Complexities in Antifungal Pharmacotherapy: A Comprehensive Pharmacovigilance Analysis
Received: 24-May-2024 / Manuscript No. IJRDPL-24-137173 / Editor assigned: 27-May-2024 / PreQC No. IJRDPL-24-137173 (PQ) / Reviewed: 12-Jun-2024 / QC No. IJRDPL-24-137173 / Revised: 04-Jun-2025 / Manuscript No. IJRDPL-24-137173 (R) / Published Date: 11-Jun-2025
Abstract
This project report provides a comprehensive overview of the pharmacovigilance of antifungal drugs, focusing on regulatory considerations, risk management strategies, key findings from pharmacovigilance reports and future directions in antifungal therapy. The report highlights the importance of continuous monitoring and assessment of antifungal drugs to ensure their safe and effective use. Key findings include the increased risk of gastrointestinal adverse effects, hepatotoxicity, renal impairment, allergic reactions and potential drug interactions associated with antifungal drugs. Recommendations for safe use include adherence to recommended dosages, limitation of prolonged use, awareness of potential adverse effects, individualized risk assessment and patient education. The report concludes with a call for ongoing surveillance, research and development of novel antifungal agents to address evolving challenges in antifungal therapy and ensure patient safety.
Keywords: Antifungal drugs; Pharmacovigilance; Regulatory considerations; Risk management strategies; Key findings; Future directions; Drug safety; Adverse effects; Drug interactions; Hepatotoxicity; Renal impairment; Allergic reactions
Keywords
Antifungal drugs; Pharmacovigilance; Regulatory considerations; Risk management strategies; Key findings; Future directions; Drug safety; Adverse effects; Drug interactions; Hepatotoxicity; Renal impairment; Allergic reactions
Introduction
Invasive fungal infections pose a significant global health concern, leading to approximately 1.7 million deaths annually. These infections are particularly prevalent in immunocompromised individuals, such as those undergoing chemotherapy, organ transplantation or living with acquired immune deficiency syndrome. The annual incidence rates of invasive aspergillosis, candidiasis and mucormycosis are estimated to be over 300,000, 750,000, and 10,000 cases, respectively. Including Indian data estimates, the incidence of mucormycosis may exceed 900,000 cases per year. These infections are associated with high mortality rates, which vary widely due to the lack of comprehensive global data, ranging from 30% to 95% for invasive aspergillosis and 46% to 75% for candidiasis. Disseminated scedosporiosis and fusariosis have an overall incidence of one or six cases per 1000 hematopoietic stem cell transplant recipients [1].
Clinicians and veterinarians currently use four classes of antifungal drugs for systemic treatment. These classes target different components of the fungal cell. The polyene class includes Amphotericin B (AMB), which interacts with ergosterol, a major component of the fungal cell membrane. AMB is highly effective against Candida species, Aspergillus fumigatus and A. flavus. Triazoles, including first and second-generation drugs, disrupt ergosterol biosynthesis at the lanosterol demethylation step. Triazoles are generally fungistatic against yeasts but fungicidal for Aspergillus species. Echinocandins inhibit the synthesis of β-D-glucans in the fungal cell wall and exhibit fungicidal or fungistatic effects against Candida and Aspergillus species, respectively. Flucytosine (5-FC), a pyrimidine analog, acts at the nucleus level of the fungus, affecting protein and DNA biosynthesis. The overuse of antifungal agents can increase resistance among opportunistic pathogens, a concern identified by the World Health Organization as one of the dominant threats in 2019.
Materials and Methods
The WHO developed a list of priority fungal pathogens to guide strategies against fungal infections and antifungal resistance. Fungal infections have surged recently, particularly among immunocompromised patients.
Additionally, the burden of fungal infections increased during the COVID-19 pandemic, leading to higher morbidity and mortality rates. Approximately 150 million people are infected with fungal infections annually, resulting in around 1.7 million deaths. By 2023, over 6.5 million annual cases and about 3.8 million deaths from fungal infections were recorded [2].
Scope of the report
The scope of this report is to provide a comprehensive overview of the epidemiology, clinical impact and pharmacovigilance considerations related to invasive fungal infections and the use of antifungal drugs for systemic treatment. The report aims to:
• Background information on antifungal drugs
• Methodology (description of data collection and analysis methods)
• Overview of antifungal drugs: Classification, mechanism and indications
• Pharmacovigilance in antifungal therapy
• Adverse events associated with antifungal drugs
• Safety profiles of major antifungal drugs
• Drug interactions
• Risk management strategies
• Special populations
• Future directions and emerging issues: Development of novel antifungal agents
• Anticipated safety challenges
Antifungal drugs: Overview
Antifungal: The pharmaceuticals used to treat fungi infections are known as antifungal drugs. Antifungal medications are available for oral consumption, topical use and intravenous drip administration
Activity of antifungal drug: Antifungal drugs often kill the fungus or prevent it from developing and proliferating. The fungal cell membrane and the fungal cell wall are among the components of the cell that the antifungal medications target. Both of these cell defence mechanisms have the potential to be damaged, causing the cell to leak and perish. Antifungal medications can target the fungi without hurting the body's cells because human bodies lack these structures [3].
Types of fungal infections
Common fungal infections
• Ringworm (Tinea/Dermatophytosis): A fungal skin infection caused by various fungi species, affecting any part of the skin, including the scalp and feet.
• Oral thrush: Caused by Candida yeast, it occurs when the mouth, throat or esophagus is disrupted.
• Vaginal yeast infection: Caused by Candida yeast, it occurs when there is an imbalance in the vaginal environment.
• Onychomycosis: Fungal infection of the nails, more commonly affecting toenails.
• Coccidioidomycosis (Valley fever): Caused by a fungus found in the soil of the south-western United States.
Less common but serious fungal infections
• Aspergillosis
• Blastomycosis
• Cryptococcus gattii Infection
• Fungal meningitis
• Fungal pneumonia
• Histoplasmosis
• Mucormycosis
• Paracoccidioidomycosis
• Talaromycosis
Symptoms of common fungal infections
• Ringworm: Ring-shaped rash, itchy, red, scaly, cracked skin.
• Oral thrush: White spots on tongue, mouth, throat, redness, pain when swallowing or eating.
• Jock itch: Red, scaly, itchy patches, mainly in the inner thighs.
• Athlete's foot: Red, swollen, itchy, occasionally peeling skin between toes.
• Vaginal yeast infection: Painful urination, painful intercourse, discomfort, abnormal vaginal discharge.
• Onychomycosis: Thick, discolored, brittle or chipped nails.
• Coccidioidomycosis (Valley fever): Fatigue, muscle aches, headache, shortness of breath, night sweats, rash, cough.
Classification of antifungal drugs
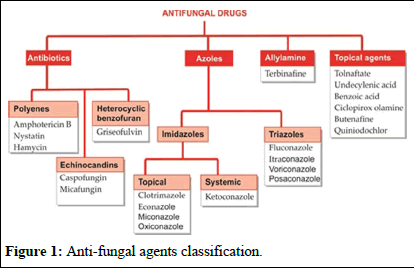
Many topical antifungals have been available since the antiseptic era. Two important antibiotics, amphotericin B, to deal with systemic mycosis, and griseofulvin to supplement attack on dermatophytes were introduced around 1960. Antifungal property or flucytosine was noted in 1970, but it could serve only as a companion drug to amphotericin. The development of imidazoles in the mid-1970s and triazoles in the 1980s provided safer and more convenient alternatives to amphotericin B and griseofulvin. Terbinafine is a novel antifungal. A group of potent semisynthetic antifungal antibiotics, the echinocandins are the latest addition (Figure 1) [4].
Figure 1: Anti-fungal agents classification.
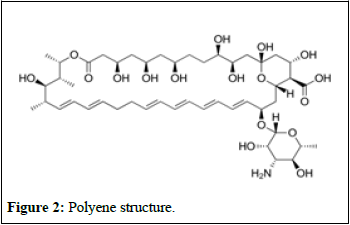
Disruptor fungal membranes: Polyenes, a group of molecules known as macrolides, have a unique structure consisting of a ring with 20-40 carbon atoms connected to a mycosamine group (Figure 2) [5].
Figure 2: Polyene structure.
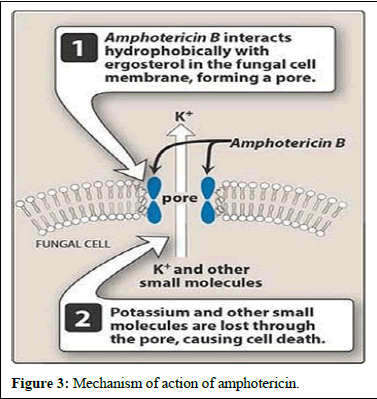
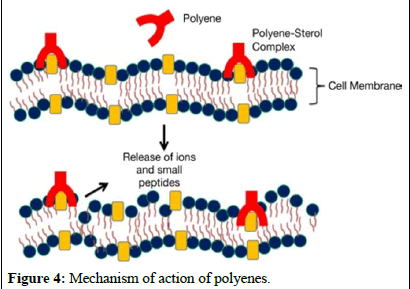
These molecules bind to ergosterol, a component of fungal cell membranes, forming complexes that create pores in the membrane, ultimately leading to cell death. Recent studies suggest that polyenes like amphotericin B bind with ergosterol, destabilizing its function and causing cell demise. Polyenes have broad-spectrum activity against fungi and are used in medical practice, with three polyenes currently used in clinical settings.
Nystatin and natamycin are effective against various bacterial species and are commonly used for vaginal and esophageal infections. Amphotericin B targets yeasts and filamentous fungi, making it a versatile antifungal agent.
Polyenes, including nystatin, natamycin and amphotericin B, share similarities with cholesterol. While nystatin and natamycin have limited absorption and significant toxicity, amphotericin B is widely used for infections and is administered intravenously due to minimal gastrointestinal absorption.
Efforts have been made to reduce the toxicity associated with polyenes by developing synthetic polyenes with improved water
solubility and lower toxicity compared to amphotericin B. Additionally, advancements in formulating amphotericin B, such as lipid-based formulations like liposomes, have been successful in enhancing efficacy while reducing harm to patients. Further research is underway to explore formulations aimed at improving the properties of amphotericin B (Figures 3 and 4) [6].
Figure 3: Mechanism of action of amphotericin.
Figure 4: Mechanism of action of polyenes.
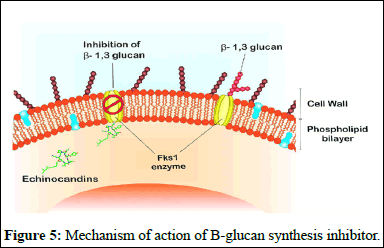
B-glucan synthesis inhibitors: Echinocandins are a type of antifungal drug that targets the cell wall synthesis of fungi. They work by inhibiting the enzyme complex responsible for making b-D glucan, a key component of the fungal cell wall. Echinocandins, including caspofungin, micafungin and anidulafungin, are derived from natural fungal products and have been modified chemically to enhance their antifungal properties [7].
These drugs are effective against a variety of fungal infections, including Candida and Aspergillus species. They are safe and have low toxicity because they target an enzyme that is absent in mammalian cells, minimizing interactions with other medications. However, they must be administered intravenously in hospital settings due to their large molecular weights and limited oral absorption.
Combining echinocandins with other antifungal drugs, such as azoles or amphotericin B, may increase their effectiveness against fungal pathogens. Development of echinocandins is important as they have shown to be more effective against a wide range of fungal infections compared to other antifungal agents (Figure 5) [8].
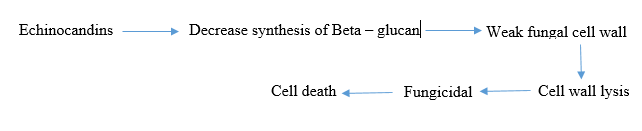
Figure 5: Mechanism of action of B-glucan synthesis inhibitor.
Chitin synthesis inhibitors: Around 3% of the cell wall synthesis is made up of chitin, a chain of N-acetylglucosamine molecules linked with glucan molecules. Enzymes called chitin synthases are responsible for producing chitin, which strengthens the cell wall and maintains osmotic balance. Since chitin is unique to fungi, it becomes a target for antifungal drugs [9].
Nikkomycins and Polyoxins are studied for their ability to inhibit chitin synthase, disrupting cell wall formation. Nikkomycin is effective against pathogens with high chitin content but not against Candida albicans or C. tropicalis. Polyoxins, on the other hand, target fungi harmful to plants.
These compounds attach to the site where chitin synthase operates and compete with UDP N-acetylglucosamine. However, their use in treatments is limited because they degrade easily and are not very effective inside living organisms. Development of polyoxins was halted in 2012, while Nikkomycin Z is currently undergoing trials.
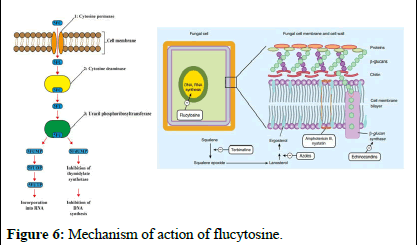
Nucleic acid synthesis inhibitors: Flucytosine (5-FC), also known as 5-fluorocytosine, is a medication that inhibits fungal growth by interfering with the use of substances like pyrimidine and proteins. It enters fungal cells through a channel called cytosine permease and converts into 5-fluorouracil (5-FU). This 5-FU disrupts RNA production by replacing the usual building block, UTP, which hampers protein synthesis and growth in fungi. Moreover, 5-FU can transform into 5-fluorodeoxyuridine monophosphate (5-FdUMP), which inhibits an enzyme called thymidylate synthase, affecting DNA synthesis and preventing proper division of fungal nuclei. Flucytosine specifically targets fungi because mammalian cells have limited ability to convert it into its active form [10].
While flucytosine is effective against Candida and Cryptococcus strains in laboratory and animal studies, its action is mainly limited to yeasts, as filamentous fungi lack the specific enzyme thymidylate synthase. Flucytosine resistance is common, so it is often used as part of a treatment regimen rather than as the primary treatment (Figure 6).
Figure 6: Mechanism of action of flucytosine.
Protein biosynthesis inhibitors: Tavaborole, approved by the FDA in 2014, treats toenail onychomycosis caused by Trichophyton rubrum and T. mentagrophytes. It combats fungi, including yeasts, molds and dermatophytes, by inhibiting leucyl synthetase, an enzyme crucial for protein synthesis. Tavaborole binds to the editing site of this enzyme alongside tRNA, blocking the transfer of acids to the ribosome and effectively halting protein synthesis [11].
Another group of agents targeting protein biosynthesis is β-amino acids, inhibiting isoleucyl synthetase. Cispentacin, derived from Bacillus cereus culture broth and its derivative Icofungipen, has strong antifungal properties against Candida albicans. Sordarin, discovered in 1969 and initially isolated from Sordaria araneosa, disrupts the function of translation Elongation Factor 2 (EF2) while sparing its equivalent. The effectiveness of sordarin analogs depends on the group (R) positioned in the 30th position.
Scientists have discovered strains producing variations of Sordarin analogs, leading to developing of semi-synthetic derivatives with unique targeting abilities. The potential of sordarin derivatives makes them an exciting prospect for further research.
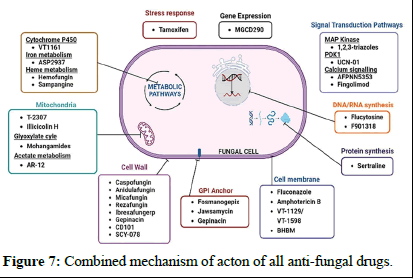
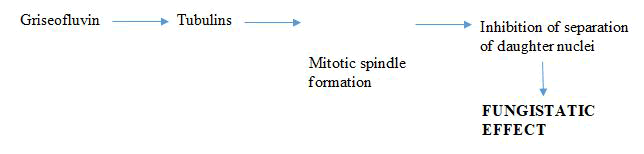
Microtubule biosynthesis inhibitors: Microtubules are structures responsible for the cell's shape, comprising alpha and beta tubulin units. Some antifungal drugs, like griseofulvin and vinblastine, disrupt these structures. Griseofulvin, isolated from Penicillium griseofulvin in 1939, is one of the earliest antifungal compounds. However, it can cause liver toxicity and is mainly effective against dermatophyte fungi, which cause conditions like ringworm and athlete's foot. Griseofulvin works by binding to tubulin, disrupting microtubule assembly and preventing cell division (Table 1 and Figure 7) [12].
| S. no. | Compound | Mode of action | Indication |
| 1 | Isavuconazole | Inhibits fungal cytochrome P450-dependent enzymes, particularly CYP51 | Invasive aspergillosis, mucormycosis |
| 2 | VT-1161 | Targets fungal CYP51 and disrupts ergosterol biosynthesis | Vulvovaginal candidiasis |
| 3 | VT1129 | Biosynthesis of ergosterol | Cryptococcal meningitis |
| 4 | Fluconazole | Inhibition of fungal cytochrome P450-dependent enzymes | Candidiasis, cryptococcal meningitis, prophylaxis in immunocompromised patients |
| 5 | Amphotericin B | Binds to fungal cell membrane ergosterol, causing membrane disruption | Invasive fungal infections, systemic candidiasis, cryptococcal meningitis |
| 6 | Caspofungin | Inhibits fungal cell wall synthesis by targeting β-(1,3)-D-glucan synthase | Invasive candidiasis, invasive aspergillosis |
| 7 | Voriconazole | Inhibits fungal cytochrome P450-dependent enzymes, particularly CYP51 | Invasive aspergillosis, candidiasis |
| 8 | Posaconazole | Inhibits fungal cytochrome P450-dependent enzymes, particularly CYP51 | Invasive aspergillosis, candidiasis |
| 9 | Echinocandins | Inhibit fungal cell wall synthesis by targeting β-(1,3)-D-glucan synthase | Invasive candidiasis |
| 10 | Nikkomycin Z | Inhibits chitin synthase, affecting fungal cell wall integrity | Experimental, preclinical studies |
| 11 | Rezafungin | Inhibits fungal protein synthesis by targeting the 60S ribosomal subunit | Invasive candidiasis, invasive aspergillosis |
Table 1: Mode of action of antifungal agents.
Figure 7: Combined mechanism of acton of all anti-fungal drugs.
Pharmacology and toxicity of antifungal agents
Antifungal drugs work in different ways and have varying effects on the body. Amphotericin B, for example, is initially combined with sodium deoxycholate to improve its solubility. Once in the body, it separates from deoxycholate, binds to lipoproteins and accumulates in the spleen and liver. It has a long half-life and is excreted unchanged in the urine and feces. However, it can cause toxic reactions due to infusion and high doses, affecting the kidneys. To reduce these side effects, new formulations of amphotericin B have been developed [13].
On the other hand, 5-flucytosine is rapidly absorbed and has good bioavailability. It is metabolized minimally by the liver and eliminated through the kidneys. However, it can cause severe side effects such as liver toxicity, myelotoxicity and gastrointestinal problems.
Triazoles, another group of antifungals, vary in their pharmacological properties. They can be taken orally or intravenously and their metabolism and side effects differ. For example, voriconazole can cause hepatotoxicity, while isavuconazole may affect the QT interval. Triazoles can also interact with other drugs due to their effect on certain enzymes (Table 2) [14].
| Triazole | FLC | ITC | VOR | POS | ISV |
| Bioavailability (%) | 90 | 55 | 90-96 | Variable | >98 |
| Protein binding (%) | 11-12 | 99 | 51-67 | >98 | >99 |
| Metabolism (CYP) | - | 3A4 | 2C19/2C9/3A4 | Glucuronidation | 3A4/3A5 |
| Half-life (h) | 27-37 | 15-42 | 6 | 15-35 | 80-130 |
| Route of elimination | Renal | Hepatic | Hepatic | Hepatic | Hepatic |
| Unchanged (%) | 64-90 | Mar-18 | <2 | 66 | 45 |
Table 2: Pharmacological properties of triazoles.
Echinocandins are only available for intravenous use. They have high protein binding and minimal metabolism by CYP450 enzymes. Each echinocandin has a different half-life and degradation process. Caspofungin has the shortest half-life, while anidulafungin has the longest. Micafungin undergoes metabolism to three metabolites. Echinocandins have fewer drug interactions due to their limited effect on CYP450 enzymes (Table 3) [15].
| Echinocandin | ANF | CSF | MCF |
| Bioavailability (%) | 2-7 (p.o.), 100 (iv.) | 100 (iv.) | 100 (iv.) |
| Protein binding (%) | 99 | 95 | >99 |
| Metabolism (CYP) | - | - | 3A |
| Half-life (h) | 40-50 | 8 | 13-20 |
| Route of elimination | Hepatic | Renal | Hepatic |
| Unchanged (%) | 10 | 1.4 | <1 |
Table 3: Pharmacological properties of echinocandins.
Dosing antifungal drugs requires careful consideration of the patient's condition and the type of infection. Dosage recommendations vary for different patient populations, such as neonates, pediatrics and adults. Pregnant women pose a challenge due to limited data on the effects of antifungals on fetal development. Amphotericin B is considered the safest option for treating systemic fungal infections in pregnant women. Dosage adjustments may be necessary for obese patients, as some antifungals are dosed based on total body weight [16].
Susceptibility of antifungal agents
Antifungal Susceptibility Testing (AST) is crucial for determining the best treatment and detecting antifungal resistance. AST is done using reference or commercial methods based on broth microdilution or agar-based assays, standardized by EUCAST and CLSI. While broth microdilution is the gold standard, commercial methods are faster and cheaper.
Microorganisms are classified as Susceptible (S), susceptible with increased exposure (I) or Resistant (R) based on the Minimum Inhibitory Concentration (MIC) of the drug. CLSI also uses a Susceptible Dose-Dependent (SDD) category. EUCAST provides breakpoints for Aspergillus and Candida, but CLSI lacks breakpoints for molds.
ESCMID guidelines recommend antifungal agents based on clinical experience, trials, and patient population. Voriconazole is recommended for Aspergillus terreus and A. niger infections and as first-line or salvage therapy for fusariosis and scedosporiosis. Echinocandins are strongly recommended for candidemia and invasive candidiasis.
Strategies to develop new antifungal compounds
A variety of drugs are available to treat fungal infections, but developing effective and safe antifungal medications remains challenging due to fungi's ability to live off living organisms. Most antifungal drugs inhibit fungal growth rather than killing them directly, with amphotericin B being a notable exception.
Researchers are exploring new drug targets unique to fungi and crucial for their growth, with a focus on inhibiting production, which contributes to fungal virulence. Inhibitors targeting enzymes like Tps2, involved in metabolism, show promise based on the crystal structure of Candida albicans Tps2.
Ras GTPases, signaling molecules important for fungal virulence, are being investigated as treatment targets. Inhibitors like farnesyltransferase inhibitors can hinder Ras function, particularly in high-temperature environments.
Calcium/calmodulin signaling, a regulator of stress responses in fungi, also affects treatment resistance. Certain calcineurin inhibitors, like tacrolimus, used as immunosuppressants, show activity against fungi, suggesting potential for developing antifungal drugs
Heat shock protein 90 (Hsp90) is another target for drug development, as it's involved in fungal resistance to azoles and echinocandins. Combining Hsp90 inhibitors like geldanamycin with existing antifungal drugs can enhance their effectiveness against fungi [17].
Results and Discussion
Adverse events associated with antifungal drugs
Invasive Fungal Infections (IFI) poses significant risks to the morbidity and mortality of immunocompromised and critically ill patients. Various factors contribute to the high incidence of IFI, including the use of broad-spectrum antibiotics antineoplastic agents, immunosuppressive drugs, hyperalimentation, graft use and prosthetic devices. Additionally, advancements in medical care have prolonged the survival time of critically ill patients, making them more susceptible to IFI [18].
Current treatment options: In clinical practice, three main classes of drugs are used to treat IFI: Azoles (such as itraconazole, fluconazole, posaconazole and voriconazole), echinocandins (including anidulafungin, micafungin and caspofungin) and polyenes. Each class targets different components of fungal cells to inhibit their growth and replication. Polyenes bind to ergosterol in fungal cell membranes, forming pores that increase membrane permeability. Azoles disrupt fungal cell membrane stability by inhibiting lanosterol demethylation, while echinocandins interfere with glucan synthesis, a critical component of fungal cell walls.
Concerns about long-term antifungal use: Although these antifungal drugs are effective in treating IFI, prolonged use raises concerns about drug tolerability and treatment-related toxicity risks. Many clinical studies have reported various adverse reactions, including hepatotoxicity, neurotoxicity, nausea and headache.
Objective of the study: This study aims to analyze the main Adverse Events (AEs) associated with commonly used antifungal agents, including Liposomal Amphotericin B (LAmB), anidulafungin, caspofungin, micafungin, fluconazole, isavuconazole, itraconazole, posaconazole and voriconazole. Due to the increasing number of available treatments, direct randomized comparisons may not cover all possible pairwise comparisons. Therefore, network meta-analysis is employed to analyze the safety comparison between multiple interventions based on indirect or combined direct and indirect results.
Safety profiles of major antifungal drugs
Preterm infants, especially those with extremely low birth weight, are vulnerable to invasive fungal infections due to factors like catheter use, antibiotics and immature immune systems. Candida species are the primary cause, leading to significant morbidity and mortality, including the risk of meningo-encephalitis in candidemia cases.
Treatment guidance for neonates is limited, relying heavily on adult studies. The Infectious Diseases Society of America suggests amphotericin B deoxycholate as the first-line therapy for neonatal candidiasis, despite safety and dosing concerns. European guidelines offer more options, including liposomal amphotericin B, fluconazole and micafungin, with safety and efficacy data supporting their use.
Other agents like flucytosine, voriconazole, posaconazole, caspofungin and anidulafungin are reserved for salvage therapy due to limited data on safety, resistance and efficacy. More research is needed, particularly on pharmacokinetics and safety, to address emerging fungal infections in neonates.
Comparison of safety profiles: Comparing the safety profiles of these three antifungal agents, fluconazole is generally considered the safest, with a low incidence of serious adverse effects. Voriconazole has a higher risk of neurotoxicity and visual disturbances, making it less suitable for long-term use or in patients requiring high doses. Amphotericin B has the highest risk of nephrotoxicity and infusionrelated reactions, limiting its use to severe fungal infections when no alternative treatments are available. The choice of antifungal agent should be based on the specific fungal infection being treated, the patient's overall health and the risk-benefit ratio of the drug.
Drug interactions
Drug interactions can occur when two or more drugs interact with each other, affecting how one or both drugs work. Antifungal drugs, like other medications, can interact with other drugs, foods or supplements, potentially leading to adverse effects or reduced effectiveness of treatment. Understanding these interactions is crucial for ensuring safe and effective therapy.
Some common interactions involving antifungal drugs:
• Cytochrome P450 interactions: Many antifungal drugs, especially azoles like fluconazole, itraconazole, voriconazole and posaconazole, can inhibit or induce cytochrome P450 enzymes in the liver. This can lead to altered metabolism of other drugs metabolized by the same enzymes, potentially leading to toxicity or reduced efficacy. For example, fluconazole can increase the levels of warfarin, leading to an increased risk of bleeding.
• Gastrointestinal motility agents: Azoles like ketoconazole and itraconazole can interact with drugs that affect gastrointestinal motility, such as metoclopramide or cisapride, potentially leading to irregular heart rhythms or other serious side effects.
• Immunosuppressants: Antifungal drugs can interact with immunosuppressants like cyclosporine or tacrolimus, which are used to prevent organ rejection in transplant patients. Azoles can inhibit the metabolism of these drugs, leading to increased levels and potential toxicity.
• Anticoagulants: Azoles can interact with anticoagulants like warfarin, increasing the risk of bleeding. Monitoring of coagulation parameters is essential when these drugs are used together.
• Statins: Azoles can increase the levels of statins like simvastatin or atorvastatin, increasing the risk of muscle toxicity or rhabdomyolysis.
• Antiretrovirals: Some azoles, particularly ketoconazole, can interact with antiretroviral drugs used to treat HIV, potentially reducing the effectiveness of HIV treatment.
• Benzodiazepines: Azoles can increase the levels of benzodiazepines like alprazolam or diazepam, leading to increased sedation or respiratory depression.
• Digoxin: Azoles can increase the levels of digoxin, a medication used to treat heart failure, increasing the risk of toxicity.
Antifungal resistance
Antifungal drugs are used to treat fungal infections by either killing the fungi or stopping their growth. Similar to how bacteria can become resistant to antibiotics, fungi can also develop resistance to antifungal drugs. This means that the drugs become less effective at killing the fungi, allowing them to continue growing.
The limited number of antifungal drugs available makes resistance a serious issue, as it can restrict treatment options. Some fungi, like Candida auris, can become resistant to all commonly used antifungal drugs. This is particularly concerning for patients with invasive fungal infections, which are severe and can affect vital organs like the blood, heart, brain or eyes [19].
Mechanisms of antifungal resistance
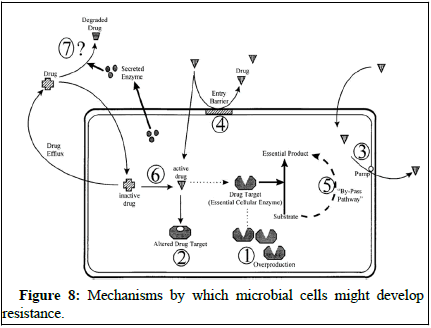
Antifungal resistance occurs when fungi adapt to survive in the presence of antifungal drugs. This adaptation is a natural process as microorganisms develop ways to counteract the effects of these drugs.
Scientists have studied how fungi become resistant to different antifungal agents and have identified three main mechanisms of resistance. One way is by reducing the concentration of the drug inside the fungal cells, which they do by increasing drug efflux. For example, Candida albicans can use transporter proteins like CDR1 and CDR2 to remove azole drugs from their cells, reducing their effectiveness. Other fungi, like Candida glabrata and Cryptococcus neoformans, have similar transporters that help them resist azole drugs [20].
Understanding these mechanisms of resistance is crucial for developing strategies to combat antifungal resistance and improve the effectiveness of antifungal treatments (Figure 8).
Figure 8: Mechanisms by which microbial cells might develop resistance.
Prevention and control of antifungal resistance
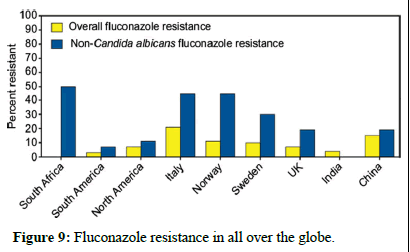
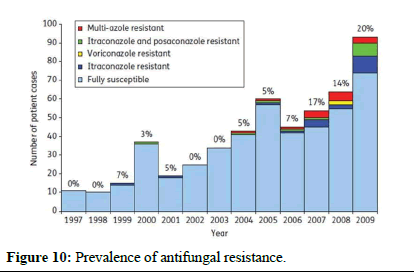
Preventing and controlling antifungal resistance is challenging, and specific strategies are still being developed. However, similar to antibacterial resistance, several approaches can be considered (Figures 9 and 10)
• Prudent use of antifungals: Avoid unnecessary or inappropriate use of antifungal drugs.
• Appropriate dosing: Ensure correct dosing to maximize effectiveness and minimize the risk of resistance development.
• Combination therapy: Consider using combinations of existing antifungal agents to prevent resistance.
• Tailored treatment: Use the most appropriate antifungal based on the known etiological agent of the infection.
• Surveillance studies: Conduct surveillance studies to monitor the frequency of antifungal resistance and inform treatment strategies.
Figure 9: Fluconazole resistance in all over the globe.
Figure 10: Prevalence of antifungal resistance.
Conclusion
While these measures are promising, more research is needed to establish their effectiveness. Additionally, advancements in rapid diagnostic methods for fungal infections can help reduce inappropriate antifungal use. Although progress in this area has been slow, the recent approval of a reference method for antifungal susceptibility testing of yeast is a positive step forward.
References
- Guarro J, Kantarcioglu AS, Horré R, Luis Rodriguez-Tudela J, Cuenca Estrella M, et al. (2006) Scedosporium apiospermum: Changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 44: 295-327.
[Crossref] [Google Scholar] [PubMed]
- Nucci M, Anaissie E (2007) Fusarium infections in immunocompromised patients. Clin Microbiol Rev 20: 695-704.
[Crossref] [Google Scholar] [PubMed]
- Carmona EM, Limper AH (2017) Overview of treatment approaches for fungal infections. Clin Chest Med 38: 393-402.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Zarychanski R, Pisipati A, Kumar A, Kethireddy S, et al. (2018) Fungicidal versus fungistatic therapy of invasive Candida infection in non-neutropenic adults: A meta-analysis. Mycology 9: 116-128.
[Crossref] [Google Scholar] [PubMed]
- Meletiadis J, Antachopoulos C, Stergiopoulou T, Pournaras S, Roilides E, et al. (2007) Differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob Agents Chemother 51: 3329-3337.
[Crossref] [Google Scholar] [PubMed]
- Geißel B, Loiko V, Klugherz I, Zhu Z, Wagener N, et al. (2018) Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat Commun 9: 3098.
[Crossref] [Google Scholar] [PubMed]
- Patil A, Majumdar S (2017) Echinocandins in antifungal pharmacotherapy. J Pharm Pharmacol 69: 1635-1660.
[Crossref] [Google Scholar] [PubMed]
- Revie NM, Iyer KR, Robbins N, Cowen LE (2018) Antifungal drug resistance: Evolution, mechanisms and impact. Curr Opin Microbiol 45: 70-76.
[Crossref] [Google Scholar] [PubMed]
- Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D (2020) Fungal infections in humans: the silent crisis. Microb Cell 7: 143-145.
[Crossref] [Google Scholar] [PubMed]
- Fang W, Wu J, Cheng M, Zhu X, Du M, et al. (2023) Diagnosis of invasive fungal infections: Challenges and recent developments. J Biomed Sci 30: 42.
[Crossref] [Google Scholar] [PubMed]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: Human fungal infections. Sci Transl Med 4: 165rv13.
[Crossref] [Google Scholar] [PubMed]
- Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL (2016) Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit Care 20: 1-6.
[Crossref] [Google Scholar] [PubMed]
- Koehler P, Bassetti M, Chakrabarti A, Chen SC, Colombo AL, et al. (2021) Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21: e149-162.
[Crossref] [Google Scholar] [PubMed]
- van Arkel AL, Rijpstra TA, Belderbos HN, van Wijngaarden P, Verweij PE, et al. (2020) COVID-19–associated pulmonary aspergillosis. Am J Respir Crit Care Med 202: 132-135.
[Crossref] [Google Scholar] [PubMed]
- Negm EM, Mohamed MS, Rabie RA, Fouad WS, Beniamen A, et al. (2023) Fungal infection profile in critically ill COVID-19 patients: A prospective study at a large teaching hospital in a middle-income country. BMC Infect Dis 23: 246.
[Crossref] [Google Scholar] [PubMed]
- Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, et al. (2022) COVID-19-associated fungal infections. Nat Microbiol 7: 1127-1140.
[Crossref] [Google Scholar] [PubMed]
- Dubey R, Sen KK, Mohanty SS, Panda S, Goyal M, et al. (2022) The rising burden of invasive fungal infections in COVID-19, can structured CT thorax change the game. EJRNM 53: 18.
- Villanueva-Lozano H, de J Treviño-Rangel R, González GM, Ramírez-Elizondo MT, Lara-Medrano R, et al. (2021) Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect 27: 813-816.
[Crossref] [Google Scholar] [PubMed]
- Ramadan HK, Mahmoud MA, Aburahma MZ, Elkhawaga AA, El-Mokhtar MA, et al. (2020) Predictors of severity and co-infection resistance profile in COVID-19 patients: First report from upper Egypt. Infect Drug Resist 13: 3409-3422.
[Crossref] [Google Scholar] [PubMed]
- Teixeira V, Feio MJ, Bastos M (2012) Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res 51: 149-177.
[Crossref] [Google Scholar] [PubMed]
Citation: Chaurasia A (2025) Complexities in Antifungal Pharmacotherapy: A Comprehensive Pharmacovigilance Analysis. Int J Res Dev Pharm L Sci 11: 275.
Copyright: © 2025 Chaurasia A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 293
- [From(publication date): 0-0 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 244
- PDF downloads: 49