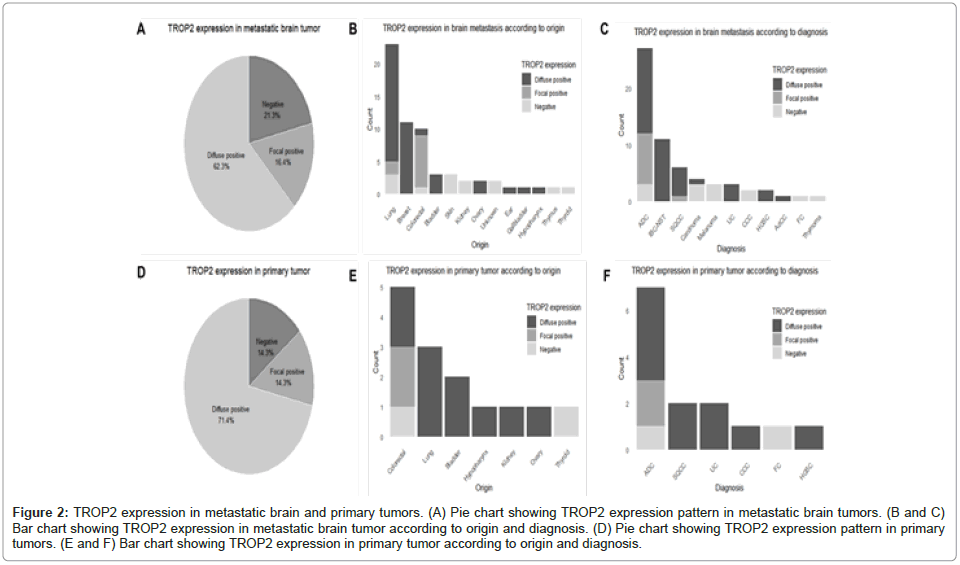
Among the 61 metastatic brain tumors, TROP2 expression was diffusely positive in 38 cases (62.3%), focally positive in 10 cases (16.4%) and negative in 13 cases (21.3%, Figure 2A). Of the tumors with diffuse positive TROP2 expression, the most common site of origin was the lung (18 cases, 47.4%), followed by the breast (11 cases, 28.9%), bladder (3 cases, 7.9%) and ovary (2 cases, 5.3%). Single cases originated from colorectal, ear, gallbladder and hypopharyngeal primaries. Among the tumors with focal TROP2 expression, 8 cases (80%) originated from colorectum and 2 cases (20%) were from the lung. In the TROP2-negative cases, lung and skin tumors accounted for 3 cases each (23.1%), kidney and tumors of unknown origin accounted for 2 cases each (15.4%) and colorectal, thyroid and thymic tumors each accounted for 1 case (7.7%, Figure 2B). The distribution of TROP2 expression according to pathologic diagnosis is illustrated in Figure 2C.
Figure 2: TROP2 expression in metastatic brain and primary tumors. (A) Pie chart showing TROP2 expression pattern in metastatic brain tumors. (B and C) Bar chart showing TROP2 expression in metastatic brain tumor according to origin and diagnosis. (D) Pie chart showing TROP2 expression pattern in primary tumors. (E and F) Bar chart showing TROP2 expression in primary tumor according to origin and diagnosis.
Notably, in the most common tumor type, adenocarcinoma, TROP2 expression was diffusely positive in 15 cases (55.6%), focally positive in 9 cases (33.3%) and negative in 3 cases (11.1%). In contrast, all 11 cases of Invasive Breast Carcinoma of No Special Type (IBC-NST) showed diffuse TROP2 positivity. Similarly, in primary tumors, TROP2 expression was diffusely positive in 10 cases (71.4%), focally positive in 2 cases (14.3%) and negative in 2 cases (14.3%, Figure 2D). Among the TROP2 diffuse positive cases, 3 cases (30%) originated from the lung, 2 cases (20%) each from the bladder and colorectum and 1 case (10%) each from the hypopharynx, kidney and ovary. For the focal TROP2 positive cases, both originated from colorectal primaries (100%). Among the TROP2-negative cases, 1 case (50%) originated from the colorectum and 1 case (50%) from the thyroid (Figure 2E).
The distribution of TROP2 expression by pathologic diagnosis showed a pattern similar to that of brain metastatic lesions, with adenocarcinoma being the most common tumor type. In adenocarcinomas, TROP2 expression was diffusely positive in 4 cases (57.1%), focally positive in 2 cases (28.6%) and negative in 1 case (14.3%). All cases of squamous cell carcinoma, urothelial carcinoma, clear cell carcinoma and high-grade serous carcinoma demonstrated diffuse TROP2 positivity, while follicular carcinoma was TROP2-negative. Detailed TROP2 expression according to tumor origin and pathologic diagnosis for both brain metastatic lesions and primary tumors are provided in (Supplementary Tables 1 and 2) and as well as shown in Figure 2A-2E.
Differences in TROP2 expression based on clinical characteristics
We analyzed whether differences in TROP2 expression were associated with characteristics of CNS metastases or patient survival. Patients were categorized into two groups based on the presentation of CNS metastases at the time of diagnosis: Single vs. multiple metastases. When stratified by TROP2 expression levels, the distribution was as follows: Among patients with diffuse positive expression, 21 had single CNS metastasis and 16 had multiple metastases. In the focal positive group, 8 had single CNS metastasis and 2 had multiple metastases. Among patients with negative expression, 7 had single CNS metastasis and 6 had multiple metastases. Fisher’s exact test did not reveal a significant association (p=0.454).
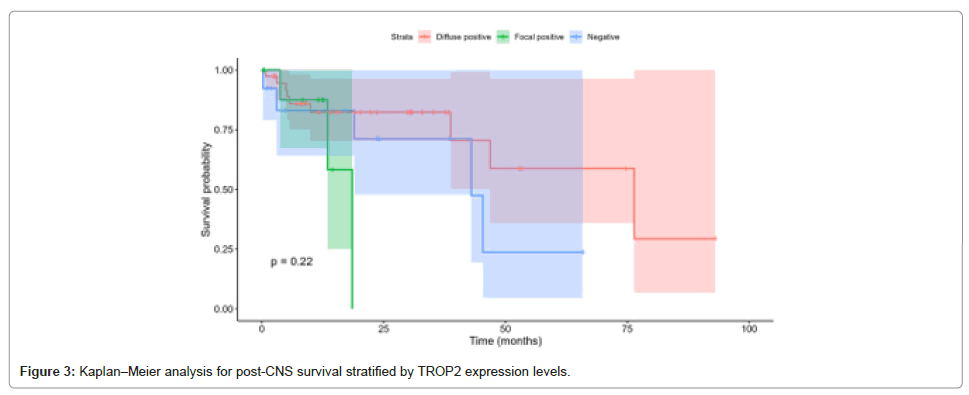
The median survival post-CNS metastasis was: Diffuse positive, 76.4 months (95% CI: 46.8-NA); focal positive, 18.5 months (95% CI: 13.5-NA); and negative, 43.0 months (95% CI: 19.0-NA). The log-rank test again showed no significant differences (p=0.218). HRs were 2.93 (95% CI: 0.72-11.94, p=0.134) for focal positive relative to diffuse positive and 1.97 (95% CI: 0.64-6.08, p=0.237) for negative relative to diffuse positive as shown in Figure 3.
Comparison of TROP2 Expression in paired primary and brain metastatic tumor
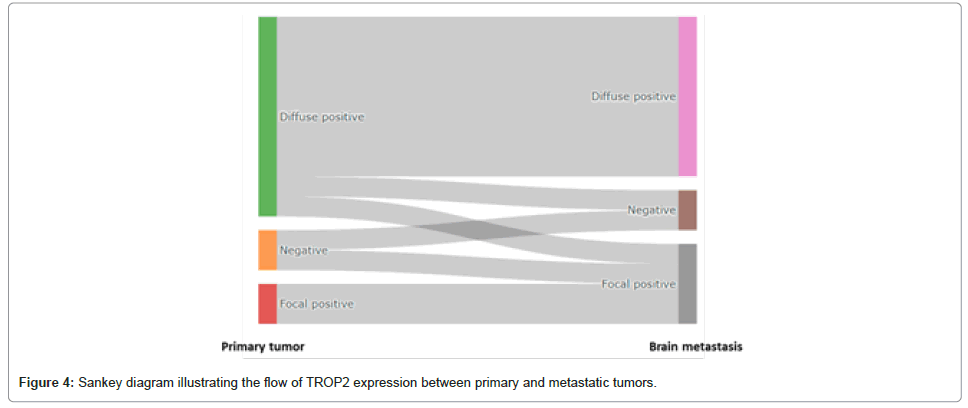
Among the 14 cases where both primary tumors and corresponding brain metastases were available for paired TROP2 expression analysis, three cases (21.4%) showed differences in TROP2 expression between the primary and metastatic sites (Figure 4, Supplementary Table 3). Specifically, in colorectal adenocarcinoma, one case shifted from diffuse positive in the primary lesion to focal positive in the metastasis and another case showed negative expression in the primary lesion but focal positivity in the metastasis. Additionally, a case of kidney clear cell carcinoma exhibited diffuse positive TROP2 expression in the primary tumor but was negative in the brain metastasis. The remaining 11 cases (78.6%) displayed consistent TROP2 expression patterns between the primary and metastatic lesions Supplementary Table 3 and as shown in Figure 4.
Discussion
This study provides important insights into the TROP2 expression in brain metastases across various primary tumor origins. Notably, the high prevalence of diffuse TROP2 positivity (62.3%) in brain metastases, particularly those originating from lung and breast cancers, highlights the potential role of TROP2 in the pathogenesis and progression of CNS metastasis.
Anti-cancer therapies targeting TROP2 have predominantly focused on the development of ADC. A representative TROP2 ADC, Sacituzumab Govitecan (SG), consists of a TROP2-targeting antibody linked to the topoisomerase I inhibitor SN-38 via a cleavable linker, with a drug-antibody ratio of 1:8, demonstrating potent anti-cancer effects [16]. In the ASCENT phase 3 clinical trial, SG significantly improved median progression-free survival (mPFS; HR 0.41, 95% CI 0.32-0.52, P<0.001) and median overall survival (mOS; HR 0.48, 95% CI 0.38-0.59, P<0.001) in patients with advanced TNBC, leading to FDA approval. In the TROPiCS-02 trial, targeting HR+/HER2- breast cancer, SG also demonstrated a significant mOS benefit compared to the control group (HR 0.79, 95% CI 0.65-0.96, P=0.020) [17]. Additionally, SG has shown therapeutic potential in minor cancer types, such as endometrial cancer, small cell lung cancer and castration-resistant prostate cancer [18]. Based on these findings, numerous combination therapy clinical trials are currently underway [19].
Datopotamab Deruxtecan (Dato-DXd) is another ADC that combines a TROP2 antibody with DXd, featuring a drug-to-antibody ratio of 1:4 [20]. In HR+/HER2- breast cancer, it demonstrated a significant improvement in mPFS compared to the control group (HR 0.63, 95% CI 0.52-0.76, P<.0001) [21]. In a phase 3 clinical trial for patients with advanced NSCLC, it also showed a significant mPFS benefit over docetaxel (HR 0.75, 95% CI 0.62-0.91, P=.004) [22]. Promising results have recently been reported in endometrial and ovarian cancer cohorts [23]. Additionally, other TROP2-targeting ADCs, such as SKB264, SHR-A1921 and LCB84, are undergoing early clinical trials based on encouraging preclinical data [24-26].
Preclinical studies suggest a potential correlation between TROP2 expression and the efficacy of ADCs. In breast cancer cell lines, increased TROP2 expression was associated with enhanced therapeutic activity of SG [27]. Similarly, clinical trials targeting TNBC demonstrated the greatest efficacy in patients with medium to high TROP2 expression, with objective response rates of 44%, 38% and 22% for high, medium and low expression levels, respectively [28]. However, clinical data across various cancer types remain limited and due to the bystander effect of ADCs and the heterogeneity of TROP2 expression, definitive conclusions cannot yet be drawn.
As cancer survival improves, recurrent CNS metastases following local treatment have become increasingly common, highlighting the need for effective systemic therapies. In cancers with high rates of brain metastases, such as breast and lung cancer, clinical trials now frequently include CNS-specific endpoints. Systemic treatment for CNS metastases remains challenging due to the BBB. While small molecules like TKIs have demonstrated CNS efficacy [29,30], larger agents like SG (160 kDa) face limited BBB penetration [31]. However, local BBB disruptions from metastases or radiotherapy may enable larger molecules to access CNS lesions, making target expression a critical determinant of efficacy. Our study supports this concept, with 78.7% of 61 brain metastases exhibiting diffuse or focal TROP2 expression. Consistency in TROP2 expression between primary and CNS lesions was observed in 78.6% of matched cases. These findings suggest a potential association between TROP2 expression and the efficacy of anti-TROP2 ADCs in CNS metastases, warranting further investigation.
This study represents the largest cohort of brain metastases evaluated for TROP2 expression using standardized IHC. All staining was performed in a single center, with a single pathologist ensuring consistency and the analysis was blinded to treatment data to minimize bias. Previous studies evaluating TROP2 expression in various cancers, including breast [3,32,33], colorectal [4,5], skin [8,34] and pancreas [9], primarily used semi-quantitative assessment methods such as H-score to evaluate TROP2 expression levels. These studies often relied on specific scoring thresholds to categorize TROP2 expression. In contrast, our study demonstrated a relatively clear differentiation of TROP2 expression into diffuse, focal and negative patterns, allowing for straightforward analysis without the need for further intensity or area-based categorization. It is important to note that factors such as the type of antibody used and the application of Tissue Microarrays (TMAs) may have influenced the observed TROP2 expression patterns. While TMAs are valuable for high-throughput analysis, they may not fully capture the tumor heterogeneity present in whole-tumor sections. Therefore, future studies examining cases with changes in TROP2 expression between primary and metastatic lesions, or those showing focal positivity, would benefit from evaluating TROP2 expression using whole-slide sections to better reflect the full spectrum of tumor heterogeneity. Comprehensive clinical data enabled robust correlations with TROP2 expression. However, the inclusion of diverse tumor types limited subgroup analyses and the lack of data from visceral metastases prevents conclusions about the specificity of TROP2 expression to CNS lesions. Furthermore, as samples were collected before TROP2 ADCs became available, intracranial efficacy based on TROP2 expression could not be assessed. Further studies are required to validate these findings.
Conclusion
In conclusion, this study demonstrated variable TROP2 expression in 61 brain metastasis samples and confirmed high consistency of TROP2 expression between primary and brain metastatic lesions. While promising, further research is required to validate the clinical efficacy of anti-TROP2 therapies in patients with CNS metastases.
Declarations
Ethics Approval and Consent to Participants
The study protocol was approved by the institutional reEthics Approval and Consent to Participants The study protocol was approved by the institutional review board of each site, including Korea University Anam Hospital (No. 2024AN0580).
Competing Interest
The authors have no conflict of interest in the study.
Funding
No funding was received for this study.
Author Contributions
The concept of the study was designed by JWK. JWL, JHK, SHL, YJC and KHP. JS and HO collected data for the study. YNS and JWK analyzed and visualized the data. YNS and JWK drafted the manuscript. All the authors have reviewed and approved the submitted data.
Data Availability
The dataset generated and/or analyzed during this study is available from the corresponding author upon reasonable request.view board of each site, including Korea University Anam Hospital (No. 2024AN0580).
References
- Shvartsur A, Bonavida B (2015) Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 6:84-105.
[Crossref] [Google Scholar] [PubMed]
- Ripani E, Sacchetti A, Corda D, Alberti S (1998) Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer 76:671-676.
[Crossref] [Google Scholar] [PubMed]
- Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, et al. (2014) Trop-2 is a determinant of breast cancer survival. PLoS One 9:e96993.
[Crossref] [Google Scholar] [PubMed]
- Moretto R, Germani MM, Giordano M, Conca V, Proietti A, et al. (2023) Trop-2 and Nectin-4 immunohistochemical expression in metastatic colorectal cancer: Searching for the right population for drugs' development. Br J Cancer 128:1391-1399.
[Crossref] [Google Scholar] [PubMed]
- Foersch S, Schmitt M, Litmeyer AS, Tschurtschenthaler M, Gress T, et al. (2024) TROP2 in colorectal carcinoma: associations with histopathology, molecular phenotype and patient prognosis. J Pathol Clin Res 10:e12394.
[Crossref] [Google Scholar] [PubMed]
- Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, et al. (2012) TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med 4:472-485.
[Crossref] [Google Scholar] [PubMed]
- Zhao W, Zhu H, Zhang S, Yong H, Wang W, et al. (2016) Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget 7:6136-6145.
[Crossref] [Google Scholar] [PubMed]
- Ito T, Tanegashima K, Tanaka Y, Hashimoto H, Murata M, et al. (2021) Trop2 expression in extramammary paget's disease and normal skin. Int J Mol Sci 22.
[Crossref] [Google Scholar] [PubMed]
- Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, et al. (2008) High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer 99:1290-1295.
[Crossref] [Google Scholar] [PubMed]
- Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, et al. (2016). Impact of TROP2 expression on prognosis in solid tumors: A systematic review and meta-analysis. Sci Rep 6:33658.
[Crossref] [Google Scholar] [PubMed]
- Cubas R, Li M, Chen C, Yao Q (2009) Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta 1796:309-314.
[Crossref] [Google Scholar] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, et al. (2021) Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med 384:1529-1541.
[Crossref] [Google Scholar] [PubMed]
- Diéras V, Weaver R, Tolaney SM, Bardia A, Punie K, et al. (2021) Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res 81:13-07.
- Upton DH, Ung C, George SM, Tsoli M, Kavallaris M, et al. (2022) Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 12:4734-4752.
[Crossref] [Google Scholar] [PubMed]
- Ali S, Górska Z, Duchnowska R, Jassem J (2021) Molecular profiles of brain metastases: A focus on heterogeneity. Cancers (Basel) 13:2645.
[Crossref] [Google Scholar] [PubMed]
- Goldenberg DM, Sharkey RM (2020) Sacituzumab govitecan, a novel, third-generation, Antibody-Drug Conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 20:871-885.
[Crossref] [Google Scholar] [PubMed]
- Rugo HS, Bardia A, Marmé F, Cortés J, Schmid P, et al. (2023) Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 402:1423-1433.
[Crossref] [Google Scholar] [PubMed]
- Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, et al. (2021) Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 32:746-756.
[Crossref] [Google Scholar] [PubMed]
- Garon EB, Liu SV, Owen SP, Reck M, Neal JW, et al. (2022) EVOKE-02: A phase 2 study of Sacituzumab Govitecan (SG) plus pembrolizumab (pembro) with or without platinum chemotherapy in first-line metastatic Non-Small Cell Lung Cancer (NSCLC). J Clin Oncol 40:9146.
- Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, et al. (2021) Datopotamab deruxtecan, a novel trop2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther 20:2329-2340.
[Crossref] [Google Scholar] [PubMed]
- Bardia A, Jhaveri K, Im SA, Pernas S, De Laurentiis M, et al. (2025) Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: Primary results from tropion-breast01. J Clin Oncol 43:285-296.
[Crossref] [Google Scholar] [PubMed]
- Ahn MJ, Tanaka K, Paz-Ares L, Cornelissen R, Girard N, et al. (2025) Datopotamab deruxtecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: The randomized, open-label phase iii tropion-lung01 study. J Clin Oncol 43:260-272.
[Crossref] [Google Scholar] [PubMed]
- Oaknin A, Ang JE, Rha SY, Yonemori K, Kristeleit R, et al. (2024) Datopotamab deruxtecan (Dato-DXd) in patients with Endometrial (EC) or Ovarian Cancer (OC): Results from the phase II TROPION-PanTumor03 study. Ann Oncol 35:S547-S548.
- Cheng Y, Yuan X, Tian Q, Huang X, Chen Y, et al. (2022) Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol 12:951589.
[Crossref] [Google Scholar] [PubMed]
- He N, Yang C, Yang Y, Xue Z, Xu J, et al. (2023) Abstract LB030: SHR-A1921, a novel TROP-2 ADC with an optimized design and well-balanced profile between efficacy and safety. Cancer Res 83:LB030.
- Kim H, Guerra E, Baek E, Jeong Y, You H, et al. (2022) LCB84, a TROP2-targeted ADC, for treatment of solid tumors that express TROP-2 using the hu2G10 tumor-selective anti-TROP2 monoclonal antibody, a proprietary site-directed conjugation technology and plasma-stable tumor-selective linker chemistry. Cancer Res 82:328.
- Cardillo TM, Rossi DL, Zalath MB, Liu D, Arrojo R, et al. (2020) Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer. Oncotarget 11:3849-3862.
[Crossref] [Google Scholar] [PubMed]
- Zhao M, DiPeri TP, Raso MG, Zheng X, Rizvi YQ, et al. (2023) Epigenetically upregulating TROP2 and SLFN11 enhances therapeutic efficacy of TROP2 antibody drug conjugate sacitizumab govitecan. NPJ Breast Cancer 9:66.
[Crossref] [Google Scholar] [PubMed]
- Lee B, Ji W, Lee JC, Song SY, Shin YS, et al. (2023) Efficacy of lazertinib for symptomatic or asymptomatic brain metastases in treatment-naive patients with advanced EGFR mutation-positive non-small cell lung cancer: Protocol of an open-label, single-arm phase II trial. Thorac Cancer 14:2233-2237.
[Crossref] [Google Scholar] [PubMed]
- Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, et al. (2020) Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated her2-positive breast cancer with brain metastases in the her2climb trial. J Clin Oncol 38:2610-2619.
[Crossref] [Google Scholar] [PubMed]
- Nakhjavani M, Samarasinghe RM, Shigdar S (2022) Triple-negative breast cancer brain metastasis: An update on druggable targets, current clinical trials and future treatment options. Drug Discov Today 27:1298-1314.
[Crossref] [Google Scholar] [PubMed]
- Jeon Y, Jo U, Hong J, Gong G, Lee HJ, et al. (2022) Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 22:1014.
[Crossref] [Google Scholar] [PubMed]
- Mertens RB, Makhoul EP, Li X, Dadmanesh F (2024) Comparative expression of trophoblast cell-surface antigen 2 (TROP2) in the different molecular subtypes of invasive breast carcinoma: An immunohistochemical study of 94 therapy-naive primary breast tumors. Ann Diagn Pathol 68:152226.
[Crossref] [Google Scholar] [PubMed]
- Tanegashima K, Tanaka Y, Ito T, Oda Y, Nakahara T, et al. (2024) TROP2 expression and therapeutic implications in cutaneous squamous cell carcinoma: Insights from immunohistochemical and functional analysis. Exp Dermatol 33:e15196.
Citation: Sung YN, Sim J, Oh H, Lee JW, Kim JH, et al. (2025) Comparative Immunohistochemical Expression of TROP2 in Brain Metastatic and PrimaryTumors. Diagnos Pathol Open 10:250.
Copyright: © 2025 Sung YN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 1612
- [From(publication date): 0-0 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 1486
- PDF downloads: 126