Cytokine levels in Plasma and PHA Stimulated T Cells in Healthy Indian Subjects
Received: 10-Sep-2018 / Accepted Date: 19-Sep-2018 / Published Date: 26-Sep-2018 DOI: 10.4172/2576-3881.1000122
Keywords: Activated T cell; IL-10; TGFβ; IFNγ; TNFα; Immune status
Introduction
Interleukins, Interferons, Tumor Necrosis Factors are small non-structural proteins which belong to the family of cytokines, released by white blood cells and have pleiotropic effects on various organs [1]. These chemical mediators are vital communication messengers responsible for cross talk between the immune cells which result in a range of physiological responses like inflammation to chemotaxis of immune cells [2,3]. Inflammatory (pro and anti) responses are mediated by interleukins expressed in white blood cells and are implicated in innate and adaptive immunity. Pro inflammatory and anti-inflammatory circuitry in the white blood cells promote chemotaxis of immune effector cells to give rise to physiological responses like wound healing, vasculogenesis and tissue repair. Interferons have shown to have antiviral properties and are implicated in antiviral responses [4].
The pro inflammatory cytokines include IFNγ, TNFα and anti-inflammatory responses include TGFβ and IL10, although it is not always the case due to the interplay and overlapping downstream effector pathways which could result in diverse physiological effects.
In the age of personalized medicine especially in cancer care, outcomes of immunotherapy using biologicals such as immune checkpoint inhibitors or cellular immune therapy such as T cells and Dendritic cells depends heavily on the general immunological status of the patient, which can be further altered by standard treatment options such as chemotherapy and radiation. The final outcomes rely on the general immune status of the patient which can be hampered if the patient is immuno-suppressed due to the malignancy and further immuno-compromised due to surgery, radiation and chemotherapy. Once the inter-dependence of cellular profile, cytokine levels, disease status and treatment status is clarified, the information could be used to create a personalized immune score for each patient. In that context, the present study attempts to develop a baseline profile for the chosen cytokines in healthy volunteers. This study will help in developing predictive models to understand the immune status of cancer patients and devise strategies to help reset this immunological clock in patients, resulting in better outcomes.
We have chosen four cytokines which traditionally can be grouped as pro inflammatory Th1: TNFα and IFNγ and anti-inflammatory Th2: TGFβ and IL-10, although as mentioned before may not be necessarily defined per se. Transforming growth factor (TGFβ) is mainly secreted by Tregs [5] and it plays a key role in suppressing the immune system thereby maintaining peripheral tolerance in autoimmunity and rejection of transplants. In cancer, TGFβ induced immune suppression plays a pivotal role as amplification of Treg cells and TGFβ levels is observed in cancer patients [6,7]. The plasma levels and PHA activated T cell supernatants were measured to obtain a reference standard in Indian healthy subjects.
Materials and Methods
Study population
The study was planned according to Central Ethics Committee (CEC) research regulations of Health Care Global (HCG) Hospitals, Bangalore, India. Ethical clearance was obtained under reference number EC/363/17/09 for the study. All healthy subjects have given informed consent prior to enrollment in the investigation.
The healthy subjects group consisted of 26 males, in the age group of 28-35. Healthy volunteers fulfilled the criteria for blood donation, hence considered healthy as per Blood Bank guidelines. They were free of infectious diseases, autoimmunity and other chronic conditions, as shown in Table 1.
| Number (n) | Healthy 26 |
| Age group (years) | 20-47 |
| Sex | Male |
Table 1: Characteristics of the healthy subjects
Blood collection
15 ml of blood was collected after venous puncture (standard blood bank procedures) in healthy subjects and was collected from patients during surgery, in EDTA vacutainer. Plasma was isolated from these samples by centrifugation at 1500 rpm for 10 mins and stored at -80°C until required. The remaining cell pellet was diluted with saline and used for mononuclear cells isolation.
Isolation and culturing of mononuclear cells from blood
Mononuclear cells were isolated using ficoll-hypaque density-gradient centrifugation. Briefly, 1:3 diluted blood was layered on ficoll-hypaque under sterile conditions and centrifuged for 20 min at 1500 rpm. The buffy coat was collected, washed twice with saline and counted. 2.5 million cells/ml in DMEM (Gibco, Invitrogen) with 10% fetal bovine serum with or without 100 ng/ml PHA (Gibco) were cultured in vitro, and incubated for 72 h for T cell activation. T cell supernatant was collected and stored at -80°C until further measurement.
Cytokines detection
TGF-β, IL-10, TNF-α and INF-γ levels were determined by ELISA (Ray biotech) as per the manufacturer's instructions.
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). Differences of the parameters between plasma and PHA stimulated T cell supernatant were analyzed by using unpaired Student’s-test. P values < 0.05 were considered to be statistically significant.
Results and Discussion
The aim of this study was to establish reference values in plasma and PHA activated T cell supernatants for two pro inflammatory (IFNγ and TNFα) and two anti-inflammatory (TGFβ and IL-10) cytokines in healthy Indian population, which could serve as a reference and help obtain an immune score for our future studies in cancer patients (Table 1). This study is one of the few studies reported where basal values of cytokines in plasma and PHA activated T cell supernatants are measured in Indian population.
Plasma levels of cytokines reflect the general status of a person and play a central role in determining outcomes for treatment modalities in cancer patients. IFNγ is a Th1 type cytokine, a pro inflammatory cytokine [8-10] associated with its anti-cancer properties, although lately debatable [11,12]. TNFα is a pro inflammatory cytokine and is involved in cancer progression [13-17]. TGFβ is a pleiotropic cytokine with regulatory and inflammatory activity [18,19] and it promotes inflammation, although its effects under inflammatory conditions are less understood. IL-10 is an anti-inflammatory cytokine and an immune-suppressor, known to be up regulated in cancer [20-22].
Zauli et al. analyzed 48 cytokines and compared their serum profiles in healthy adults (>18 years) and young children (1-6 yrs) and children (7-17 yrs) and found that there were differences in their levels. Cytokines like TNFα and IFNγ have a marked difference in production levels in children (7-17 yrs) [23].
Tumor necrosis factor is a pro inflammatory cytokine and is involved in inflammation associated carcinogenesis. It is considered, in most cases as a cancer promoter and promotes growth, proliferation, invasion, metastasis and angiogenesis of cancer cells [24]. It is secreted by many inflammatory cells like Myeloid Derived Suppressor Cells (MDSC). Positive correlation between progression of disease and TNFα levels were reported in chronic lymphocytic leukemia, Barrett's adenocarcinoma, prostate cancer, breast cancer, and cervical carcinoma [13-17]. In a study by Ferrajoli et al. plasma TNFα levels in CLL patients were significantly elevated as compared to healthy individuals (16.4 pg/ml versus 8.7 pg/ml). Plasma values more than 14 pg/ml in patients correlated with significant decreased survival times [25].
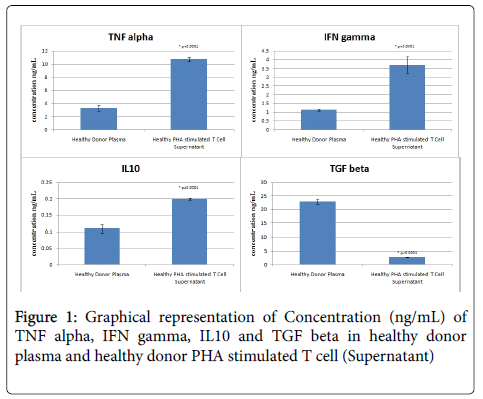
Our studies demonstrate a mean TNFα plasma level of 3.285 ± 0.443 ng/ml which increases significantly to 10.718 ± 0.294 ng/ml (p<0.0001) after PHA activation of T cells. This suggests that activated T cells in normal subjects secrete sufficient amounts of TNFα on activation, which is a natural course during infection, which could be deregulated in cancer patients, due to activation of MDSC (Table 2 and Figure 1).
| TNF alpha (ng/mL) | IFN gamma (ng/mL) | IL10 (ng/mL) | TGF beta (ng/mL) | |
|---|---|---|---|---|
| Healthy Donor Plasma | 3.285 ± 0.443 | 1.127 ± 0.071 | 0.108 ± 0.013 | 22.965 ± 0.967 |
| Healthy PHA stimulated T Cell Supernatant | 10.718 ± 0.294 | 3.694 ± 0.476 | 0.199 ± 0.004 | 2.690 ± 0.117 |
Table 2: Summary of plasma and PHA stimulated T Cell Supernatant cytokine concentration of TNF alpha, IFN gamma, IL10 and TGF beta.
In a study by Conti-Freitas et al. in laryngeal (Head and Neck) cancers, it was found that IFNγ was expressed in lower amounts in unstimulated T cells (mononuclear cells) confirming that these patients had a suppressed immune system due to tumor induced immune suppression. Patient derived T cells after in vitro stimulation with Con A resulted in stimulation and increased IFNγ levels, although they found better stimulation in BCG stimulated cells when compared to Con A. In vitro stimulated T cells showed functional recovery in terms of IFNγ secretion [26]. Direct correlations of IFNγ level in cancer has been elucidated in a number of studies and have been shown to have a protective role to play against tumor development [27,28] and cancer editing [29,30]. Although recent studies have indicated contrary roles of IFNγ and is implicated in "tumor sculpting"; and induces generation of more aggressive tumor clones, which are also evasive to the immune system [31].
Our results show a mean value of 1.127 ± 0.071 ng/ml in PHA activated T cell supernatant which is significantly higher than plasma levels 3.694 ± 0.476 ng/ml (p<0.0001) as seen in healthy subjects. These results indicate an immune system tilted towards Th1 activation on the onset of T cell activation, which could be compromised (as reported in several studies) in cancer patients (Table 2 and Figure 1).
A study by Kim et al. demonstrated high levels of serum IL-10 in plasma of patients with cutaneous cancers, strongly suggesting an immune system prone to anti-inflammatory responses leading to immune suppression and immune evasion by the tumor. It was observed that increased IL-10 levels lead to decreased antigen presentation and tumor escape [32]. Head and neck cancer patients with nodal metastasis (T3/T4 stages) show increased serum IL-10 levels, implicating advanced disease with Th2 type immune response [33]. In a study which analyzed inhibitory cytokine levels in 219 head and neck cancer patients and 64 metastatic lymph nodes, it was observed that tumor cells released significant quantities of TGFβ, PGE2 and IL-10, which correlated with decreased CD8+ T cells within the tumor, which could be a reason for immune suppression and immune evasion by the tumor [34].
Our results show significant decrease in IL-10 plasma levels (when compared to other cytokines) and an increase in IL-10 levels upon T cell activation (0.108 ± 0.013 ng/ml vs. 0.199 ± 0.004 ng/ml, p<0.0001) , although significantly lower than TNF α and INF γ levels, indicating reduced activity of IL10 in healthy individuals (Table 2 and Figure 1). This profile is reported to be altered in several malignancies, as mentioned earlier.
Transforming growth factor β (TGFβ) is a pleiotropic cytokine with potent regulatory and inflammatory activity [18,19]. TGFβ is secreted by Tregs, which constitute around 5-10% of CD4+ T cells and play a pivotal role in maintaining peripheral tolerance. There was a significant decrease in TGFβ levels in PHA stimulated T cells (2.690 ± 0.117) compared to plasma levels (22.965 ± 0.967) (p<0.0001), indicating no Treg mediated immune-suppression in healthy subjects (Table 2 and Figure 1).
This indicates that PHA activation does not result in further expansion of TGFβ producing Tregs, although higher levels of TGFβ in the plasma indicates homeostasis, as Tregs and TGFβ are necessary to maintain peripheral tolerance and prevent autoimmunity [35,36]. TGF β levels in the periphery are related to Treg induced immune suppression, and hence these values are indicative of expected values in cancer patients.
Present study outcomes demonstrate certain measured levels of plasma TNFα and IFNγ, which increases significantly on T cell activation, in healthy individuals. TGFβ levels in plasma is significantly higher than PHA activated T cell supernatant, whereas IL-10 level in plasma is negligible, when compared to pro inflammatory cytokine levels.
One has to bear in mind the complex inter-connections and interdependence observed within these cytokine circuitry and the physiological responses thereof should be considered as a whole and not by considering individual cytokine levels only. Further experiments will explore the correlation of these cytokines in patients and obtain a prognostic predictable immunological score based on the type of cancer, the stage of disease, tumor burden and treatment modalities.
References
- Zhang JM, An J (2007) Cytokines, inflammation, and pain. Int Anesthesiol Clin 45: 27-37.
- Leonard WJ, Lin JX (2000) Cytokine receptor signaling pathways. J Allergy Clin Immunol 105: 877-888.
- O’Shea JJ, Gadina M, Kanno Y (2011) Cytokine Signaling: Birth of a Pathway. J Immunol 187: 5475-5478.
- Huang SC, Wei PC, Hwang-Verslues WW, Kuo WH, Jeng YM, et al. (2017) TGF-β1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up-regulation of IL-17RB. EMBO Mol Med 9: 1660-1680.
- Wang Y, Liu T, Tang W, Deng B, Chen Y, et al. (2016) Hepatocellular Carcinoma Cells Induce Regulatory T Cells and Lead to Poor Prognosis via Production of Transforming Growth Factor-β1. Cell Physiol Biochem 38: 306-318.
- Â Kotsakis K, Koinis F, Katsarou A, Gioulbasani M, Aggouraki D, et al. (2016) Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci Rep 6: 39247.
- Kursunel MA, Esendagli G (2016) The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev 31: 73-81.
- Â Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13: 95-109.
-  Haabeth OA, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, et al. (2011) Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 2: 240.
- Zaidi MR, Merlino G (2011) The two faces of interferon-γ in cancer. Clin Cancer Res 17: 6118-6124.
-  Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, et al. (2016) Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin Cancer Res 22: 2329-2334.
- Â Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, et al. (2002) The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood 100: 1215-1219.
- Â Ahmed MI, Salahy EE, Fayed ST, El-Hefnawy NG, Khalifa A (2001) Human papillomavirus infection among Egyptian females with cervical carcinoma: relationship to spontaneous apoptosis and TNF-alpha. Clin Biochem. 34: 491-498.
- Â Michalaki V, Syrigos K, Charles P, Waxman J (2004) Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90: 2312-2316.
- Â Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, et al. (2006) Tumour necrosis factor-alpha in Barrett's oesophagus: a potential novel mechanism of action. Oncogene 21: 6071-6081.
-  GarcÃa-Tuñón I, Ricote M, Ruiz A, Fraile B, Paniagua R, et al. (2006) Role of tumor necrosis factor-alpha and its receptors in human benign breast lesions and tumors (in situ and infiltrative). Cancer Sci 97: 1044-1049.
- Li MO, Flavell RA (2008) Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 28: 468-476.
- Li MO, Flavell RA (2008) TGF-beta: a master of all T cell trades. Cell 134: 392-404.
- Khan H, Changkija B, Konwar R (2012) Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 133: 11-21.
-  Llanes-Fernández L, Alvarez-Goyanes RI, Arango-Prado Mdel C, Alcocer-González JM, Mojarrieta JC, et al. (2006) Relationship between IL-10 and tumor markers in breast cancer patients. Breast 15: 482-489.
-  Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ (2003) Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 48: 82-84.
- Â Giulio Kleiner, Annalisa Marcuzzi, Valentina Zanin, Lorenzo Monasta, Giorgio Zauli (2013) Cytokine Levels in the Serum of Healthy Subjects. Mediators of Inflammation 434010: 6.
- Wang X, Lin Y (2008) Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin 29: 1275-1288.
-  Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, et al. (2002) The clinical significance of tumor necrosis factor-α plasma level in patients having chronic lymphocytic leukemia. Blood 100: 1215-1219.
- Conti-Freitas LC, Foss-Freitas MC, Mamede RC, Foss NT (2012) Interferon-gamma and interleukin-10 production by mononuclear cells from patients with advanced head and neck cancer. Clinics 67: 587-590.
-  MartÃn F, Santolaria F, Batista N, Milena A, González-Reimers E, et al. (1999) Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 11: 80-86.
- Â Lee IC, Huang YH, Chau GY, Huo TI, Su CW, et al. (2013) Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer 133: 2895-2902.
- Â Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13: 95-109.
-  Horras CJ, Lamb CL, Mitchell KA (2011) Regulation of Hepatocyte Fate by Interferon-γ. Cytokine Growth Factor Rev 22: 35-43.
- Â Porter GA, Abdalla J, Lu M, Smith S, Montgomery D, et al. (2001) Significance of Plasma Cytokine Levels in Melanoma Patients With Histologically Negative Sentinel Lymph Nodes. Ann Surg Oncol 8: 116-122.
- Â Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, et al. (1995) IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol 155: 2240-2247.
- Sparano A, Lathers DM, Achille N, Petruzzelli GJ, Young MR, et al. (2004) Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 131: 573-576.
- M. Rita I. Young, Mark A. Wright, Yvonne Lozano, John P. Matthews, Janet Benefield, M. Margaret Prechel, et al. (1996) Mechanisms of immune suppression in patients with head and neck cancer: Influence on the immune infiltrate of the cancer. International Journal of Cancer 67: 333-338.
- Li MO et al. (2006) Transforming Growth Factor-b Controls Development, Homeostasis, and Tolerance of T Cells by Regulatory T Cell-Dependent and -Independent Mechanisms. Immunity 25: 455-471.
- Marie JC, Liggitt D, Rudensky AY (2006) Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25: 441-454.
Citation: Manapat A, Umrao A, Purushothama H, Rao GA, Rao JA (2018) Cytokine levels in Plasma and PHA Stimulated T Cells in Healthy Indian Subjects. J Cytokine Biol 3: 122. DOI: 10.4172/2576-3881.1000122
Copyright: © 2018 Manapat A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6469
- [From(publication date): 0-2018 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 5510
- PDF downloads: 959