Critical Role for Inflammatory Macrophages in Driving Antigen-dependent Th17Cell Responses?
Received: 04-Mar-2016 / Accepted Date: 31-Mar-2016 / Published Date: 05-Apr-2016 DOI: 10.4172/2576-3881.1000105
Abstract
Macrophages are heterogeneous cells with diverse phenotypes and sometimes opposing functions. These activities are dictated by activating stimuli in their microenvironment. For example, it is well described how CD4 + T helper (Th) cell-derived cytokines result in different macrophage-activation states. However, much less is known on how differentially-activated macrophages, presenting antigen, can drive the major types of CD4 + Th subpopulations, especially in human systems. Many studies have focussed on dendritic cells as the major antigen-presenting cell shaping T cell responses or on murine macrophage-secreted cytokines in the presence of mitogenic-stimuli, such as CD3/CD28, to induce Th polarization. Recent literature is, however, providing evidence that activated antigen- presenting macrophages can be as efficient as dendritic cells in polarising Th cells, especially Th17, and whilst both these cell types co-exist within inflamed tissue, macrophages are more abundant. The bias towards polarization of particular T cell subsets is strongly dependent on the activation state of macrophages. The concept of targeting macrophages to downregulate inflammatory responses may therefore have further reaching consequences by also abrogating pathogenic Th cells in autoimmune or inflammatory diseases.
Commentary
Macrophages have a key role in regulating immune responses such as inflammation, wound healing, and maintenance of tissue homeostasis [1]. Macrophages are characterized by remarkable plasticity and diversity [2]. They rapidly respond to microenvironmental signals, for example, cytokines and acquire distinct phenotypes and functions. Key phenotypes have been characterized in vitro, as M1-activated proinflammatory/microbicidal macrophages induced by microbial products and IFNg, and, M2 antiinflammatory/ tissue healing/immunomodulatory macrophages induced by IL-4/IL-13/IL-10 [1,2]. These are, however, extremes on a continuum of the phenotypes found in vivo . The nature and balance of macrophage activation-states is important and their dysregulation has been linked to inflammatory diseases, autoimmunity or, at the other extreme, cancer and chronic wounds. Macrophages are professional antigen-presenting cells (APCs) and recent evidence suggests the heterogeneous nature of macrophages impacts their role in CD4+ Tcell polarization [3-6].
CD4+ T helper (Th) cells orchestrate adaptive immune responses. Soluble and cell-contact dependent signals from APCs result in T cell differentiation into distinct functional phenotypes including Th1, Th2, Th17, and regulatory T (Treg) cells, characterised by cytokine output and transcription factor expression [7]. Human M1 and M2 subsets differentially produce cytokines modulating T-cell polarization [8], however, their role in antigen presentation and downstream CD4+ T cell polarization remains poorly studied. Dendritic cells are still commonly viewed as the most potent APC, especially driving naïve T cells to Th1 [9]. Macrophages, however, express MHC class-II and costimulatory molecules and efficiently present antigen to T cells in vitro . A greater abundance of macrophages is present within inflamed or immune-activated tissue such as the gut, where numbers of macrophages but not dendritic cells correlate with Th17 activity [10]. New evidence suggests that they are at least as capable of presenting antigen and driving Th17 cell responses as dendritic cells and this is highly dependent on the macrophage activation status and the cytokines they produce [3]. A recent hypothesis supports this notion, suggesting that APCs in lymphoid tissue act in concert with tissueresident APC to shape T cell responses [11]. Thus, in diseased tissue where macrophages outnumber dendritic cells by orders of magnitude, local antigen presentation and CD4+ T cell polarization by macrophages may be significant.
Evidence for the ability of macrophages to drive Th cell responses has recently been shown by several independent groups. CD4+ T cells co-cultured in vitro with activated macrophages and specific antigen were skewed towards Th1 or Th2 depending on the macrophage stimuli [4]. Macrophages from the red pulp and from the gut lamina propria favored the generation of Foxp3+ Treg cells [5,6]. Strikingly, dendritic cells in gut mucosa were dispensable for bacterial antigenspecific Th17 production and intestinal macrophages presenting antigen were essential for segmented filamentous bacteria-specific Th17 responses [12,13]. Similar trends have been shown in the human system, for example LPS or LPS/IFN-γ–activated, antigen-loaded human macrophages skewed Th cells toward Th17 or Th1, respectively [3]. Taken together, these investigations highlight the essential role of macrophages in CD4+ T cell polarization in which activation state and antigenic stimuli are critical.
The question arises as to how activated macrophage as APCs control T cell polarization. In a direct comparison between differentially activated human macrophages presenting antigen to autologous T cells, it was shown that the cytokine output profile is critical [3]. This study had the advantage in that the interactions between antigenloaded, activated macrophages and autologous T cells more closely recapitulate the physiological responses in vivo , in contrast to previous studies
Evidence for the ability of macrophages to drive Th cell responses has recently been shown by several independent groups. CD4+ T cells co-cultured in vitro with activated macrophages and specific antigen were skewed towards Th1 or Th2 depending on the macrophage stimuli [4]. Macrophages from the red pulp and from the gut lamina propria favored the generation of Foxp3+ Treg cells [5,6]. Strikingly, dendritic cells in gut mucosa were dispensable for bacterial antigenspecific Th17 production and intestinal macrophages presenting antigen were essential for segmented filamentous bacteria-specific Th17 responses [12,13]. Similar trends have been shown in the human system, for example LPS or LPS/IFN-γ–activated, antigen-loaded human macrophages skewed Th cells toward Th17 or Th1, respectively [3]. Taken together, these investigations highlight the essential role of macrophages in CD4+ T cell polarization in which activation state and antigenic stimuli are critical.
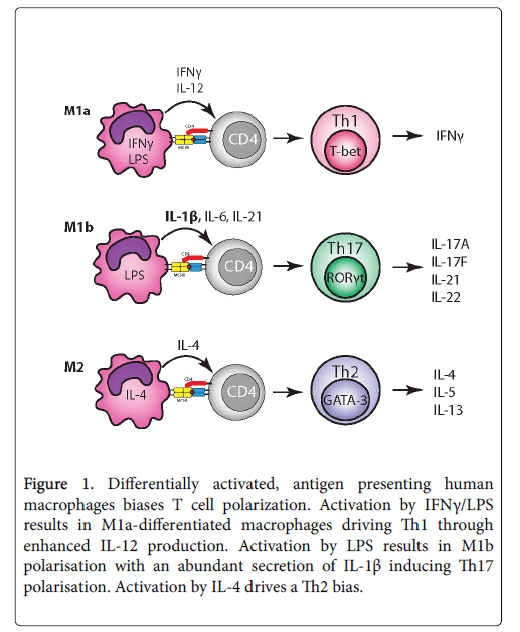
The question arises as to how activated macrophage as APCs control T cell polarization. In a direct comparison between differentially activated human macrophages presenting antigen to autologous T cells, it was shown that the cytokine output profile is critical [3]. This study had the advantage in that the interactions between antigenloaded, activated macrophages and autologous T cells more closely recapitulate the physiological responses in vivo , in contrast to previous studies that relied on adding cytokines together with mitogenic stimuli to induce T cell polarization. Antigen-loaded IFNg/LPS-activated macrophages were most efficient at driving Th1, associated with their upregulated production of IL-12, whilst LPS-activation induced the highest levels of IL-1b, that is known to be an important driver in human Th17 polarization [3]. By contrast, IL-4-activated macrophages did not result in significant polarization of either Th1 or Th17, or secretion of IL-12/IL-1b, in line with their recognised antiinflammatory/ tissue reparative nature (Figure 1).
Figure 1: Differentially activated, antigen presenting human macrophages biases T cell polarization. Activation by IFNγ/LPS results in M1a-differentiated macrophages driving Th1 through enhanced IL-12 production. Activation by LPS results in M1b polarisation with an abundant secretion of IL-1β inducing Th17 polarisation. Activation by IL-4 drives a Th2 bias.
In the same study, LPS-activated macrophages were at least as potent at inducing Th17 as dendritic cells from the same human donor, supporting the notion that macrophages make an important contribution to driving antigendependent Th17 responses within inflamed tissue. Th17 and Th1 polarization again correlated strongly with the output of the polarizing cytokines IL-1b and IL-12 by activated macrophages and dendritic cells, respectively. Likewise, human monocytes were reported to be more potent than dendritic cells at inducing Th17 expansion when activated by anti-CD3 and monocyte-derived factors, with IL-1b again identified as a key polarising cytokine [14]. A limitation of these studies was that macrophage polarising stimuli were restricted to LPS, IFNg/LPS, IL-10 and IL-4 that showed significant differences in their ability to secrete factors driving Th17. However, in vivo , the activating stimuli at a particular point in time are likely to be more complex with, a multitude of different ligands causing activation of macrophages in response to infection. It will therefore be important in future in vitro and in vivo studies, to assess how combinations of bacterial components, TLR ligands and other macrophage activating stimuli compare in their capacity to polarize Th cells. There are, however, precedents for macrophages activated in vivo driving Th17. One example is during fungal infection where antigen presentation and macrophage activation through engagement of multiple receptors (e.g. TLR4, dectin1, mannose receptor) has been shown to result in efficient Th17 polarization to eliminate infection [15]. While much of the studies are limited to monocytes/macrophages derived from blood from healthy volunteers, tissue-derived macrophages from different sources may also have different biology. Further studies on macrophages from other tissues and from disease settings are necessary to consolidate results in vitro . Interestingly, human tumorassociated macrophages were the prominent antigen presenting cells in tumours and most efficient at inducing Th17 cells, potentially by high IL-1b secretion [16,17]. Moreover, a unique macrophage subset distinct from M1/M2 macrophages displaying a characteristic IL-1ßhigh phenotype, supported the expansion of Th17. An additional study pointing towards the importance of IL-1ß as a Th17 driver in vivo demonstrated that IL-1ß but not IL-6 was required for the induction of Th17 cells by intestinal macrophages [18].
Thus, activated macrophages presenting antigen at sites of inflammation may play a greater role in driving T cell responses than initially anticipated. T cell polarization is dependent on their preactivation status as well as the local antigenic stimuli and importantly the macrophage-derived cytokines. For human cells, enhanced IL-1ß is strongly associated with Th17 differentiation while IL-12 is inhibitory, at least in vitro . It is notable that activated human macrophages show phenotypic and functional differences as compared to activated macrophages in the murine system e.g. iNOS expression [19]. While the focus here is on human studies, differences in murine macrophage activation and the resultant ability to drive Th polarization is also of interest, especially given that generation of murine Th17 relies more on IL-6 and TGF-b than on IL-1. The predominance of Th17 at inflammatory sites in human studies might be explained by the presence of activated macrophages providing an essential Th17 polarizing environment. This highlights new potential avenues to target Th1 and Th17 cell driven inflammatory and autoimmune diseases through regulating macrophage phenotypes, or to boost activity in cancer where Th1 cell skewing would benefit. Knockdown of suppressor of cytokine signalling (SOCS3) expression in macrophages, for example, effectively decreases their IL-1b secretion and ability of antigen-loaded macrophages to drive Th1 and Th17 responses [20]. Further work is, however, needed in order to determine the efficacy of macrophage-targeting therapies to regulate aberrant T cell responses.
References
- Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723-737.
- Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787-795.
- Arnold CE, Gordon P, Barker RN, Wilson HM. (2015) The activation status of human macrophages presenting antigen determines the efficiency of Th17 responses. Immunobiology 220(1):10-19.
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM (2006) Biochemical and functional characterization of three activated macrophage populations. J LeukocBiol 80: 1298-1307.
- Kurotaki D, Kon S, Bae K, Ito K, Matsui Y, et al. (2011) CSF-1-dependent red pulp macrophages regulate CD4 T cell responses. J Immunol 186: 2229-2237.
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8: 1086-1094.
- Gutcher I, Becher B (2007) APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest 117: 1119-1127.
- Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH (2006) Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J LeukocBiol 79: 285-293.
- Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327: 291-295.
- Allam JP, Duan Y, Heinemann F, Winter J, Götz W, et al. (2011) IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J ClinPeriodontol 38: 879-886.
- Ley K (1000) The second touch hypothesis: T cell activation, homing and polarization. Version 2 .
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, et al. (2014) Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40: 594-607
- Panea C, Farkas AM1, Goto Y1, Abdollahi-Roodsaz S1, Lee C1, et al. (2015) Intestinal Monocyte-Derived Macrophages Control Commensal-Specific Th17 Responses. Cell Rep 12: 1314-1324.
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F (2007) Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 8: 942-949.
- van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R, et al. (2010) Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J LeukocBiol 88: 227-232.
- Foucher ED, Blanchard S, Preisser L, Descamps P, Ifrah N, et al. (2015) IL-34- and M-CSF-induced macrophages switch memory T cells into Th17 cells via membrane IL-1α. Eur J Immunol 45: 1092-1102.
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, et al. (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114: 1141-1149.
- Tatano Y, Shimizu T1, Tomioka H1 (2014) Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.Sci Rep 4: 4146.
- Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, et al. (2014) A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology 141: 96-110.
- Mestas J, Hughes CC (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731-2738.
Citation: Arnold CE, Barker RN, Wilson HM (2016) Critical Role for Inflammatory Macrophages in Driving Antigen-dependent Th17 Cell Responses?. J Cytokine Biol 1: 105. DOI: 10.4172/2576-3881.1000105
Copyright: © Arnold CE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13659
- [From(publication date): 5-2016 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 12635
- PDF downloads: 1024