Cutaneous Neurofibroma of the Ear Pinna: A Report of Two Case
Received: 08-Jul-2021 / Accepted Date: 27-Sep-2021 / Published Date: 04-Oct-2021 DOI: 10.4172/2161-119X.1000461
Abstract
Aim & Objective: To present the current considerations, natural history and treatment modalities of cutaneous neurofibromas (cNF)
Methods: Cutaneous Neurofibroma experts from various research and clinical backgrounds reviewed the terms currently in use for cNF as well as the clinical, histologic, and radiographic features of these tumors using available data.
Aim & Objective: To present the current considerations, natural history and treatment modalities of cutaneous neurofibromas (cNF)
Methods: Cutaneous Neurofibroma experts from various research and clinical backgrounds reviewed the terms currently in use for cNF as well as the clinical, histologic, and radiographic features of these tumors using available data.
Results: Neurofibromas develop within nerves, soft tissue, and skin. The primary distinction between cNF and other neurofibromas is that cNF are limited to the skin whereas other neurofibromas may involve the skin, but are not limited to the skin. There are important cellular, molecular, histologic, and clinical features of cNF. Each of these factors is discussed in consideration of a clinicopathologic framework for cNF.
Conclusion: The development of various treatment options for neurofibroma requires formulation of diagnostic criteria that encompass the clinical and histologic features of these tumors. However, the rear several areas of overlap between cNF and other neurofibromas that make distinctions between cutaneous and other neurofibromas more difficult, requiring careful deliberation with input across the multiple disciplines that encounter these tumors and ultimately, prospective validation. The ultimate goal of this work is to facilitate accurate diagnosis and meaningful therapeutics for cNF.
Keywords: Neurofibroma; Neurofibromatosis (NF); Schwann’s cells; Neurons; Biopsy; Haemorrhagic
Introduction
Neurofibromas are circumscribed but non-encapsulated neoplasms of the nervous system. They can arise in all peripheral nerve elements, including Schwann’s cells, neurons, fibroblasts and perineural cells. Neurofibromas are usually benign. Malignant transformation has been reported to occur in 2 to 16% of cases [1].
The most common and prominent location of neurofibromas is the skin. Discrete lesions are often referred to as dermal or cutaneous neurofibromas. CNF are benign and, unlike plexiform neurofibromas (pNF), are not known to have any malignant potential. Neurofibromas may also develop within oral mucous membrane [1,2].
Neurofibromas are defined as histologically benign (WHO grade I) tumors composed of multiple cell types including Schwann cells, fibroblasts, immune cells (such as mast cells and macrophages) and other elements of nerve. Regardless of their location, all neurofibromas share certain histologic and cellular characteristics [3].
It was proposed at the European NF meeting by Ortonne et al. and Stemmer Rachamimov et al., in which a comprehensive synthesis of clinical and pathologic features was attempted defining neurofibromas by their clinical appearance and histologic features: growth pattern (diffuse/infiltrating or localized), relationship to nerve (intraneural or extra neural, without any perineural capsule), and anatomic location (cutaneous, cutaneous/subcutaneous, deep).The resulting categories proposed were cutaneous NF (discrete) and larger NF involving skin and subcutaneous tissue (diffuse, with or without atypia) [4] (Table 1).
|
Proposed classification |
Proposed by |
|---|---|
|
Cutaneous neurofibronias |
Jouhilahti et al.[3] |
|
Localized cutaneousneurofibromas |
Carroll and Ratner[4] |
|
Localized neurofibromas |
Rosenberg[5] |
|
Dermal neurofibromas Nodular neurofibromas |
Huson[6] |
|
Discrete cutaneous neurofibromas of 1he dennis and epidermis (endoneurial neurofibroma) |
Friedman and Riccardi[7] |
|
Cutaneous neurofibromas |
Goldblum et al[8] |
|
Localized cutaneous neurofibromas Localized subcutaneous neurofibromas |
Stemmer-Rachamimmov. wolkenstein. and Ortonne (unpublished, 2008)[1] |
Table 1: Clinicopathologic classification of neurofibromas.
Clinical Presentation
Case 1
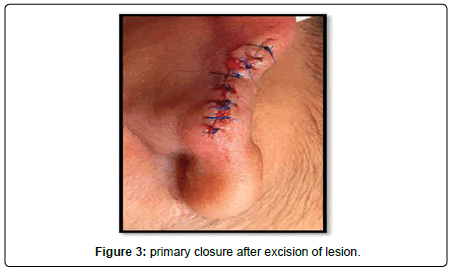
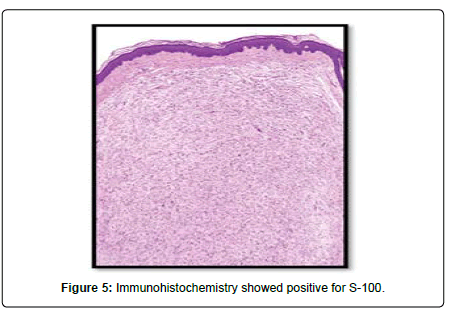
A 23-year-old lady presented to the Oral and Maxillofacial surgery outpatient department of our hospital with complaint of a swelling over the right pinna since past 6 months (Figure 1). It was insidious in onset and slowly increasing in size. Upon undertaking the clinical history patient gave history of piercing the ear at an ornament shop. There was no history of bleeding from the swelling and no history of such lesions elsewhere in the body. There was no significant family history. Examination of pinna showed single 1× 1 cm reddish brown swelling with an irregular surface seen on medial and lateral surface of the lobule. It was firm in consistency, tender, did not bleed on touch, and overlying skin was involved. Extraoral, Intraoral, Nose and throat examination was normal. There was no significant finding on systemic examination. Bilingual written informed consent was taken prior to the procedure. After the baseline investigation. The lesion was excised under local anaesthesia with approximately 0.5 cm margin of the healthy tissue and wound was closed primarily (Figure 2). The wound healed within a week. There was no recurrence seen in 1 year follow up. Histopathological examination showed a lesion composed of spindle shaped cells arranged in short fascicles, whorling and storiform patterns. Nuclei were ovoid to elongated, vesicular and show mild pleomorphism. No atypical or necrosis was seen. The lesion was infiltrating the dermal collagen and entrapping the skin appendages. Subcutaneous fat was free. On immunohistochemistry tumor cells were strongly positive for S-100, suggestive of neurofibroma (Figure 3).
Case 2
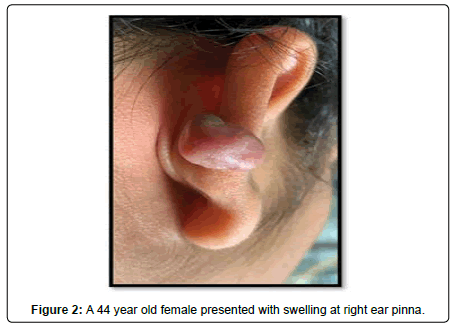
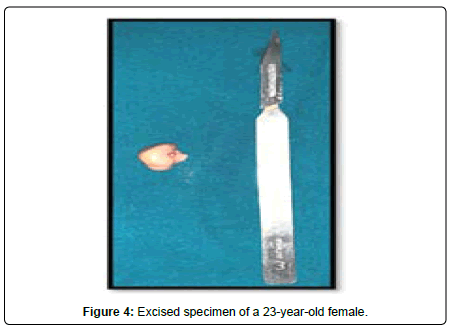
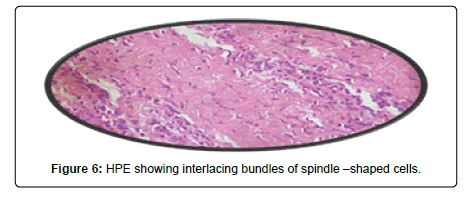
A 44-year-old female presented with a 3-year history of swelling at the pinna of left ear. In the examination, a solid mass of approximately 2×1 cm was found, involving the skin (Figure 4). The skin over the mass was somewhat bluish. The mass was tender upon palpation. There was no significant family history. Similar clinical history of ear piercing like previous case was obtained. Extraoral, Intraoral, Nose and throat examination was normal. There was no significant finding on systemic examination. Baseline investigation were done. Bilingual written informed consent was taken prior to the procedure. The facial nerve function was normal, and there was no sensory deficit in the pinna skin. A biopsy of the mass in the postauricular area was somewhat haemorrhagic and revealed microscopic features of a neurofibroma. The surgical excision under local anaesthesia with approximately 0.5 cm margin of the healthy tissue was done and wound was closed primarily (Figure 5). There was no recurrence seen in 1-year follow-up. Histopathological examination showed non-encapsulated lesion in the dermis. The tumor consists of interlacing bundles of spindle –shaped cells. The cells had wavy serpentine nuclei and pointed ends (Figure 6).
Discussion
Neurofibromas (NF) are one of the most common disorders and can affect anyone, regardless of family history, race, gender, or ethnic background. Neurofibromatosis disorders, including neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis, are complex genetic conditions that can affect many different organ systems in the body. Neurofibromatosis type 1 is the most common form of neurofibromas. In about half of people with neurofibromatosis type 1, the disorder is inherited from a parent. In the other half, the disorder is the result of a new, or spontaneous, mutation or loss of a portion of the neurofibromatosis 1 gene. The most common signs of the condition include flat, brown spots on the skin called café-au-lait spots. These usually appear within the first year of life and are often present at birth [1].
Neurofibromatosis type 2 is much less common than type 1, occurring in about 1 in 40,000 births. Only a minority of people with neurofibromatosis type 2 have café-au-lait spots or skin tumors resembling those seen in type 1. In neurofibromatosis type 2, tumors also frequently occur on nerves other than the eighth cranial nerve which can cause ringing in the ears, problems with balance, and progressive hearing loss. Tumors can grow large enough to cause headaches and press on nearby nerves, causing problems with facial weakness. Schwannomatosis is a rare form of neurofibromatosis that was first recognized in the 1990s. It may affect as many as 1 in 40,000 people. People who have schwannomatosis do not develop vestibular schwannomas, nor do they typically develop other types of tumors associated with neurofibromatosis type 1 or neurofibromatosis type 2, such as meningiomas, ependymomas, or neurofibromas [1-4].
Neurofibromas are tumours derived from Schwann cells, fibroblasts and the supporting cells known as perineural cells. They can grow anywhere in the body where there are nerve cells. This includes nerves just under the surface of the skin, as well as nerves deeper within the body, spinal cord, and/or brain. A nerve is a necessary component for proliferation, development, and maintenance of NF1-deficient Schwann cells through the perineural microenvironment that releases factors such as Neuregulin 1 (NRG1) [4].
Histologically, all neurofibromas are mixed tumors consisting of cells of diverse lineages.
They are composed of Schwann cells and non-neoplastic elements including mast cells and fibroblasts, perineurial cells, hair follicles, exocrine sweat glands, sebaceous glands, melanocytic cells & adipocytes. The diverse cellular components of neurofibromas are embedded in an abundant collagenous and often myxoid extracellular matrix. All neurofibromas elsewhere in the body show similar histological features and cell composition. (Figure 7) Solitary lesions are not usually associated with systemic manifestations unlike multiple lesions which are commonly seen in patients with neurofibromatosis or von Recklinghausen’s disease. A single or few neurofibromas alone do not indicate NF [5, 6]
Anatomical classification of cNF is ordered by stage according to appearance [7].
• During their nascent stage, cNF cannot be seen by the visible eye, but ultrasound or other forms of imaging can detect the dermal mass.
• The sessile stage of the cNF occurs when a visible papule is located on the skin.
• Subsequently, it moves to the globular stage, which is a larger nodule with a 20–30 mm height and comparable base.
• The final stage is the pedunculated stage signified by the extrusion of dermal cNF contents into a mass above the skin attached by a stalk.
Potential unified classification system for cutaneous neurofibromas [8, 9]
A. Cutaneous neurofibromas (discrete)
1. Clinical variants:
• Nascent (seen on ultrasound or similar imaging only)
• Flat
• Sessile
• Globular
• Pedunculated
B. Extensive neurofibromas (with or without atypia)
2. Histologic variants:
• Diffuse with or without particular differentiation (melanocytic, Meissner/round cells)
• Diffuse associated with plexiform (plexiform and diffuse)
• Diffuse, not otherwise specified
• Plexiform intraneural
cNF are common and a major clinical burden for people, prominently affecting emotional well-being as well as physical comfort. Currently, the only way of treating cNF is removal by procedural methods such as electrodessication, CO2 laser, or surgery. Developing effective medical therapies for existing cNF and prevention of cNF are priorities for the majority of adults. Development of a reliable and widely accepted literature based on clinical genetic, and radiologic features of cNF is a crucial step in investigating these tumors to better understand their development and start to uncover opportunities for tumor specific therapies that will allow reduction in the clinical symptoms and burdens of cNF. The work presented here creates a framework for prospective validation studies to more accurately define cNF. Ultimately, this will facilitate score diagnosis of these lesions and the efficient development of therapeutics [10, 11].
Most tumours caused by NF need no treatment. But tumours that are painful, disfiguring, growing rapidly, impairing function or compressing other body parts may need attention [12].
Physical destruction of cNF may also be performed by thermal necrosis through other forms including electrodessication and diathermy loop excision [13].
Electrodessication, a form of radiofrequency ablation, uses needletip cautery at alternating electrical currents to illicit minimal thermal damage to tissue with instant hemostasis. This may be used to treat >500 cNF at one sitting but will require general anaesthesia under these circumstances. Electrodessication should not be employed as the primary mode of cNF removal in patients with limited cNF burden due to epidermal and dermal damage, which will result in dyspigmented scarring as well as in patients with larger cNF. It is also important to note that patients with cardiac pacemakers should not undergo electrodessication on the trunk, back, or neck [14].
Monopolar diathermy loop uses a heated, metal loop to simultaneously remove and necrotize the cNF tissue and provide cauterization for healing by secondary intention. This technique is not recommended in cosmetically sensitive areas due to inevitable depigmented scarring at each site of removal [15].
Conclusion
It has been suggested that subtotal resection of neurofibromas is preferable to a complete excision as the latter procedure may result in a loss of normal tissue and still not prevent a recurrence. Trevisani et al wrote that ‘complete surgical excision of these lesions is virtually impossible; if not contraindicated’. Crikelair and Cosman emphasized that amelioration rather than complete eradication should be the therapeutic goal [15].
Ketotifen, noncompetitive H1 antihistamine antagonist and mast cell stabilizer, has seen off-label utility by blocking degranulation of mast cells. A topical application of 5% imiquimod has shown decrease in tumor volume (measured by calipers) and the degree of infiltrating inflammatory cells around the region of application in duration of 4 months [15].
References
- Ortonne N, Wolkenstein P, Blakeley J, Korf B, Plotkin S, et al. (2018) Cutaneous Neurofibromas: Current Clinical and Pathologic Issues. Neurol 91: S5-S13.
- Jo V, Fletcher C Â (2014) WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathol 46: 95-104.
-  Jouhilahti E, Peltonen S, Callens T, Jokinen E, Heape A, et al. ( 2011)The Development of Cutaneous Neurofibromas. Am J Pathol  178: 500-505.
- Carroll S, Ratner N (2008) How does the Schwann cell lineage form tumors in NF1? Glia 56: 1590-1605.
- Rosenberg A (2013) WHO Classification of Soft Tissue and Bone, Fourth Edition: summary and commentary. Curr Opin Oncol 25: 571-3.
- Huson S (1994) Neurofibromatosis 1: a clinical and genetic overview. The neurofibromatoses: a pathogenetic and clinical overview. 167–168.
- Friedman JM, Riccardi VM (1999) An overview of NF-1: dysplasia and neoplasia.
- Goldblum JR, Folpe AL, Weiss SW, Enzinger FM, Weiss SW (2013) Enzinger and Weiss’s Soft Tissue Tumors. Philadelphia: Saunders.
- Verma S, Riccardi V, Plotkin S, Weinberg H, Anderson R, et al. (2018) Considerations for development of therapies for cutaneous neurofibroma. Neurol 91: S21-S30.
- Brenaut E, Marcorelles P, Genestet S, Ménard D, Misery L (2015)  Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol  72: 328-32.
- Cannon A, Jernigan K, Korf B, Widemann B, Casey D, et al. (2018) Clinical Trial Design for Cutaneous Neurofibromas. Neurol 91: S31-S37.
- Brosseau J, Pichard D, Legius E, Wolkenstein P, Lavker R, et al.(2018) The biology of cutaneous neurofibromas: consensus recommendations for setting research priorities. Neurol 91: S14-S20.
- Pinna V, Lanari V, Daniele P, Consoli F, Agolini E, et al. (2015) Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Neurofibromas 23: 1068-71.
- Ferner R, Thomas M, Mercer G, Williams V, Leschziner G, et al. (2017) Â Evaluation of quality of life in adults with neurofibromatosis 1 (NF1) using the Impact of NF1 on Quality Of Life (INF1-QOL) questionnaire. Health Qual Life Outcomes. Neurofibromatosis 15: 34.
- Bicudo N, De Menezes Neto B, Da Silva de Avó L, Germano C, Melo D (2016) Quality of Life in Adults with Neurofibromatosis 1 in Brazil. J Genet Couns Neurofibromatosis 25: 1063-74.
Citation: Bhende R, Abdul N, Kshirsagar R, Patankar S, Banerjee P, et al. (2021) Cutaneous Neurofibroma of the Ear Pinna: A Report of Two Cases. Otolaryngol (Sunnyvale) 11: 461. DOI: 10.4172/2161-119X.1000461
Copyright: © 2021 Bhende R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3013
- [From(publication date): 0-2021 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 2101
- PDF downloads: 912