Current Issues Regarding the Care of the Fibromyalgia Patient
Received: 25-Apr-2017 / Accepted Date: 16-May-2017 / Published Date: 22-May-2017
Abstract
Fibromyalgia (FM) is a chronic disorder that has been found to negatively impact quality-of-life, physical, emotional, and social functioning, personal relationships, and working productivity. Employees with FM are 2 to 3 times less productive than healthy workers. FM-related workplace absenteeism accounts for an average of 30 days each year. As their severity of illness rises, FM workers are often forced to change job positions, work site tasks, and the number of hours worked. FM poses a significant economic burden costing society tens of thousands of dollars per patient each year. FM patients frequently suffer with higher levels of pain, significant disruptions in sleep, and require more medication when compared to other chronic pain conditions. Timely diagnosis continues to be a problem most likely due to the diversity of presentation, comorbid illnesses, absence of quantitative measurements (e.g. laboratory, imaging), and poor understanding of diagnostic criteria. Moreover, prescribing patterns vary considerably and treatment often remains inadequate. Research indicates that only 31% of FM patients are prescribed medications of proven efficacy including pregablin, amitriptyline, cyclobenzaprine, duloxetine, gabapentin, tramadol, or milnacipran within the first year of diagnosis. In order to address the potential shortcomings of pharmacotherapy related to prescribing, poor response rates, and patient dissatisfaction, it becomes very important to incorporate a combination of both pharmacological and non-pharmacological approaches. Additionally, a patient-centered, multi-disciplinary strategy which uses education and non-pharmacologic interventions such as aerobic and strengthening exercise should be a mainstay for all treatment plans.
Keywords: Fibromyalgia; Comorbidities; Quality-of-life; Multidisciplinary; Pharmacotherapy; Exercise; Cognitive behavioral therapy; Education
4942Introduction
Fibromyalgia (FM) affects about 3 to 6% of the world’s population including 10 million US citizens; 80% of those diagnosed are women [1-3]. The illness presents as chronic widespread pain (CWP) and fatigue, non-restorative sleep, insomnia, disturbances in cognition also known as “Fibro-fog”, and morning stiffness [4,5]. FM patients experience 4.2 comorbidities on average including sleep disturbance (most commonly), depression, anxiety, chronic fatigue, and headache/ migraine [6-9]. Additionally, rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome can occur concomitantly with FM and should be considered when constructing a treatment regimen [5]. The chronicity of FM creates a tremendous burden on work/life including significant impacts on physical and mental functioning, personal relationships, activities of daily living (ADLs), work productivity, career advancement, and health-related quality-of-life (HRQOL) [9-12]. It also poses a significant economic burden to society costing tens of thousands of dollars per patient each year with expenses increasing in concert with disease severity and the presence of comorbidities [13].
FM is currently considered a neurosensory disorder involving a combination of central sensitization and peripheral mechanisms as its underlying pathophysiology [14]. Symptoms are expressed via central magnification of pain perception and include hyperalgesia, a heightened response to painful stimuli, and allodynia, an enhanced sensitivity to non-painful stimuli [4,15]. As a central sensitivity syndrome, it belongs to a group of several coinciding illnesses responsible for diffuse pain including chronic fatigue syndrome, myofascial pain syndrome, bladder pain syndrome, irritable bowel syndrome, tension headache, functional dyspepsia, posttraumatic stress disorder, rheumatoid arthritis, and Gulf War Syndrome [4,16-18]. Evidence suggests that the etiology of FM is multifactorial including genetic predisposition as well as environmental triggers such as physical injury or psychological trauma [19-22].
Given the diversity of presentation, presence of comorbidities, nonspecific symptomatology and absence of quantitative diagnostics (e.g. laboratory, imaging), management of FM can be difficult. Many FM sufferers remain inadequately treated due to wide variations in prescribing patterns [23,24]. Moreover, health care providers (HCPs) often underutilize or provide inadequate doses of appropriate pharmacotherapy and continue to prescribe medications that have limited to no efficacy [9,12,23]. This review will focus on important issues to consider when caring for the FM patient.
Challenges in diagnosis
In 1990 the American College of Rheumatology (ACR) defined FM as chronic widespread pain lasting 3 months or more with associated painfulness in 11 out of 18 defined ‘tender points’ (TPs) [25]. The ACR criteria underwent revision in 2010 and modification in 2011 in order to better focus on the wide ranging symptomatology and common comorbidities [26,27]. The traditional TP examination was replaced with a patient self-report questionnaire that included symptom severity scales (SSS) and widespread pain index (WPI); positive diagnosis equated to SSS ≥ 5 and WPI ≥ 7 or SSS ≥ 9 and WPI=3-6 [27,28]. The 2011 modification to the ACR criteria dropped the estimation of somatic symptoms and expanded the WPI to a new 0-31 point FM symptoms (FS) scale. The FS scale includes 9 pain locations and 6 self-reported symptoms (e.g. reduced cognition, fatigue, sleeping difficulties, headache, depression, and abdominal pain) with positive diagnosis defined as FS scores of ≥ 13 [27]. The 2016 revision to the 2010/2011 ACR diagnostic criteria reported that the median sensitivity and specificity of the 2010/2011 criteria were 86% and 90%, respectively [29]. The newly published 2016 criteria adjusts the scoring for the WPI, adds a generalized pain criterion, clarifies the FM diagnosis in relation to other disorders, and includes the FS scale (sum of the SSS and WPI), as a full component. Adults must have at least 3 of the following criteria for a diagnosis of FM: 1) WPI ≥ 7 and SSS score ≥ 5 or WPI of 4-6 and SS score of ≥ 9; 2) generalized pain (excluding chest, jaw, and abdominal) must be present in at least 4 of 5 regions; 3) symptoms have been present most of the time for a minimum of 3 months; and 4) the diagnosis of FM is valid regardless of other diagnoses and a FM diagnosis does not exclude the existence of other clinically significant illnesses [29].
There is currently no “gold standard” or FM specific biomarker and diagnosis by exclusion continues to occur. Research indicates that HCPs do not routinely utilize the ACR criteria for diagnosis of FM; most likely resulting from inadequate knowledge and lack of confidence in distinguishing FM from other illnesses with similar presentations [10,30,31]. Thus, it takes 5 years on average to diagnose FM and up to 75% of FM patients remain undiagnosed and inadequately treated [1,4]. Further delays in diagnosis can occur because of comorbid conditions with overlapping symptomatology [7,32,33]. Even with a confirmed diagnosis approximately 69% of FM patients do not receive adequate pharmacotherapy [12]. Delays and lack of diagnosis ultimately results in patient dissatisfaction and frustration, suboptimal medical care, poor adherence, and increased health care burden [4,34,35].
Patients are often positively impacted once a diagnosis is made because their suffering becomes legitimized; they have improved coping, and less stigmatization [35-38]. Newly diagnosed FM patients actually report improved health satisfaction and less long-term symptoms [39]. Unfortunately, FM patients find that their care from HCPs is lacking in respect and support, important disease-related information, and continuity of care [40]. Attitudes of HCPs are also concerning as they believe that their time and efforts caring for FM patients are wasted and hence adjustments in their clinical commitments may be necessary [40]. Overall, FM patients describe their journey to diagnosis as fraught with numerous medical consultations involving multiple specialists causing prolonged delays, frustration, dissatisfaction, and nonadherence to prescribed therapy [35,37,40].
Quality of life impacts
FM symptom severity significantly impacts HRQOL. FM patients often suffer with higher levels of pain, reduced physical and social functioning, significant disruptions in sleep, and require more medication when compared to other chronic pain conditions (e.g. rheumatoid arthritis, osteoarthritis, myofascial pain syndrome systemic lupus erythematosus) [9,23,41-43]. Research has shown that FM patients also experience higher interference in their overall functioning than those with non-FM related CWP [9]. Significant psychiatric and physical impairments in FM patients have been reported on the Short-Form (SF)-12, SF-36, and EuroQol 5 dimension 3-level (EQ-5D-3L) HRQOL instruments [9,44]. Concomitant major depressive disorder and disease severity were found to be significantly and directly related to poorer outcomes on EQ-5D-3L compared to healthy controls and those with other chronic illness such diabetes, headache, cancer, asthma, osteoarthritis, rheumatoid arthritis, hypertension, myocardial infarction, coronary atherosclerosis, and congestive heart failure [44].
Nearly all FM patients report major disruptions in their sleep quality which, in turn, affects symptom severity and pain management [9,45-47]. FM patients experience reduced sleep quality and higher sleep interruptions, daytime sleepiness, shortness of breath, and snoring when compared to those with non-FM related CWP and the general US population [9,48]. Recent research involving 1,044 participants diagnosed with FM found that frequent use and extended duration (over 30 minutes) of napping during the day was associated with worsening symptoms (e.g. pain, fatigue, memory difficulties, sleep disturbance), as well as, higher levels of comorbid depression and anxiety compared to those napping for less than 30 minutes [47]. Interestingly, this study found that younger adults with FM had higher frequencies and durations of daytime napping as a result of pain and irritability compared to those over the age of 60. Sleeping difficulties and levels of fatigue were the highest contributors to predicting who would engage in daytime napping in FM which is consistent with other studies [7,45,47,49,50].
In addition to sleeping difficulties, pain, and fatigue, FM sufferers face social isolation which further erodes their HRQOL by promoting depressed mood, loneliness, frustration, and fear [51]. Strains in family member relationships and the loss of friends results from others lack of knowledge and understanding of the illness process and its impact on daily living [51]. Reductions in recreational, community, and social activities resulting from FM symptoms have been found to directly affect their ability to maintain healthy relationships [52]. A small qualitative study reported that HCPs, family, and friends believed that FM was fictitious or a result of psychological illness which promoted an atmosphere of skepticism, stigmatization, and frustration [52]. Opinions and disbeliefs such as these were often linked to disease-related diagnostic challenges (e.g. delayed, misdiagnosis, absence of objective testing), lack of directly visible or hidden symptoms, and gender stereotypes [52].
Economic burden
FM is associated with a significant economic burden for both patients and society [9,13,53]. Research using administrative claims database from 31 large self-insured US companies reported that the total annual expenditures among employees with FM was similar to those with osteoarthritis [54]. A study involving patient self-report survey data from the US found that the 3-month total pain-related costs per participant were significantly higher in the FM cohort ($4,291) when compared to those without CWP ($1,531) and non-FM related CWP ($3,107) [9]. Medical record and patient self-report data from the US and France, and Germany found that the annual adjusted direct costs per subject differed significantly in the US ($7,087) as compared to Germany ($2,417) and France ($481) [55]. Direct costs primarily involved prescription medications, patient out-of-pocket expenses, and HCP office visits. FM patients in the US also saw their HCPs about 17 times each year versus 19 visits in Germany and 12 visits in France [55].
FM-related health care expenditures have been reported to rise significantly in concert with increases in severity of illness due to a greater need for more HCP office visits and medications [56]. A patient self-report study (N=203) from 20 US community-based physician practices found that the mean annualized direct and indirect expenditures (in 2009 US$) for mild, moderate and severe FM were $10,219, $26,217, and $42,456, respectively [56]. Indirect costs in this study represented the greatest proportion of the mean total costs and rose considerably with increasing disease severity (52.5%-mild, 78.4%- moderate, and 78.1%-severe).
Issues with employment
FM is associated with various levels of disability which causes poor HRQOL and leads to considerable productivity deficits [57,58]. Worsening severity of illness forces FM patients to frequently change job positions, work site tasks, and the number of hours worked [57,59]. They are 2 to 3 times less productive than healthy workers [48,54,60]. A study of 203 FM patients in the US found that inadequate or unrefreshing sleep, fatigue, CWP, and comorbid illness (e.g. anxiety and depression) were the most common causes for reductions in productivity [48]. Disease severity significantly (p<0.001) affected employment status; 71.4% of mild FM sufferers were employed full or part-time employment versus 61.2% for moderate FM and 28.5% for severe FM (Table 1) [48]. Moreover, being disabled, unemployed, and/or taking early retirement were also correlated to FM severity with the level of absenteeism reported as 52.6% (severe), 22.4% (moderate), and 9.6% (mild). None of the mild FM sufferers reported being disabled however 36.1% of those with severe and 14.3% of those with moderate FM described themselves as fully disabled [48]. Two other patient surveys from the US reported that only 38-40% of FM patients were employed for pay [9,56]. Additionally, findings from FM patients living in Puerto Rico and the US indicated that 21.6% were no longer working and nearly 30% were receiving disability pay over an average of about 11 months [23]. Research indicates that employees with FM are absent from the workplace about 23 to 58 days each year as a direct result of their illness [48,54,60]. Presenteeism is also a major concern. Those who are employed report working with FM symptoms 15.6 days over a 4-week period [48]. A recent study that used the 6-item Work Productivity Activity Impairment questionnaire assessing absenteeism and presenteeism found that FM patients in the US were absent from work, on average, 9.8% of their scheduled work time and had impairment in their working abilities (presenteeism) at work 43.3% of the time [9].
| Status | Mild (N=21) | Moderate (N=49) | Severe (N=133) |
|---|---|---|---|
| Employed, full time | 61.9% | 36.7% | 19.5% |
| Employed, part time | 9.5% | 24.5% | 9% |
| Disabled, not working | - | 14.3% | 36.1% |
| Full-time, homemaker | 19% | 10.2% | 12% |
| Unemployed | 4.8% | 2% | 10.5% |
| Retired | 4.8% | 6.1% | 6% |
| Other | - | 4.1% | 4.5% |
| Student | - | 2% | 2.3% |
| Adapted from Schaefer C, et al. [48] | |||
Table 1: Employment status by disease severity.
Based on 2008 and 2009 patient self-report data, disability- and absenteeism-related lost productivity related to FM was estimated to cost about $10,001 in Germany, $8,718 in France, and $6,431 in the US each year which equated to 80.5%, 94.8%, and 47.6% of the total expenditures, respectively [55]. Another survey of US FM patients found that annual indirect costs resulting unemployment / early retirements and disability were estimated at $6,956 and $6,776, respectively [9]. Indirect costs including lost productivity in FM patients working for pay and having unpaid caregiver time was reported at $11,948 annually and accounted for 67.5% of the total disease related costs [9].
Treatment plan considerations
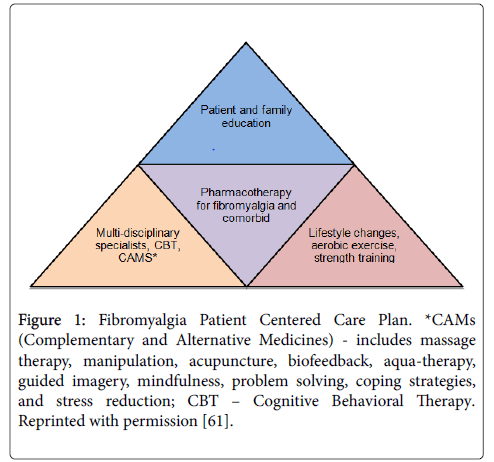
Care of the FM patient should be individualized, patient centered, multidisciplinary, and multifaceted. The most successful treatment plans address both the FM and comorbid conditions. These plans integrate lifestyle changes, patient and family member illness education, aerobic exercise, sleep hygiene, strength training, dietary consults targeting weight loss, complementary and alternative medicine (CAM), cognitive behavioral therapy (CBT), and pharmacotherapy (Figure 1) [61].The most successful outcomes (e.g. reduced pain and fatigue; improved mood and HRQOL) happen when patients take an active role in their overall care [62].
Figure 1: Fibromyalgia Patient Centered Care Plan. *CAMs (Complementary and Alternative Medicines) - includes massage therapy, manipulation, acupuncture, biofeedback, aqua-therapy, guided imagery, mindfulness, problem solving, coping strategies, and stress reduction; CBT – Cognitive Behavioral Therapy. Reprinted with permission [61].
Fibromyalgia-induced fatigue, CWP, depressed mood can easily promote inactivity which, in turn, works to accentuate these symptoms. Poor aerobic fitness, flexibility, and strength are common in FM. Symptoms such as CWP, fatigue, and sleeping difficulties are frequently associated with deconditioning [63]. Individualized, low to moderate intensity strength and/or flexibility exercises (e.g. yoga, Tai Chi), low impact water and land aerobics have been shown to improve functioning, muscle stiffness, sleeping difficulties, and mood, as well as, reduce CWP and fatigue in those with FM [64-69]. Resistance training can also be used to improve muscle strength, tenderness, CPW, and overall functioning [64]. There are currently no specific recommendations for training regimens regarding frequency, intensity, timing, or progression. Patients should be instructed to be consistent in their exercise practices in order to limit muscle soreness, keep away from high powered workouts (e.g. CrossFit, high intensity interval training, boot camps, pylometrics), work within their normal range of motion and avoid pain producing postures, not to engage in strength training when FM symptoms are elevated, avoid the urge to over-train when experiencing better wellness, and select an exercise activity with something they find to be enjoyable to promote sustained practice [70].
Along with exercise, current FM treatment guidelines recommend incorporating patient and family education along with behavioral and psychological interventions (e.g. CBT, problem solving, coping skills) [71,72]. Evidence highly supports educational interventions with the greatest success occurring with the use of groups that include patients, caregivers, and family members [73,74]. Education then becomes the cornerstone for illness comprehension, self-care, coping, exercise practices, as well as pharmacologic and psychologic interventions. Group or individual CBT is a major component in combating mental health related comorbidities [5,75]. The best outcomes occur when CBT is done in conjunction with other treatments such as exercise, education, pharmacotherapy, and mind-body [76,77]. There is some supporting evidence for biofeedback, hypnosis and mindfulness-based interventions (MBIs) such as acceptance and commitment therapy, mindfulness-based stress reduction (MBSR), and meditation awareness training in the treatment of FM [72,78-83]. MBIs are safe, usually well tolerated, beneficial add-on therapy, and can be useful when constructing the overall care plan. Short-term improvements in pain management and HRQOL were reported in a systematic metaanalysis of six trials comparing a MBSR intervention to active controls and usual care [81]. There is also limited evidence that MBIs reduce FM-related symptom severity, improve sleep, and lower psychological distress [82,83]. Research remains ongoing in order to best define the MBI-related sustainability as it concerns patient outcomes.
The US National Institutes of Health published consensus statement supports the use of acupuncture for various pain conditions stating, “Acupuncture may be useful as an adjunct treatment or an acceptable alternative or may be included in a comprehensive management program [84,85].” A 2013 Cochrane database systematic review reported that manual- and electro-acupunctures where not significantly better than sham acupuncture for relief of most FM symptoms including fatigue, pain, sleeping difficulties, and global wellbeing over the short term (one month) but did improve muscular stiffness [86]. However, a newly published RCT found that the real acupuncture cohort had significant improvement in pain, depressed mood, and functional status after 1 or 2 months when compared to sham acupuncture. In general, acupuncture is well tolerated and should be considered in the FM treatment regimen. An important consideration is that acupuncture may not be as effective in those taking opioids on a daily basis due to its ability to stimulate endogenous opioids [5].
Pharmacotherapy choices
There is currently no recognized algorithm to guide medication selection in FM. Thus, pharmacotherapy choices should be individualized and based on severity of illness and comorbidities. Amitriptyline, cyclobenzaprine, duloxetine, milnacipran, pregabalin and tramadol have proven effective for the treatment of various FM symptoms; their respective therapeutic class, dosage range, place in therapy, and common adverse effects are provided in Table 2 [5,71,87]. Tramadol lowers the seizure threshold and should be avoided in those with seizure disorders [88]. It is important to note that selective serotonin reuptake inhibitors (SSRIs) are effective adjuncts for treatment of anxiety and depressive symptoms but have been found to be ineffective in relieving CWP experience by FM sufferers [10,87-89].
| Medication | Class | Dosage (mg/day) | Uses | Common adverse effects |
|---|---|---|---|---|
| Amitriptyline | TCA | 10-50 | pain, fatigue, sleep disturbance | somnolence, weight gain, constipation, dizziness, headache, blurred vision |
| Cyclobenzaprine | muscle relaxant | 5-30 | muscle relaxation, sleep disturbance | somnolence, dizziness, xerostomia, constipation |
| Duloxetine | SNRI | 60-120 | pain, depressed mood, sleep disturbance | nausea, dizziness, dry mouth, hyperhidrosis |
| Milnacipran | SNRI | 100-200 | pain, fatigue, cognitive dysfunction | headache, dizziness, nausea, diaphoresis, hypertension, tachycardia, palpitations, hyperhidrosis, constipation |
| Pregabalin | α2δ ligand | 300-600 | pain, sleep disturbance | dizziness, weight gain, drowsiness, peripheral edema |
| Tramadol | opioid | 100-300 | pain | somnolence, nausea, vomiting, dizziness, constipation, insomnia, pruritus, headache, flushing, xerostomia |
| Reprinted with permission [61]. | ||||
Table 2: Efficacious medications for fibromyalgia.
At present, prescribing patterns vary considerably and many FM sufferers do not receive optimal pharmacotherapy [23,24]. Robinson and colleagues reported that specialist including rheumatologist were more likely to prescribe pregabalin, duloxetine and milnacipran compared to primary care providers [23]. A recent study found that only 31% of FM patients were appropriately prescribed pregablin, amitriptyline, cyclobenzaprine, duloxetine, gabapentin, tramadol, or milnacipran within the first year of diagnosis [12]. Unfortunately, there is also evidence that nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, benzodiazepines, and opioids other than tramadol (e.g. codeine, hydrocodone, oxycodone, and morphine) continue to be prescribed for the treatment of FM despite their lack of efficacy [9,23].
Ineffective pharmacotherapy, medication-related adverse reactions, poor compliance, and high rates of discontinuance can result in significant levels of treatment dissatisfaction within the FM patient population [11,90,91]. A multinational patient survey of 800 FM patients found that 35% of participants reported poorly treated CWP [11]. Another international study using patient self-reported surveys (N=900) found that 70-80% of participants received inadequate pharmacotherapy that resulted in extremely distressing CWP, fatigue, and poor sleep, 70% also reported self-medicating with over-the-counter pain medications increasing their risk for adverse events [91]. Finally, a cross-sectional survey (N=1,651) reported that 46.5% of participants had little to no treatment satisfaction with 70.6% indicating a worsening in health since diagnosis and under the current treatment regimen [90].
In order to address the potential shortcomings of pharmacotherapy related to prescribing, response, and dissatisfaction, it becomes very important to incorporate a combination of both pharmacological and non-pharmacological approaches. The European League against Rheumatism (EULAR) revised recommendations support a multi-disciplinary approach which uses education and non-pharmacologic interventions such as aerobic and strengthening exercise as first line treatment [92]. This is followed by additional individualized therapy (e.g. pharmacotherapy, hydrotherapy, CBT, and/or acupuncture), if needed, designed to improve HRQOL and tailored to pain intensity, fatigue, sleep disturbance, patient preferences, and psychiatric comorbidities [92]. CBT and pharmacotherapy are recommended as well and especially for those with moderate to severe comorbid anxiety and depression.
Summary
FM is a chronic illness that adversely impacts physical and mental functioning, ADLs, personal relationships, work productivity, career advancement, and HRQOL. It also poses a significant economic burden to society with total expenditures for mild to moderate illness averaging about $20,000 to just over $42,000 for those with severe cases. Despite the ACR guidelines, it can take an average of 5 years for diagnosis and up to 75% of FM patients remain undiagnosed and inadequately treated. Delays and lack of diagnosis ultimately results in patient dissatisfaction and frustration, suboptimal medical care, poor adherence, and increased health care burden. The most successful outcomes occur when patients take an active role in their overall care. Treatment plans that integrate lifestyle changes, patient and family member illness education, aerobic exercise, sleep hygiene, strength training, dietary consults targeting weight loss, CAMs, CBT, and pharmacotherapy (e.g. amitriptyline, duloxetine, milnacipran, tramadol, pregabalin, and cyclobenzaprine) are highly recommended. Unfortunately, nearly 70% of FM patients do not receive adequate pharmacotherapy indicating significant improvements are still needed in order to improve recognition, prescribing habits, management, and patient outcomes of this potentially debilitating disease.
References
- Branco JC, Bannwarth B, Failde I, AbelloCarbonell J, Blotman F, et al. (2010) Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 39: 448-453.
- Queiroz LP (2013) Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 17: 356.
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, et al. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58: 26-35.
- Arnold LM, Clauw DJ, Mc Carberg BH (2011) Improving the recognition and diagnosis of fibromyalgia. Mayo ClinProc 86: 457-464.
- Â Berger A, Dukes E, Martin S, Edelsberg J,Oster G (2007) Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J ClinPract 61: 1498-508.
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L (2007) An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord 8: 27.
- Ghavidel-Parsa B, Bidari A, Amir Maafi A, Ghalebaghi B (2015) The Iceberg Nature of Fibromyalgia Burden: The Clinical and Economic Aspects. Korean J Pain 28: 169-176.
- Schaefer C, Mann R, Masters ET, Cappelleri JC, Daniel SR (2016) The Comparative Burden of Chronic Widespread Pain and Fibromyalgia in the United States. Pain Pract 16: 565-579.
- Smith HS, Barkin RL (2011) Fibromyalgia syndrome: a discussion of the syndrome and pharmacotherapy. Dis Mon 57: 248-285.
- Choy E, Perrot S, Leon T, Kaplan J, Petersel D (2010) A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res 10: 102
- Liu Y, C Qian, M Yang (2016) Treatment Patterns Associated with ACR-Recommended Medications in the Management of Fibromyalgia in the United States. J Manag Care Spec Pharm 22: 263-271.
- Skaer TL (2014) Fibromyalgia: disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics 32: 457-466.
- Baek SH, Seok HY, Koo YS, Kim BJ (2016) Lengthened Cutaneous Silent Period in Fibromyalgia Suggesting Central Sensitization as a Pathogenesis. PLoS One 11: e0149248
- Chinn S, Caldwell W, Gritsenko K (2016) Fibromyalgia Pathogenesis and Treatment Options Update. Curr Pain Headache Rep 20: 25.
- Suresh E (2015) How to diagnose fibromyalgia. Br J Hosp Med 76: 696-702.
- Yunus MB (2007) Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 36: 339-356
- Theoharides TC (2013) Atopic conditions in search of pathogenesis and therapy. ClinTher 35: 544-547.
- Jones GT (2016) Psychosocial Vulnerability and Early Life Adversity as Risk Factors for Central Sensitivity Syndromes. Curr Rheumatol Rev 12: 140-153.
- Kato K, Sullivan PF, Evengård B, Pedersen NL (2009) A population-based twin study of functional somatic syndromes. Psychol Med 39: 497-505.
- Ablin JN, D Buskila (2015) Update on the genetics of the fibromyalgia syndrome. Best Pract Res ClinRheumatol 29: 20-28.
- Robinson RL, Kroenke K, Mease P, Williams DA, Chen Y, et al. (2012) Burden of illness and treatment patterns for patients with fibromyalgia. Pain Med 13: 1366-1376
- McNett M, Goldenberg D, Schaefer C, Hufstader M, Baik R, et al. (2011) Treatment patterns among physician specialties in the management of fibromyalgia: results of a cross-sectional study in the United States. Curr Med Res Opin 27: 673-683.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160-172.
- Wolfe F, Walitt BT, Häuser W (2014) What is fibromyalgia, how is it diagnosed, and what does it really mean? Arthritis Care Res (Hoboken) 66: 969-971
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, et al. (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38: 1113-1122.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62: 600-610.
- Wolfe F,Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, et al. (2016) Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 46: 319-329
- Hayes SM, Myhal GC, Thornton JF, Camerlain M, Jamison C, et al. (2010) Fibromyalgia and the therapeutic relationship: where uncertainty meets attitude. Pain Res Manag 15: 385-391.
- Perrot S, Choy E, Petersel D, Ginovker A, Kramer E (2012) Survey of physician experiences and perceptions about the diagnosis and treatment of fibromyalgia. BMC Health Serv Res 12: 356
- Buskila D, Atzeni F, Sarzi-Puttini P (2008) Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun Rev 8: 41-43.
- Mease P (2005) Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J RheumatolSuppl 75: 6-21.
- Rodham K, Rance N, Blake D (2010) A qualitative exploration of carers and patients experiences of fibromyalgia: one illness, different perspectives. Musculoskeletal Care 8: 68-77.
- Asbring P, Narvanen AL (2002) Women's experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qual Health Res 12: 148-160.
- Hellström O, Bullington J, Karlsson G, Lindqvist P, Mattsson B (1999). A phenomenological study of fibromyalgia. Patient perspectives. Scand J Prim Health Care 17: 11-16.
- Raymond MC, Brown JB, Experience of fibromyalgia. Qualitative study. Can Fam Physician 46: 1100-1106.
- Lachapelle DL, Lavoie S, Boudreau A (2008) The meaning and process of pain acceptance. Perceptions of women living with arthritis and fibromyalgia. Pain Res Manag 13: 201-210.
- White KP, Nielson WR, Harth M, Ostbye T, Speechley M (2002) Does the label "fibromyalgia" alter health status, function, and health service utilization? A prospective, within-group comparison in a community cohort of adults with chronic widespread pain. Arthritis Rheum 47: 260-265
- Briones-Vozmediano E, Vives-Cases C, Ronda-Pérez E, Gil-González D (2013) Patients' and professionals' views on managing fibromyalgia. Pain Res Manag 18: 19-24.
- Hoffman DL, Dukes EM (2008) The health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12. Int J ClinPract 62: 115-126
- Salaffi F, Sarzi-Puttini P, Girolimetti R, Atzeni F, Gasparini S, et al. (2009) Health-related quality of life in fibromyalgia patients: a comparison with rheumatoid arthritis patients and the general population using the SF-36 health survey. ClinExpRheumatol 27: 67-74.
- Wolfe F, Michaud K, Li T, Katz RS (2010) EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J Rheumatol 37: 296-304.
- Luo X, Cappelleri JC, Chandran A (2011) The burden of fibromyalgia: assessment of health status using the euroqol (EQ-5D) in patients with fibromyalgia relative to other chronic conditions. Outcomes Res Med 2: 203-214.
- Theadom A, Cropley M, Humphrey KL (2007) Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res 62: 145-151.
- Affleck G, Urrows S, Tennen H, Higgins P, Abeles M (1996) Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain, 68: 363-368.
- Theadom A, Cropley M, Kantermann T (2015) Daytime napping associated with increased symptom severity in fibromyalgia syndrome. BMC Musculoskelet Disord 16: 13.
- Schaefer C, Chandran A, Hufstader M, Baik R, McNett M, et al. (2011) The comparative burden of mild, moderate and severe fibromyalgia: results from a cross-sectional survey in the United States. Health Qual Life Outcomes 9: 71.
- Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russell IJ, et al. (2008) Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 59: 952-960.
- Osorio CD, Gallinaro AL, Lorenzi-Filho G, Lage LV (2006) Sleep quality in patients with fibromyalgia using the Pittsburgh Sleep Quality Index. J Rheumatol 33: 1863-1865.
- Arnold LM, Crofford LJ, Mease PJ, Burgess SM, Palmer SC, et al. (2008) Patient perspectives on the impact of fibromyalgia. Patient EducCouns 73: 114-120.
- Armentor JL (2015) Living With a Contested, Stigmatized Illness: Experiences of Managing Relationships Among Women With Fibromyalgia. Qual Health Res.
- Vervoort VM, Vriezekolk JE, Olde Hartman TC, Cats HA, van Helmond T, et al. (2016) Cost of illness and illness perceptions in patients with fibromyalgia. ClinExpRheumatol 34: S74-82.
- White LA, Birnbaum HG, Kaltenboeck A, Tang J, Mallett D, et al. (2008) Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med 50: 13-24.
- Knight T, Schaefer C, Chandran A, Zlateva G, Winkelmann A, et al. (2013) Health-resource use and costs associated with fibromyalgia in France, Germany, and the United States. Clinicoecon Outcomes Res 5: 171-80.
- Chandran A, Schaefer C, Ryan K, Baik R, McNett M, et al. (2012) The comparative economic burden of mild, moderate, and severe fibromyalgia: results from a retrospective chart review and cross-sectional survey of working-age U.S. adults. J Manag Care Pharm 18: 415-426.
- Mannerkorpi K, Gard G (2012) Hinders for continued work among persons with fibromyalgia. BMC Musculoskelet Disord 13: 96.
- Grodman I (2011) Understanding fibromyalgia and its resultant disability. Isr Med Assoc J 13: 769-772.
- Henriksson CM, Liedberg GM, Gerdle B (2005) Women with fibromyalgia: work and rehabilitation. DisabilRehabil 27: 685-694.
- Kivimäki M, Leino-Arjas P, Kaila-Kangas L, Virtanen M, Elovainio M, et al. (2007) Increased absence due to sickness among employees with fibromyalgia. Ann Rheum Dis 66: 65-69.
- Skaer TL (2016) Treatment recommendations for fibromyalgia. Intl J Pharma Res Rev 5: 19-28.
- Russell IJ (2008) Fibromyalgia syndrome: approach to management. CNS Spectr 13: 27-33.
- Â White KP, Harth M (1996) An analytical review of 24 controlled clinical trials for fibromyalgia syndrome (FMS). Pain 64: 211-219.
- Bidonde J, Busch AJ, Bath B, Milosavljevic S (2014) Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. CurrRheumatol Rev 10: 45-79.
- Busch AJ, Webber SC, Brachaniec M, Bidonde J, Bello-Haas VD, et al. (2011) Exercise therapy for fibromyalgia. Curr Pain Headache Rep 15: 358-367.
- Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, et al. (2013) Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 12.
- Häuser W, Klose P, Langhorst J, Moradi B, Steinbach M, et al. (2010) Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 12: 79.
- Langhorst J, Klose P, Dobos GJ, Bernardy K, Häuser W (2013) Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. RheumatolInt 33: 193-207.
- Langhorst J, Häuser W, Lauche R, Perrot S, Alegre C, et al. (2014) Complementary and alternative medicine for the treatment of fibromyalgia. Evid Based Complement Alternat Med.
- Jones KD (2015) Recommendations for resistance training in patients with fibromyalgia. Arthritis Res Ther 17: 258.
- Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, et al. (2012) Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag 18: 119-126.
- Häuser W, Thieme K, Turk DC (2010) Guidelines on the management of fibromyalgia syndrome - a systematic review. Eur J Pain 14: 5-10.
- Burckhardt CS (2006) Multidisciplinary approaches for management of fibromyalgia. Curr Pharm Des 12: 59-66.
- Goldenberg DL, Burckhardt C, Crofford L (2004) Management of fibromyalgia syndrome. JAMA, 2004. 292: 2388-2395.
- Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, et al. (2010) Psychological treatments for fibromyalgia: a meta-analysis. Pain 151: 280-295
- Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W (2013) Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev.
- Sil S, Kashikar-Zuck S (2013) Understanding why cognitive-behavioral therapy is an effective treatment for adolescents with juvenile fibromyalgia. Int J Clin Rheumtol.
- Skaer T (2015) Research findings using mindfulness-based interventions for chronic pain. Pain Studies and Treatment 3: 38-45.
- Theadom A, Cropley M, Smith HE, Feigin VL, McPherson K (2015) Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev
- Van Gordon W, Shonin E, Dunn TJ, Garcia-Campayo J, Griffiths MD, et al. (2017) Meditation awareness training for the treatment of fibromyalgia syndrome: A randomized controlled trial. Br J Health Psychol 22: 186-206.
- Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S, et al. (2013) A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res 75: 500-510.
- Lakhan SE, Schofield KL (2013) Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PLoS One 8: e71834.
- Henke M, Chur-Hansen A (2014) The effectiveness of mindfulness-based programs on physical symptoms and psychological distress in patients with fibromyalgia: a systematic reived. Int J Wellbeing 4: 28-45.
- Singh BB, Wu WS, Hwang SH, Khorsan R, Der-Martirosian C, et al. (2006) Effectiveness of acupuncture in the treatment of fibromyalgia. AlternTher Health Med 12: 34-41.
- Deare JC, Zheng Z, Xue CC, Liu JP, Shang J, et al. (2013) Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev 5: CD007070.
- Smith HS, Bracken D, Smith JM (2011) Pharmacotherapy for fibromyalgia. Front Pharmacol 2: 17.
- Firestone KA, Holton KF, Mist SD, Wright CL, Jones KD, et al. (2012) Optimizing fibromyalgia management. Nurse Pract 37: 12-21.
- Häuser W, Wolfe F, Tölle T, Uçeyler N, Sommer C, et al. (2012) The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 26: 297-307.
- Lauche R, Häuser W, Jung E, Erbslöh-Möller B, Gesmann M, et al. (2013) Patient-related predictors of treatment satisfaction of patients with fibromyalgia syndrome: results of a cross-sectional survey. ClinExpRheumatol 31: S34-40.
- Clark P, Eduardo S P, Ginovker A, Salomón PA (2013) A patient and physician survey of fibromyalgia across Latin America and Europe. BMC Musculoskelet Disord 14: 188.
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, et al. (2017) EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 76: 318-328.
Citation: Skaer TL (2017) Current Issues Regarding the Care of the Fibromyalgia Patient. Fibrom Open Access 2: 120.
Copyright: © 2017 Skaer TL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 6785
- [From(publication date): 0-2017 - Aug 23, 2025]
- Breakdown by view type
- HTML page views: 5780
- PDF downloads: 1005