Cyclin A1 Expression is Reciprocally Controlled by the Transcription Factor ZNF217 and miRNAs in Invasive Breast and Prostate Cancer Cells: An In Silico Analysis
Received: 09-Feb-2018 / Accepted Date: 26-Feb-2018 / Published Date: 05-Mar-2018
Abstract
Cyclins and their partner cyclin-dependent kinases (CDKs) play crucial roles in proliferation, initiation of DΝΑ replication, and mitosis. Human cyclin Α1 is expressed at its highest levels in spermatocytes and is re-expressed in leukemic cell lines, in cells from acute myeloid leukemia (ΑΜL) and acute promyelocytic leukemia (ΑΡL), as well as in metastatic breast cancer, hepatocarcinomas and prostate cancer. Transgenic mice engineered to express cyclin Α1 in myeloid precursor cells develop ΑΜL with low penetrance and long latency. In this work we have mined and analyzed data from the Gene Expression Omnibus (GEO) that potentially reveal novel cyclin A1 mechanisms of action in metastatic breast and prostate tumors. The data suggest that in breast and prostate tumors, cyclin A1 expression is repressed by two miRNAs and activated by the transcription factor ZNF217, and that cyclin A1 is overexpressed in invasive prostate cancer but not in pre-invasive carcinoma. Down-regulation of the miRNAs and overexpression of ZNF217 correlate with epithelial-to-mesenchymal transition (EMT), suggesting that EMT may be partly mediated by ZNF217-mediated re-expression of cyclin A1. Collectively, these data argue that re-expression of cyclin A1 protein may contribute to the mesenchymal-to-epithelial transition in the aforementioned tumor types. Since cyclin A1 protein is not expressed in normal adult tissues, except germ cells, it may be a promising target for intervention. We discuss implications of these findings in further dissecting the role of cyclin A1 protein in cancer development and for its targeting.
Keywords: Spermatocytes; Cyclin A1 protein; Breast cancer; Prostate cancer; Acute myeloid leukemia; Acute promyelocytic leukemia
Abbreviations
AML: Acute Myeloid Leukemia; ATRA: All-trans Retinoic Acid; CDK: Cyclin Dependent Kinase; MDM2: Mouse Double Minute; miRNA: microRNA; ZNF217: Zinc Finger Protein 217.
Introduction
Cyclin A1 in human myeloid leukemias
The human cyclin Α1 was first identified in the mouse and in human ΑΜL cell lines by virtue of its high levels of expression [1-3]. It is expressed in nearly 99% of all AML samples and its mRNA has been detected in several myeloid leukemia cell lines, including ML-1, U937, ΝΒ4, KG-1, and ΤΗIΖ1. Molecular mechanisms underlying the oncogenic activation of cyclin A1 in various types of cancers as well as its role remain unknown. Cyclin A1 is required for normal meiosis in the mouse [4] and its forced overexpression in transgenic mice leads to a myelodysplastic syndrome and eventually to leukemia, although with low penetrance and long latency [5]. In normal hematopoietic cells cyclin A1 is predominantly localized in the nucleus however it is found in the cytoplasm in cells from AML patients, suggesting that its involvement in the transformation of leukemic cells may be linked to forming predominantly alternative complexes with the cyclin dependent kinases, CDK1 and CDK2 and with novel substrates, activating their kinase activity [6,7]. It has been shown that cyclin A1 plays a role in mediating the response of leukemic cells to ATRAtreatment. Treatment with ATRA enables the relocalization of cyclin A1 from the cytoplasm to the nucleus [8,9]. Several studies have demonstrated that cyclin A1 is important in DNA repair mechanisms by binding to the nuclear proteins that are required for DNA repair. Altered expression and modification of cyclin A1 protein is associated with sensitivity to apoptosis induced by anti-tumor therapeutic agents [10,11]. These data suggest that cyclin A1 expression plays a role in the etiology of myeloid leukemias and is likely to be a downstream target of the aberrant fusion proteins that characterize APL.
Role of cyclin A1 in solid tumors
The role of cyclin A1 in solid type tumors is less well known. Data exist for breast cancer cell lines warranting further exploration given its tissue-restricted expression. Recently it was shown that cyclin A1 contributes to prostate cancer cell invasion via interactions with the VEGF and AR pathways [12,13]. Cyclin A1 tumorigenic pathways are closely linked with developmental networks such as pathways where the six1 homeobox protein is active. Oncogenic induction of six1 causes cyclin A1 levels to increase and correlates with enhanced proliferation of mammary carcinoma cells whereas knockdown of ccnA1 in the same cells prevents their proliferation when six1 is ectopically overexpressed.
Role of miRNAs in solid tumors
Metastatic burden can be heavy in cancer. An understanding of molecular mechanisms of metastasis may lead to the identification of suitable targets, especially among the miRNAs some of which have metastasis-promoting or metastasis-inhibiting roles. Several pathways are affected by interactions with miRNAs. Specifically, miR-335 and miR-205 are found to be either deleted or under-expressed in breast and prostate cancers with miR-335 commonly deleted in human breast cancers. Notably, whereas miR-335 is critical for breast cancer reinitiation [14], miR-205 is involved in invasive cancer. For example, compared to matched normal prostatic tissues, miR-205 expression levels are reduced in prostate cancer cell lines as well as in tumor, especially in patients with local-regionally disseminated disease [15].
The restoration of the expression of miR-205 in prostate cancer cells results in reversal of the mesenchymal-to-epithelial transition. This is indicated by up-regulation of E-cadherin, an epithelial cell marker, and by reduction of cell mobility and invasion. Also, it results in the downregulation of several oncogenes, such as interleukin 6, caveolin-1 and EZH2 , which are known to be involved in disease progression. Interestingly, enforced expression of the miR-200 family alone is sufficient to prevent TGF-beta-induced EMT, the reverse of MET. Ectopic overexpression of mir-200 microRNAs in mesenchymal cells reverses the mesenchymal to epithelial transition by targeting ZEB. Expression of these microRNAs is lost in invasive breast cancer cell lines with the mesenchymal phenotype. Notably, expression of miR-200 family members is also lost in metaplastic breast cancer specimens lacking E-cadherin [16].
Role of the zinc-finger, transcription factor ZNF217 in solid tumors
The Krüppel-like zinc finger protein ZNF217 has been established as an oncogene in breast cancer and it may play critical roles in other solid tumors. Enhanced expression of ZNF217 mRNA correlates with poor prognosis and the development of metastases in breast cancer. Moreover, its overexpression in breast cancer cells stimulates migration and invasion in vitro and induces lung or node metastases in mice in vivo .
Notably, ZNF217 also induces epithelial-to-mesenchymal transition (EMT) in human mammary epithelial cells, through the TGF-β- activated, SMAD signaling pathway [17]. Also, in lung carcinoma cell lines, ZNF217 may act as an oncogene by recruiting deacetylase/ demethylase complexes by interacting with MDM2 to inhibit acetylation of p53 [18].
In this work, we have mined gene expression microarray data which demonstrate the following:
A) CcnA1 mRNA levels are reduced by expressed miR-335 and miR-205 in breast and prostate cancer cells.
B) knock-down of either miRNA with siRNA increases the levels of ccnA1 mRNA, suggesting a role in its regulation.
C) Cyclin A1 expression levels correlate with invasive prostate cancer
D) That the transcription factor ZNF217 increases expression of ccnA1 mRNA, in invasive breast cancer cells.
We discuss plausible mechanisms of control of the epithelial-tomesenchymal transition by cyclinA1 and ZNF2117.
Materials and Methods
Using the term “cyclin A1 and invasive and cancer ” as a query in the Gene Expression Omnibus, GEO, database, returned 50 studies of which only three fulfilled the criterion of ccnA1 mRNA expression levels being significantly changed by re-expression of miRNAs (Table 1). Data were downloaded as SOFT files in Excel and subjected to statistical analysis using Student’s t-test in order to evaluate the significance of change in expression levels of the ccnA1 gene. Gene expression data for ccnA1 from study GDS 3138 [14,19] were analyzed with Student’s t-test for significance.
| GEO STUDY | VARIABLE | 1st AUTHOR | PMID |
|---|---|---|---|
| GDS3138 | MIR-335 | Tavazoi, Png | 18185580, 21289068 |
| GDS3634 | MIR-205 | Gavidline | 19244118 |
| GDS4885 | ZNF217 | Vendrell | 25593193 |
Table 1: GEO gene expression studies showing significant changes in ccnA1 mRNA levels by miRNAs and ZNF217 .
The null hypothesis was that there is no significant difference between control and experimental samples. Y-axis: arbitrary normalized transcriptomic microarray expression values. Three expression values were used for each group and a p-value was computed for each one.
Results
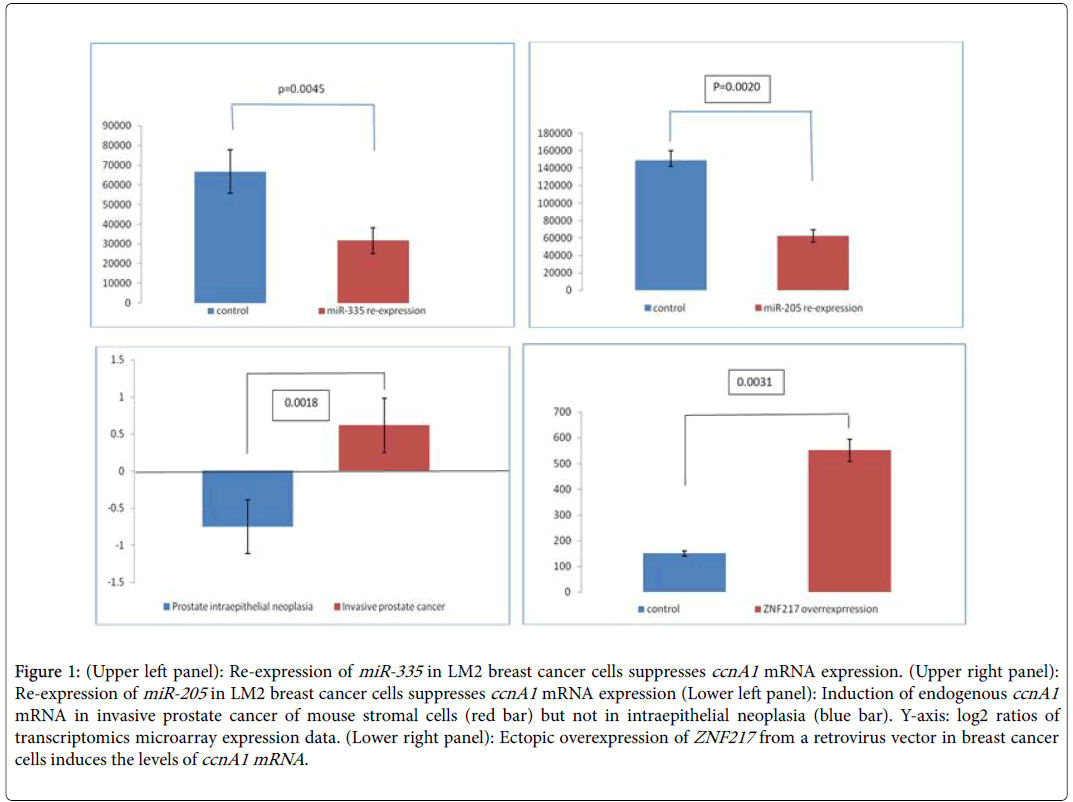
The epithelial-to-mesenchymal transition (EMT) in invasive breast and prostate cancers is a complex phenomenon [20-22]. It is critical for cancer invasive behavior, and is currently studied with the aim of finding suitable targets [23,24]. In this work we have mined publicly available data from breast and prostate cancer cell lines to identify links between microRNA signatures, the transcriptional factor ZNF2117 and cyclin A1 in invasive breast and prostate cancers. Our rationale was based on the fact that miRNAs suppress cyclin A1 expression levels in these tumors and counteract the activating effect of the transcription factor ZNF217 . We identified three datasets (Table 1) in GEO that report results of the effect of overexpressing miR-335 (breast) and miR-205 (breast and prostate cancer respectively) on the levels of cyclin A1 mRNA. We extracted the data for cyclin A1 and subjected them to elementary statistical analysis in order to validate that significant differences exist in cyclin A1 levels between noninvasive and advanced invasive cancers. Re-expression of miR-335 in LM2 breast cancer cells reduces the levels of cyclin A1 mRNA by at least 50% (Figure 1A, p=0.0045) and this correlates with a decrease in invasive behavior. In DU145 prostate cancer cells, re-expression of miR-205 (Figure 1B, p=0.0020) also leads to significantly decreased ccnA1 mRNA levels. In a study of stromal cells in a mouse model on breast cancer invasiveness (Table 1, GDS2443), the levels of ccnA1 mRNA were significantly increased in invasive prostate cancer cells (Figure 1C) but were reduced in intraepithelial, non-invasive prostate neoplasia (Figure 1C, p=0.0018). Since the levels of these miRNAs are substantially reduced in these invasive cancers, we conclude that they might act as tumor suppressors and that in their absence, ccnA1 mRNA levels are increased, contributing to invasive growth. Notably, in both tumor types, the absence of these miRNAs and overexpression of cyclin A1 correlate with metastasis. On the other hand, restoring the expression of these microRNAs in malignant LM2 breast cancer cells suppresses lung and bone metastasis by human cancer cells in vivo [14,19].
Figure 1: (Upper left panel): Re-expression of miR-335 in LM2 breast cancer cells suppresses ccnA1 mRNA expression. (Upper right panel): Re-expression of miR-205 in LM2 breast cancer cells suppresses ccnA1 mRNA expression (Lower left panel): Induction of endogenous ccnA1 mRNA in invasive prostate cancer of mouse stromal cells (red bar) but not in intraepithelial neoplasia (blue bar). Y-axis: log2 ratios of transcriptomics microarray expression data. (Lower right panel): Ectopic overexpression of ZNF217 from a retrovirus vector in breast cancer cells induces the levels of ccnA1 mRNA.
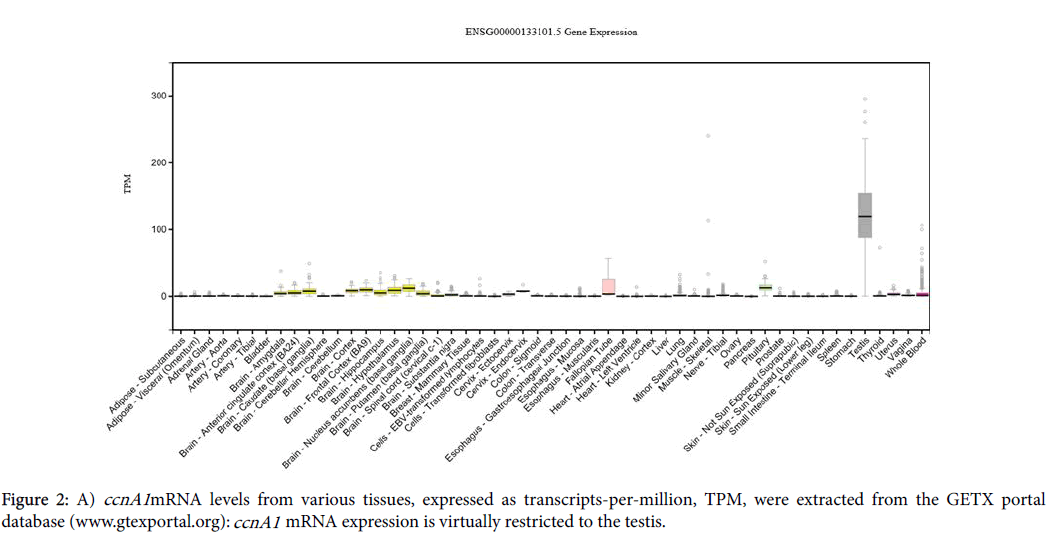
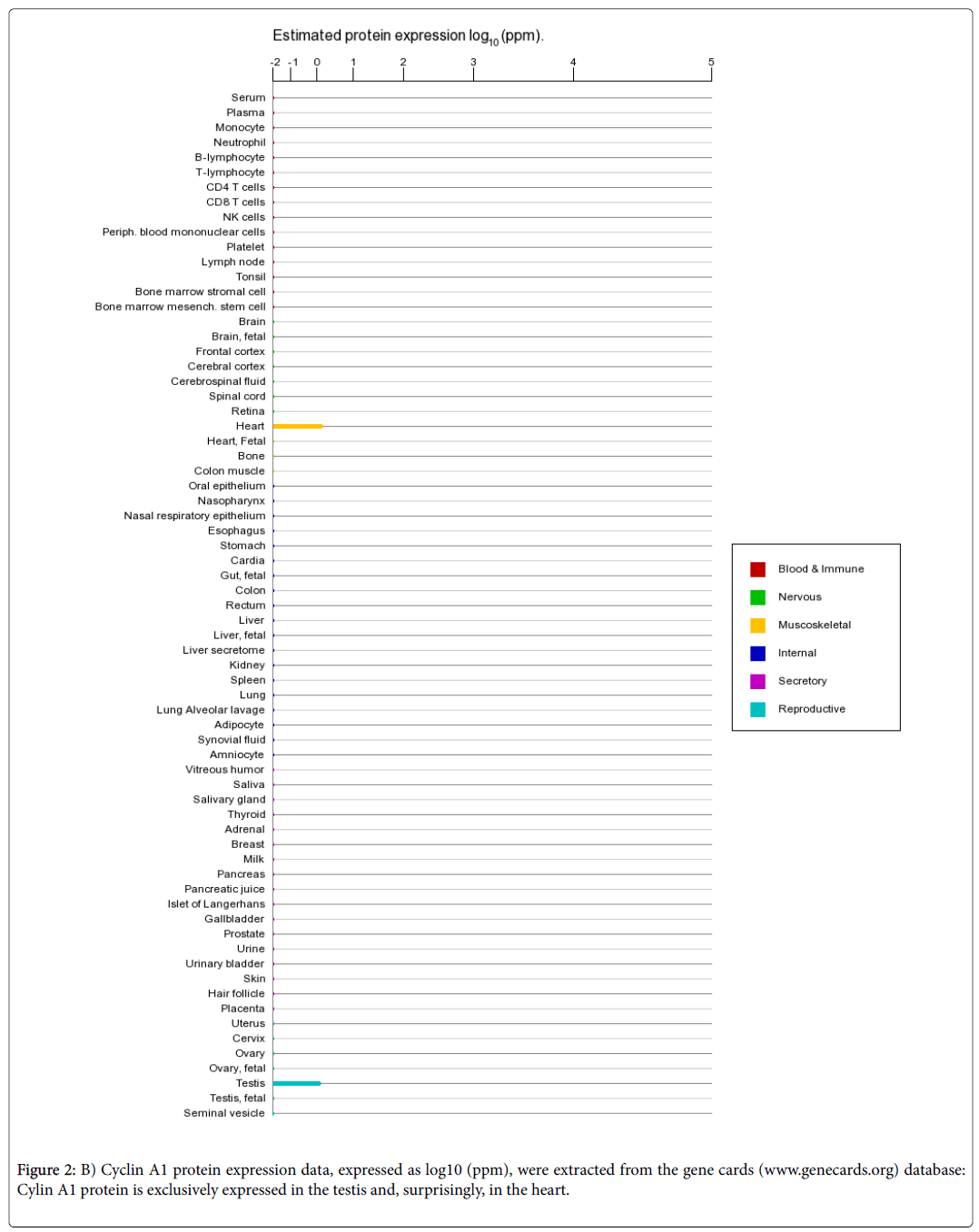
In conclusion, we have identified two miRNAs that suppress ccnA1 expression in two cancer cell lines with invasive properties and also we have found that overexpression of ZNF217 correlates with activation of ccnA1 mRNA expression. When viral constructs of miR-335 and miR-205 are introduced into these cells, they reduce ccnA1 mRNA and protein levels significantly and inhibit invasiveness of LM2 cells. Moreover, miR-205 not only down-regulates expression of ccnA1 , but also, it promotes the mesenchymal-to-epithelial transition and reduces tumorigenicity in DU145 prostate cancer cells [17]. In terms of message and protein expression levels in adult cells, ccnA1 mRNA is expressed predominantly in testis and only at low levels in other tissues (Figure 2A), whereas the protein is exclusively expressed in developing spermatocytes in the testis (Figure 2B). Surprisingly, protein also is found in the heart (Figure 2B), however its significance remains unknown.
Discussion
Expression of miR-335 is lost in the majority of primary breast tumors from patients who relapse, and the loss of expression of microRNA is associated with poor, distal metastasis-free survival. Therefore, restoring the expression of these microRNAs in malignant LM2 cells could suppress lung and bone metastasis by human cancer cells in vivo . In agreement with these findings, re-expression of miR-335 in breast cancer cell lines leads to loss of invasive ability decreased cyclin A1 mRNA and reversal of EMT. MiR-335 is thus identified as a metastasis suppressor miRNA in human breast cancer cell lines and miR-205 in both breast and prostate cells. The correlation of miR-335 and cyclin A1 expression is indicative of their opposite roles in the biology of breast cancer cells. The data suggest that expression of miR-335 is part of a network of growth control that counteracts expression programs of growth in which cyclin A1 participates.
In breast or prostate cancer cells, EMT, and its reverse, MET, are linked to invasion and are controlled by, among other factors, the transcription factor ZNF217 . Overexpression of ZNF217 in breast cancer cells enhances in vitro migration and invasion and in vivo it contributes to the development of lung or node metastases in mice. ZNF217 is capable of promoting EMT in human mammary epithelial cells, and activates the TGF-β-activated, SMAD signaling pathway. We found that in breast cancer cells, overexpression of ZNF217 in nonmetastatic breast cancer cells, leads to cyclin A1 mRNA overexpression and this correlates well with invasive behavior and EMT suggesting that cyclin A1 may be instrumental in promoting EMT by overexpressed ZNF217 (Figure 1D, p=0.0031).
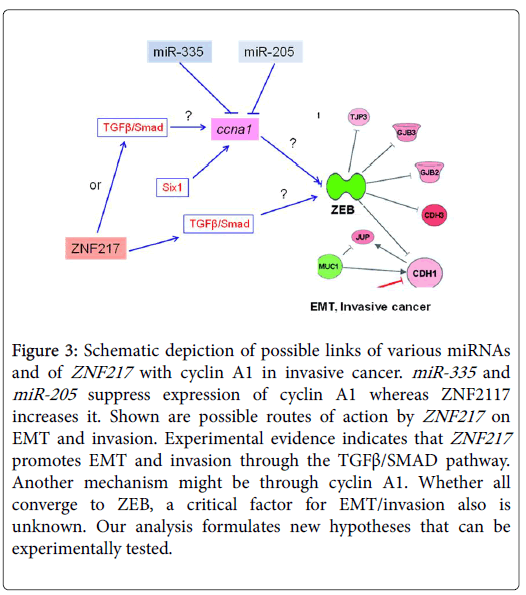
In both invasive breast and prostate cancer, cyclin A1 might be reciprocally regulated by mir-335 , miR-205 and ZNF217 and this could be relayed to ZEB which is a key factor in controlling EMT (Figure 3). Lower miR-335/205 expression levels and increased ZNF217 levels, could act as an additional signal which leads to cyclin A1 overexpression thus forcing cells to transit to an invasive phenotype. Since ZNF217 induces metastasis through the TGFβ/SMAD pathway ([17,24], we propose that it may do this partly via cyclin A1. As cyclin A1 expression is activated by the six1 oncogene, leading to EMT, this might be an alternative pathway of action by ZNF217 /cyclin A1. Whether these pathways converge to ZEB, a key factor in EMT and invasion, and whether cyclin A1 acts through ZEB remain to be established (Figure 3). As a corollary, the restoration of miR-335/205 expression in breast and prostate cancer cells could open an approach for reducing invasiveness. It is known that re-expression of these miRNAs results in cell rearrangements consistent with reversal as indicated by up-regulation of E-cadherin and reduction of cell locomotion and invasion, as well as in the down-regulation of several oncogenes known to be involved in disease progression (i.e., interleukin 6, caveolin-1, EZH2 ). The evidence suggests that these events are driven by the concurrent repression of specific predicted miR-205 targets, such as N-chimaerin, ErbB3 , E2F1 , E2F5 , ZEB2 , and protein kinase Cε. In conclusion, we have determined that miR_335 , mir-205 and ZNF217 control the levels of the cyclin A1 mRNA and this may implicate this cyclin in the control of EMT in invasive breast and prostate cancer cells.
Figure 3: Schematic depiction of possible roles of various miRNAs and ZNF217 on cyclin A1 role in invasive cancer. MiR-335 and miR-205 are inferred to suppress expression of cyclin A1 whereas ZNF2117 increases its expression. Shown are possible routes of action by ZNF217 on EMT and invasion. It has been experimentally established that ZNF217 promotes EMT and invasion through the TGFβ/SMAD pathway. Another mechanism might be through cyclin A1. Whether all converge to ZEB, a critical factor for EMT/invasion is unknown. Our analysis formulates new hypotheses that can be experimentally tested.
Acknowledgements
The corresponding author gratefully acknowledges grant # 88133 from the program for young investigators by the Aristotle University of Thessaloniki, Macedonia, Greece.
Conflict Of Interest
The authors declare no conflict of interest of any kind.
References
- Ekberg J, Holm C, Jalili S, Richter J, Anagnostaki L, et al. (2005) Expression of cyclin A1 and cell cycle proteins in hematopoietic cells and acute myeloid leukemia and links to patient outcome. Eur J Haematol 75: 106-115
- Holm C, Ora I, Brunhoff C, Anagnostaki L, Landberg G, et al. (2006) Cyclin A1 expression and associations with disease characteristics in childhood acute lymphoblastic leukemia. Leuk Res 30: 254-261.
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, et al. (1996) A distinct cyclin A is expressed in germ cells in the mouse. Development 122: 53-64.
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, et al. (1998) Cyclin A1 is required for meiosis in the male mouse. Nat Genet 20: 377-380.
- Liao C, Wang XY, Wei HQ, Li SQ, Merghoub T, et al. (2001) Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin A1. Proc Natl Acad Sci U.S.A. 98: 6853-6858.
- Panigrahi SK, Manterola M, Wolgemuth DJ (2017) Meiotic failure in cyclin A1-deficient mouse spermatocytes triggers apoptosis through intrinsic and extrinsic signaling pathways and 14-3-3 proteins. PLoS ONE 12: e0173926.
- Persson JL, Zhang Q, Wang XY, Ravnik SE, Muhlrad S, et al. (2005) Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction 130: 411-422.
- Salazar G, Liu D, Liao C, Batkiewicz L, Arbing R, et al. (2003) Apoptosis in male germ cells in response to cyclin A1-deficiency and cell cycle arrest. Biochem Pharmacol 66: 1571-1579.
- Salazar G, Joshi A, Liu D, Wei H, Persson JL, et al. (2005) Induction of apoptosis involving multiple pathways is a primary response to cyclin A1-deficiency in male meiosis. Dev Dyn 234: 114-123.
- Federico M, Symonds CE, Bagella L, Rizzolio F, Fanale D, et al. (2010) R-Roscovitine (Seliciclib) prevents DNA damage-induced cyclin A1 upregulation and hinders non-homologous end-joining (NHEJ) DNA repair. Mol Cancer 9: 208.
- Müller-Tidow C, Ji P, Diederichs S, Potratz J, Bäumer N, et al. (2004) The cyclin A1- CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol 24: 8917-8928.
- Syed Khaja AS, Dizeyi N, Kopparapu PK, Anagnostaki L, Härkönen P, et al. (2013) Cyclin A1 modulates the expression of vascular endothelial growth factor and promotes hormone-dependent growth and angiogenesis of breast cancer. PLoS ONE 8: e72210.
- Wegiel B, Bjartell A, Ekberg J, Gadaleanu V, Brunhoff C, et al. (2005) A role for cyclin A1 in mediating the autocrine expression of vascular endothelial growth factor in prostate cancer. Oncogene 24: 6385-6393.
- Png KJ, Yoshida M, Zhang XHF, Shu W, Lee H, et al. (2011) MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev 25: 226-231.
- Gandellini P, Folini M, Longoni N, Pennati M, Binda M, et al. (2009). miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res 69: 2287-2295.
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, et al. (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593-601.
- Vendrell JA, Thollet A, Nguyen NT, Ghayad SE, Vinot S, et al. (2012) ZNF217 is a marker of poor prognosis in breast cancer that drives epithelial-mesenchymal transition and invasion. Cancer Res 72: 3593-3606.
- Mantsou A, Koutsogiannouli E, Haitoglou C, Papavassiliou AG, Papanikolaou NA, et al. (2016) Regulation of expression of the p21CIP1 gene by the transcription factor ZNF217 and MDM2. Biochem Cell Biol 94: 560-568.
- Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, et al. (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451: 147-152.
- Bhatia S, Monkman J, Toh AKL, Nagaraj SH, Thompson EW, et al. (2017) Targeting epithelial-mesenchymal plasticity in cancer: clinical and preclinical advances in therapy and monitoring. Biochem J 474: 3269-3306.
- Davis FM, Stewart TA, Thompson EW, Monteith GR (2014) Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol Sci 35: 479-488.
- Weidle UH, Dickopf S, Hintermair C, Kollmorgen G, Birzele F, et al. (2018) The role of micro RNAs in breast cancer metastasis: Preclinical validation and potential therapeutic targets. Cancer Genomics Proteomics 15: 17-39.
- Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18: 128-134.
- De Craene B, Berx G (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13: 97-110.
Citation: Tsolkas G, Komninou D, Papanikolaou NA (2018) Cyclin A1 Expression is Reciprocally Controlled by the Transcription Factor ZNF217 and miRNAs in Invasive Breast and Prostate Cancer Cells: An In Silico Analysis. J Biochem Cell Biol 1: 104.
Copyright: 2018 Tsolkas G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5282
- [From(publication date): 0-2018 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 4300
- PDF downloads: 982