Research Article Open Access
Comparison among the Efficiency of Different Bioremediation Technologies of Atrazine–Contaminated Soils
| Ebtesam El-Bestawy1,2*, Sabir J2, Mansy AH3 and Zabermawi N2 | |
| 1Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, 163 Horria Ave. El-Shatby, P.O. Box 832, Alexandria, Egypt | |
| 2Department of Life Sciences, Faculty of Science, King AbdulAziz University. P.O. Box 42805 Jeddah 21551, Kingdom of Saudi Arabia | |
| 3Central Laboratory of Pesticides, Agricultural Research Center at Sabahia, Alexandria, Egypt | |
| Corresponding Author : | Ebtesam El-Bestawy Department of Life Sciences Faculty of Science, King AbdulAziz University P.O. Box 42805 Jeddah 21551, Kingdom of Saudi Arabia Tel: (203) 4801499/48014553/48014174 Fax: 203-4285792 E-mail: ebtesamelbestawy@yahoo.com |
| Received June 25, 2014; Accepted July 31, 2014; Published August 04, 2014 | |
| Citation: El-Bestawy E, Sabir J, Mansy AH, Zabermawi N (2014) Comparison among the Efficiency of Different Bioremediation Technologies of Atrazine–Contaminated Soils. J Bioremed Biodeg 5:237 doi:10.4172/2155-6199.1000237 | |
| Copyright: © 2014 El-Bestawy E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In a previous study by the authors, toxicity screening of Atrazine-resistant soil bacteria from different contaminated soils resulted in 23-soil isolate best grown in the presence of herbicide Atrazine. They were identified according to their 16S rDNA sequencing into Enterobacter (E. cloacae), Bacillus (B. cereus and B. anthracis), Pseudomonas (P. aeruginosa, P. balearica, P. indica and P. otitidis), Ochrobactrum (O. intermedium) and Providencia (P. vermicola). The 23 resistant isolates were enriched in nutrient broth medium amended with 2 folds the recommended dose (RD) of Atrazine. The enrichment technique resulted in the selection of seven bacterial species belong to 4 genera (Enterobacter, Pseudomonas, Bacillus and Providencia) that were superior in their resistance to Atrazine and exhibited remarkable growth stimulation (70.7-88.7%). These four acclimatized and highly Atrazine-resistant strains have been selected and efficiently used for the degradation of Atrazine-contaminated soil. They were employed in different proposed bioremediation technologies including biostimulation (addition of nutrients to enhance the growth and activity of the indigenous microorganisms), bioaugmentation (seeding the most promising indigenous and exogenous Atrazine degraders to accelerate and help the indigenous bacterial population to achieve high and fast remediation of the contaminated soil) and finally a combination of biostimulation and bioaugmentation technologies to investigate the synergistic or suppressive effects of the two techniques. Results proved that bioaugmentation coupled with biostimulation is the most promising bioremediation technology since it is powerful, economical and environmentally friendly technique for decontamination of Atrazine-contaminated soils.
Most herbicides decompose rapidly in soils via soil microbial decomposition, hydrolysis, or photolysis. Many modern chemical herbicides for agriculture are specifically formulated to decompose within a short period after application [7]. Atrazine half-life in soil ranges from 13 to 261 days [8]. Its fate in soil depends upon many different factors including sorption to soil component, uptake by plants, transport via runoff and leaching, biodegradation, photodegradation, volatilization and chemical degradation [9]. In soil, many microorganisms and plants play the major role in biodegradation and elimination of Atrazine and other pesticides converting them into simpler non-toxic compounds whereas physical and chemical forces have limited effects on pesticides degradation [10,11]. Type of soil determines the mobility and fate of Atrazine through sorption to soil particles. Atrazine is more readily adsorbed on muck or clay soils than on soils of low clay and organic matter content; therefore, adsorption to certain soil constituents significantly limits the downward movement or leaching [12-14].
The best-characterized organisms that degrade Atrazine are of Pseudomonas spp. as well as other bacteria [15,16], especially when experienced previous exposure to the pesticide or its analogue [17]. Bioremediation technique is more environmentally safe practice particularly for plant protection and its use should lead to an almost complete disappearance of pesticide pollution [18,19]. For biodegradation of Atrazine, bioaugmentation or biostimulation are the two options exist [20]. This was confirmed by the study of Chelinho et al., [16] when combining bioaugmentation with Pseudomonas sp. ADP and biostimulation with citrate as an efficient bioremediation tool for Atrazine-contaminated soils. Also plant species with well-developed fibrous rooting systems are most effective in enhancing degradation rates and increasing microbial counts within their rhizosphere [11]. Co-application of glufosinate with nitrogen fertilizers may alter Atrazine cometabolism and extended the herbicide’s residual weed control in adapted soils [21,22]. Similarly, the presence of other herbicide such as alachlor altered Atrazine persistence in soil compared to other herbicides [23].
The main objective of the present study was to investigate the remediation capability of the indigenous microbial population in loam-sand soil cultivated with corn maize crop and treated with Atrazine. Levels of degradation using enriched soil natural microorganisms in simulated field conditions system was monitored during cultivation course and the dissipation rate of the Atrazine was estimated.
Cell (I): Control (sterile soil, i.e. no indigenous or exogenous microbes)
Cell (II): Biostimulation (i.e. soil with only the indigenous microorganisms+the addition of nutrients).
Cell (III): Bioaugmentation (i.e. soil with the indigenous microorganisms augmented by exogenous microorganisms to help the indigenous species in the biodegradation of the herbicide without the addition of nutrients).
Cell (IV): A combination between Biostimulation and Bioaugmentation (i.e. indigenous soil microbes+exogenous microorganisms+addition of nutrients).
Bioremediation of contaminated Egyptian (SoilE) and Saudi (SoilH) in system I and II: Atrazine-free Egyptian (soilE) and Saudi (soilH) was divided and dispensed into 8 cells (25x25x15 cm plastic basin each) where different bioremediation technologies were applied. Cell (I) was considered as control with sterile soil autoclaved at 121°C for 20 minutes to kill and prevent the growth of the indigenous microorganisms. In cell (II) biostimulation technology was applied where soil was enriched with nutrients ((NH4)2SO4] and K2HPO4 at 250 and 100 mg/kg respectively) to enhance the growth and activity of the indigenous microorganisms [25]. Bioaugmentation technology was applied to the soil in cell (III) where the most promising indigenous and exogenous Atrazine degraders were seeded at definite ratios to accelerate and help the indigenous bacterial population to achieve high and fast remediation of the contaminated soil. A combination of biostimulation and bioaugmentation technologies was applied to the soil of cell (IV) to investigate the synergistic or suppressive effects of the two techniques [26]. Each cell was filled with (5 kg) of soil where pre-emerge recommended dose of Atrazine was applied (750 g/200 to 600 L water/Fadden).
Bacterial seeding in cells III and IV was carried out by preparing bacterial mixed culture (125 ml nutrient broth) diluted in 250 ml saline solution (0.85%) and poured evenly all around the cell to ensure that they reached the whole cell. Since all the investigated bacteria are aerobic, oxygen supply was very important for microbial growth so that tilling technique was introduced to the 8 cells 5 times per week by spade to provide maximum oxygen. Sterilized distilled water was added to the 8 cells once a week to sustain wetness and avoid dryness or flooding both of which have deleterious effect on bioremediation process and microbial growth. Water poured evenly all around the cells and mixed well with the soil to ensure even distribution and avoid accumulation of water in the bottom of the cell. The total volume of solution added (inoculum, Atrazine, water) was calculated to bring the soil water content to 60% of its moisture holding capacity. Then soils in the 8 cells were thoroughly mixed with Atrazine to form homogenous medium, and incubated in the dark at room temperature. Four replicates of 50 g soil sample each were collected from each cell at 3 days interval till the end of the experiment duration (4 weeks) where Atrazine residues were extracted and determined using GC. Removal efficiency of Atrazine by the selected technologies was calculated and compared.
Extract clean up: The clean-up procedure was done according to the method of Mills et al., [28]. A mixture of an elution solvent system (50% methylene chloride, 1.5% acetonitrile, 48.5% hexane (v/v/v) was used. A chromatographic column containing to gm activated florisil, then the residues from the column were eluted with 200 ml of this mixture. The eluent was evaporated just to dryness as previously described and the residues were ready for chromatographic determination after being re-dissolved in an appropriate volume of ethyl acetate.
Gas liquid chromatography determination: Hewlett Packard GC model 6890 equipped with nitrogen phosphorus Ni63 electron capture detector was used for Atrazine analysis. GC conditions were as follows: PAS-5 capillary column (30 m length×0.32 mm internal diameter (id) ×0.52 µm film thickness), 5% phenyl methyl polysiloxane. N2 at flow rate of 3 ml/min was used as carrier gas. Injector and detector temperature were 230°C and 280°C respectively. The initial column temperature was 180°C for 2 min, raised at 3°C/min, and then held at 200°C for 15 min.
Calibration curve: Stock solution (100 ppm) of Atrazine was prepared in ethyl acetate. Matrix matched calibration standard at the concentration of 2.5, 5, 10 and 40 mg/kg were prepared. Each concentration was injected under the mention chromatographic conditions. The peak area was plotted against each concentration values of r2 was 0.9994.
Preparation of blank solution: Solvent and the anhydrous sodium sulphate used in the extraction and clean up were subjected also to recovery test to detect any possible traces of the Atrazine in the solvents.
As a general trend, Atrazine residues (RC) decreased with increasing exposure times regardless soil type or treatment used.
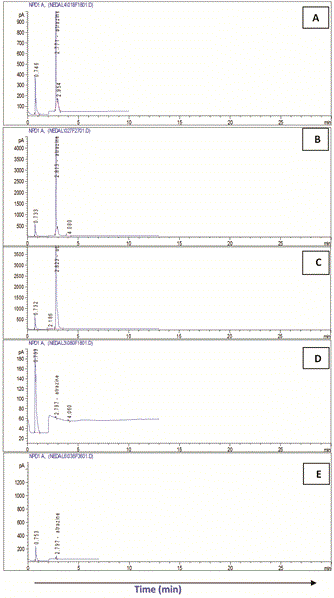
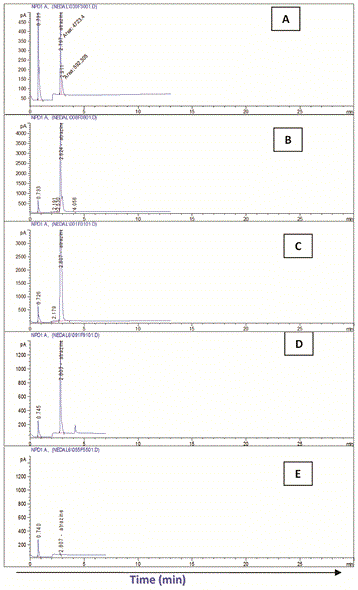
The highest Atrazine residue levels (RC) were detected at the zero time in all treatments. In system I (soilE), Atrazine residue ranged between the highest (30.94 mg/l) in cell I and the lowest (27.43 mg/l) in cell II while in system II (soilH) it was almost the same in the four treatment (30.03-30.85 mg/l).
With time, regular biodegradation took place leading to continuous reduction in Atrazine levels in both systems. On the other hand, significant variations were detected in Atrazine biodegradation among the different treatment in both systems.
In both systems (soilE and soilH), treatment no IV considered the most effective recording the highest removals of Atrazine followed by treatment III and finally II compared to the control which showed the lowest removals.
In that respect, biodegradation of Atrazine and removal efficiency (RE) in soilE using treatment IV recorded 99.95% after 15 day. This was followed by treatment III (RE: 99.90%) and finally II (RE: 99.85%) compared to the control which showed the lowest removals (RE: 99.77%) after the same exposure time.
Similarly, in soilH, treatment IV recorded Atrazine RE of 99.96% after 15 day followed by treatment III (RE: 99.94%) and finally II (RE: 99.92%) compared to the control which showed the lowest removals (RE: 99.1%) after the same exposure time.
In both systems, Atrazine was not detected after 18 days except in the control soils and in soilH using treatment II.
In conclusion, the combination between biostimulation and bioaugmentation remediation technologies considered the most efficient in removing Atrazine contaminants in both systems compared to the other tested technologies. This combination reduced the residues to its lowest level 0.015 mg/l in soilE (system I) and 0.012 mg/l in soilH (system II) after only 15 day.
Comparing these results indicated that, removal of the Atrazine herbicide from the contaminated soils achieved by the investigated remediation technologies in the following efficiency order: Biostimulation and Bioaugmentation>Bioaugmentation> Biostimulation>Natural (volatilization).
Cell (I); control; Cell (II): Biostimulation; Cell (III): Bioaugmentation; Cell (IV): Acombination between Biostimulation & Bioaugmentation;ND: Not detected below the detection limit (0.01 mg/l)
Results indicated clearly that combination between biostimulation and bioaugmentation remediation technologies considered the most efficient and best choice in removing Atrazine contaminants in both systems compared to the other tested technologies. This combination reduced the residues to its lowest levels of 0.015 mg/l in soilE (system I) and 0.012 mg/l in soilH (system II) after only 15 day. These results are consistent with those of other workers where microbial biodegradation is considered one of the most viable options for remediating heavy metal-contaminated groundwater [33] and organophosphate pesticides-contaminated environment [34,35].
The initial level of soil contamination controls to a large extent the rate of bioremediation and biodegradation of atrazine. Lima, et al., [30] reported that bioaugmentation with Pseudomonas sp. ADP (9 ± 1×107 CFU g-1) resulted in rapid atrazine removal (99% from an initial level of 7.2 ± 1.6 µg g-1) after 8 d independent of citrate. Elevating the initial level to 200×RD of atrazine but with coupling bioaugmentation of Pseudomonas sp. ADP with 2.4 mg g-1 citrate amendment (biostimulation) resulted in improved biodegradation efficiency of atrazine (87%) compared to bioaugmentation alone (79%).
These results suggested that bioremediation is valuable tool for efficient removal of atrazine from contaminated field soils to minimize atrazine and its chlorinated derivatives from reaching water compartments. In a similar manner, the combination of bioaugmentation-biostimulation approach coupled with rye cultivation showed the most profound effect on Trinitrotoluene (TNT) degradation, a commonly used explosive for military and industrial applications, which cause serious environmental pollution after 28 day [31].
The high efficiency of biostimulation and/or bioaugmentation was also confirmed for biodegradation of petroleum hydrocarbons. Amendment of indigenous microbial populations with a lignocellulosic substrate and bioaugmentation with two strains of white-rot fungi (i.e., Trametes versicolor and Lentinus tigrinus) enhanced the native microbiota which promoted the highest biodegradation of creosote, even of those with five aromatic rings after 60 treatment days [32]. Importance of biostimulation in the bioremediation of soils contaminated with Atrazine is well documented. Degradation of 14C ring labelled atrazine was monitored in laboratory incubations of soils supplemented with 0, 10, 100 and 1000 µg g-1 sucrose concentrations. Another experiment was carried out to determine the effect of carbon amendment on total microbial biomass and soil respiration with different concentrations of sucrose and non-labelled atrazine. Atrazine dealkylation was enhanced in treatments with 100 and 1000 µg g-1of sucrose added. Hydroxyatrazine (HA) metabolite was formed in the control (no sucrose) and in the presence of 10 µg g-1 of sucrose, whereas desethylatrazine (DEA) was only detected in treatment with 1000 µg g-1 sucrose. Results indicate that total microbial biomass increased significantly (P<0.001) with the addition of 1000 µg g-1 sucrose [36].
Bioaugmentation may be done by single or many microorganisms. Bioaugmentation of soil with mixed bacterial consortium enhanced the rate of atrazine degradation in a highly polluted soil [37]. Zhang et al. (2012) [38] reported isolation of four-member mutualistic bacterial consortium (DNC5) capable of metabolizing atrazine from corn-planted soil. Arthrobacter sp. DNS10 found to be the primary organism capable of mineralizing atrazine to cyanuric acid. Bacillus subtilis DNS4 and Variovorax sp. DNS12 are secondary strains that utilized cyanuric acid during atrazine degradation process. The growth and the degradation rate of the consortium DNC5 were faster than that of the single strain DNS. The high degradation ability of the consortium showed good potential for atrazine biodegradation. This information contribute toward a better understanding about metabolic activities of atrazine degrading consortium, which are generally considered to be responsible for atrazine mineralization in the natural environment.
Therefore, the present results supported by other workers clearly indicated that bioaugmentation coupled with biostimulation is a powerful, economical and environmentally friendly bioremediation technique that is very promising for decontamination of Atrazine-contaminated soils.
References
- European Parliament EU (2010) Sustainable use of pesticides: STOA study. Brussels.
- Randall -Amster AJD (2010) Silent Spring Has Sprung: Truth out PhD Synonyms and brand names. Peace and Justice Studies Association.
- Ackerman F (2007) The economics of atrazine. See comment in PubMed Commons below Int J Occup Environ Health 13: 437-445.
- Extension Toxicology Network (1996) Pesticide Information Profile: Atrazine (Cooperative Extension Offices of Cornell University, Oregon State University, the University of Idaho, and the University of California at Davis and the Institute for Environmental Toxicology, Michigan State University.
- Duhigg C (2009) Debating how much weed killer is safe in your water glass. The New York Times.
- Dos Santos LB1, Abate G, Masini JC (2004) Determination of atrazine using square wave voltammetry with the Hanging Mercury Drop Electrode (HMDE). See comment in PubMed Commons below Talanta 62: 667-674.
- Reigart JR, Roberts JR (1999) Recognition and Management of Pesticide Poisonings. (5th edition), Washington, DC, USA.
- United States Environmental Protection Agency (US EPA) (2003) Guidelines on ecological risk assessment: Atrazine CAS # 1912-24-9, Agency for Toxic Substances and Disease Registry (ATSDR): September 2003.
- Swann RL, Eschenroeder AE (1983) Fate of chemicals in the environment: American Chemistry Symposium. Series 225, American Chemistry Society, Washington, DC, USA.
- (2009) Soil microorganisms in biodegradation of pesticides and herbicide. My agriculture information bank.
- Marecik R, Króliczak P, Czaczyk K, Białas W, Olejnik A, et al. (2008) Atrazine degradation by aerobic microorganisms isolated from the rhizosphere of sweet flag (Acorus calamus L.). See comment in PubMed Commons below Biodegradation 19: 293-301.
- Dehghani M, Nasseri S, Amin S, Naddafi K, Yunesian M, et al. (2005) Atrazine adsorption desorption behaviour in Darehasaluie Kavar corn field soil in Fars province of Iran. Iran J Environ Health Sci Eng 2: 221-228.
- Correia FV, Macrae A, Guilherme LR, Langenbach T (2007) Atrazine sorption and fate in a Ultisol from humid tropical Brazil. See comment in PubMed Commons below Chemosphere 67: 847-854.
- Danrong Z, Haixia D, Yiqing G, Yanan T (2009) Comparison of atrazine sorption and degradation properties in packed and intact soil columns: Proceedings of 3rd International Conference on Bioinformatics and Biomedical Engineering, June 11-13, Beijing, 1-3.
- Zeng Y, Sweeney CL, Stephens S, Kotharu P (2004) Atrazine pathway map. Wackett, LP. Biodegradation Database.
- Chelinho S, Moreira-Santos M, Lima D, Silva C, Viana P, et al. (2010) Cleanup of atrazine-contaminated soils: ecotoxicological study on the efficacy of a bioremediation tool with Pseudomonas sp. ADP. J Soil Sediment 10: 568-578.
- Krutz LJ, Shaner DL, Accinelli C, Zablotowicz RM, Henry WB (2008) Atrazine dissipation in s-triazine-adapted and nonadapted soil from Colorado and Mississippi: implications of enhanced degradation on atrazine fate and transport parameters. See comment in PubMed Commons below J Environ Qual 37: 848-857.
- Hobbs PR, Sayre K, Gupta R (2008) The role of conservation agriculture in sustainable agriculture. See comment in PubMed Commons below Philos Trans R Soc Lond B Biol Sci 363: 543-555.
- Gregoire C, Elsaesser D, Huguenot D, Lange J, Lebeau T, et al. (2008) Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems: A Review. Environmental Chemistry Letters 2: 293-338.
- Wackett LP , Sadowsky MJ, Martinez B, Shapir N (2002) Biodegradation of atrazine and related s-triazine compounds: from enzymes to field studies. See comment in PubMed Commons below Appl Microbiol Biotechnol 58: 39-45.
- Zablotowicz RM, Krutz LJ, Weaver MA, Accinelli C, Reddy KN (2008) Glufosinate and ammonium sulfate inhibit atrazine degradation in adapted soils. Biology and Fertility of Soils 45: 19-26.
- Ouyang Y, En Zhang J, Lin D, Liu GD (2010) A Stella model for the estimation of atrazine runoff, leaching, adsorption, and degradation from an agricultural land. J Soil Sediments 10: 263-271.
- Chirnside AEM, Ritter WF, Radosevich M (2009) Biodegradation of aged residues of atrazine and alachlor in a mix-load site soil. Soil Biol Biochem 41: 2484-2492.
- El-Bestawy E, Saber J, Mansy AH, Zabermawi N (2013) Isolation, identification and acclimatization of Atrazine-resistant soil bacteria. Annals of Agricultural Science 58: 119-130.
- Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresource Technology 96: 1049-1055.
- Chaineau CH , Yepremian C, Vidalie JF, Ducreux J, Ballerini D (2004) Bioremediation of crude oil-polluted soil: Biodegradation, leaching and toxicity assessments. Water, Air & Soil Pollution 144: 419-440.
- Polese L, Dores EFGC, Jardim EFG, Navickene S, Ribeiro ML (2002) Determination of herbicides residues in soil by small scale extraction. Eclética Química 27, São Paulo.
- Mills PA, Bong BA, Kamps LR, Burke JA (1972) Elution solvent system for florisil column cleanup in organochlorine pesticide residue analyses. See comment in PubMed Commons below J Assoc Off Anal Chem 55: 39-43.
- Tyagi M, da Fonseca MM, de Carvalho CC (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. See comment in PubMed Commons below Biodegradation 22: 231-241.
- Lima D, Viana P, André S, Chelinho S, Costa C, et al. (2009) Evaluating a bioremediation tool for atrazine contaminated soils in open soil microcosms: the effectiveness of bioaugmentation and biostimulation approaches. See comment in PubMed Commons below Chemosphere 74: 187-192.
- Nolvak H, Truu J, Limane B, Truu M, Cepurnieks G, et al. (2013) Microbial community changes in TNT spiked soil bioremediation trial using biostimulation, phytoremediation and bioaugmentation. J Environmental Engineering and Landscape Management, Vilnius Gediminas Technical University 2013. High Beam Research.
- Llado S, Covino S, Solanas AM, Viñas M, Petruccioli M, et al. (2013) Comparative assessment of bioremediation approaches to highly recalcitrant PAH degradation in a real industrial polluted soil. See comment in PubMed Commons below J Hazard Mater 248-249: 407-14.
- Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. See comment in PubMed Commons below J Environ Manage 92: 2355-2388.
- Burbank MB, Weaver TJ, Williams BC, Crawford RL (2012) Urease activity of ureolytic bacteria isolated from six soils in which calcite was precipitated by indigenous bacteria. Geomicrobiology Journal 29: 389-395.
- Chishti Z, Hussain S, Arshad KR, Khalid A, Arshad M (2013) Microbial degradation of chlorpyrifos in liquid media and soil. See comment in PubMed Commons below J Environ Manage 114: 372-380.
- Ngigi AN, Getenga ZM, Dörfler U, Boga HI, Kuria B, et al. (2013) Effects of carbon amendment on in situ atrazine degradation and total microbial biomass. See comment in PubMed Commons below J Environ Sci Health B 48: 40-48.
- Hashemi H (2013) Study of the bioremediation of atrazine under variable carbon and nitrogen sources by mixed bacterial consortium isolated from corn field soil in Fars Province of Iran. J of Environmental and Public Health.
- Zhang Y, Cao B, Jiang Z, Dong X, Hu M, et al. (2012) Metabolic ability and individual characteristics of an atrazine-degrading consortium DNC5. See comment in PubMed Commons below J Hazard Mater 237-238: 376-81.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16336
- [From(publication date):
September-2014 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 11610
- PDF downloads : 4726
