Case Report Open Access
Delayed Distant Metastasis of Tonsillar Squamous Cell Carcinoma Origin- A Case Report
Hermann Simo1, Louis De Las Casas2, Vasuki Anandan2, Michal Preis3 and Reginald Baugh4*1The University of Toledo College of Medicine & Life Sciences, The University of Toledo Medical Center, Toledo, OH 43614, USA
2Department of Pathology, The University of Toledo Medical Center, The University of Toledo Medical Center, Toledo, OH 43614, USA
3Department of Otolaryngology, Maimonides Medical Center, The University of Toledo Medical Center, Toledo, OH 43614, USA
4Department of Surgery, Division of Otolaryngology, Head & Neck Surgery, University of Toledo Medical Center, The University of Toledo Medical Center Toledo, OH 43614, USA
- Corresponding Author:
- Reginald F. Baugh
Department of Surgery
Division of Otolaryngology
The University of Toledo Medical Center
States3000 Arlington Avenue, Toledo, OH 43614, USA
Tel: 419-383-6834
E-mail: Reginald.Baugh@utoledo.edu
Received date: April 11, 2014; Accepted date: April 28, 2014; Published date: May 05, 2014
Citation: Simo H, Casas LDL, Anandan V, Preis M, Baugh R (2014) Delayed Distant Metastasis of Tonsillar Squamous Cell Carcinoma Origin- A Case Report. Otolaryngology 4:170 doi:10.4172/2161-119X.1000170
Copyright: © 2014 Simo H et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Otolaryngology: Open Access
Abstract
Introduction: The incidence of distant metastases from head and neck Squamous Cell Carcinoma (SCC) is reportedly low; reports of distant metastases from tonsil carcinoma are rare. 85% of distant metastases of SCC in head and neck cancers usually become apparent within two years of primary diagnosis, but can take up to five years before diagnosis.
Background: Metastases from tonsillar cancers are uncommon, with less than 1% reported to go to subcutaneous tissues. Metastases are reported to occur within 1-48 months after initial treatment.
Methods and results: A case report is presented of a patient seen with an isolated temporal scalp Squamous Cell Carcinoma (SCC) lesion 8 years after treatment for a tonsillar SCC and negative annual PET scans thereafter. The comparative immunehistochemical study and in situ hybridization done between the scalp tumor and the previous tonsil tumor eight years earlier, showed similarities, thus suggesting a metastasis from the tonsil tumor.
Conclusions: A tonsillar SCC metastasis presenting as a temporal scalp lesion 8 years after primary tumor treatment and locoregional control achievement is a uniquely rare event. The case highlights the need for a method to identify and track tumor cell lineage, and the need for better understanding of cancer stem cells role in head and neck SCC.
Keywords
Distant metastasis; Oral pharyngeal cancer; Stem cell; Squamous cell carcinoma; Tonsillar cancer
Introduction
Primary cutaneous squamous cell carcinoma tumors often arise from the overlying epidermis or display an epidermal connection, the epidermis will show in-situ component or dysplasia, and the tumors will be located more superficially. In contrast squamous cell carcinoma tumors originating from a metastasis lack connection with the epidermis and the overlying epidermis will lack an in-situ component or dysplasia and they are often located in the deeper dermis or subcutaneous fat. Once we know that the tumor is a metastatic squamous cell carcinoma, the next question we need to address is whether the metastasis is originating from the primary head and neck cancer, or is it a nodal presentation of a yet unknown primary tumor?
We report a rare presentation of delayed distant metastasis of tonsillar squamous cell carcinoma to the temporal scalp, eight years after the primary tumor was treated and loco-regional control was achieved. Comparative immunehistochemical stain and in situ hybridization between the current tumor and the previous tumor eight years earlier showed similarities.
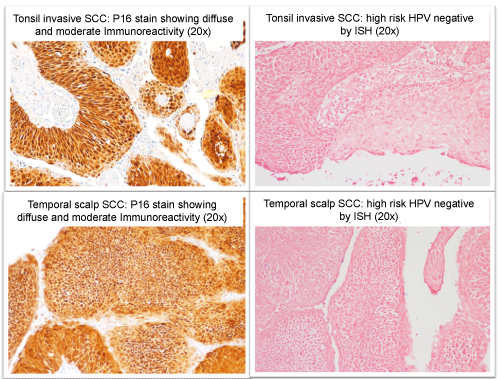
In light of patients clinical history of previous tonsilar carcinoma, combined with the facts that the current tumor has a similar morphology as the previous tonsilar tumor, immunohistochemical and In-situ hybridization studies (P16 and high risk HPV) of both tumors had similar pattern; we felt confident concluding that we were dealing with a metastasis from the previous tonsil SCC tumor as opposed to a metastasis of a yet unknown primary.
This occurrence highlights the importance for identifying cancer stem cell lineage and the need for better methods and tools for testing and detection of sub-pathological distant metastases.
Case Report
A sixty-five year old male was referred to our Otolaryngology clinic for follow-up after being diagnosed with subcutaneous squamous cell carcinoma of the right temporal scalp at an outside facility.
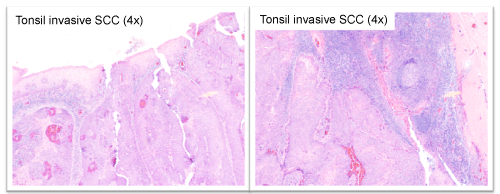
The patient was originally diagnosed in 2003 with a 3cm keratinizing, moderately to poorly differentiated squamous cell carcinoma of the left tonsil, which had spread to the left neck (Figure 1). He underwent radical tonsillectomy and left radical neck dissection. Two lymph nodes were positive, 4.5 cm maximal diameter, with extra nodal extension; the internal jugular vein and sternocleidomastoid muscle were free of tumor. Following surgery he received radiation therapy (6580cgy). Locoregional control was achieved.
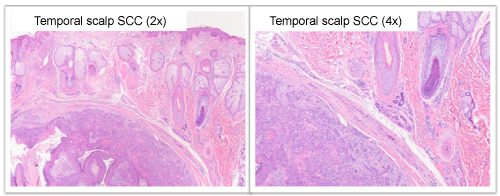
Annual PET scans were negative for local recurrence or metastasis until 2012 when an uptake was noted in the right temporal scalp; this uptake was interpreted as an infected sebaceous cyst.
The lesion gradually grew, and in September 2012, started secreting a clear, sometimes blood-stained discharge. The lesion was biopsied and the pathological report showed subcutaneous squamous cell carcinoma involving all surgical margins; consistent with metastasis (Figure 2). The patient was referred to our care, we completed a wide local excision with 2cm circumferential margin, the deep margin was superficial to the temporalis fascia by 3mm; the temporalis fascia was not involved but was removed as part of the specimen as an extra measure of protection. The pathology report showed SCC of similar histology as the primary tonsil tumor; a comparative immunohistochemical stain of p16 and high-risk HPV in situ hybridization between the current tumor and the previous tumor eight years ago showed similarities, further supporting a distant metastasis from the original tonsil tumor (Figure 3). Both original tumor and supporting metastasis stained negative for high-risk HPV.
Discussion
The detection of a delayed distant metastasis in head and neck SCC poses a challenge to the physician: first of all is it a primary cutaneous tumor or a metastatic tumor? Secondly is it a metastasis originating from the primary head and neck cancer, or is it a nodal presentation of a yet unknown primary tumor?
The tonsil is the most common primary site for carcinoma of the oropharynx. The incidence of distant metastasis for oropharyngeal cancer varies extensively in the literature, ranging between 4%-31% in clinical studies [1-5]. Metastases to the skin are extremely rare, occurring in 1-2% of patients with head and neck SCC [6,7]. They are often associated with oral cavity cancers and have been reported to account between 10-15% of all distant metastatic lesions [2,5-9]. The incidence of metastasis is influenced by various factors including location of primary tumor, initial staging, histological differentiation and adequacy of loco-regional control at the primary site [1-5]. The occurrence of skin metastases after treatment of head and neck SCC has been reported to be extremely rare, developing in less than 0.8% of patients with oropharyngeal head and neck SCC; with a time of occurrence between 1-36 months after initial treatment [10].
Tumors with advanced loco-regional extension at the primary site carry an increased risk for distant metastatic spread [11]. Patients having achieved loco-regional control at the primary site are considered cured of the cancer. When they later develop distant metastases; it poses a challenge to the physician- were these occult distant metastases present at the time of loco-regional treatment? What processes lead them to develop into clinically apparent disease?
For hematologic and lymphatic spread to occur so long after the primary tumor was treated with curative intent is quite intriguing and unlikely. In our case, the fact that no enlarged lymph nodes were palpated and the PET scan did not show any lymph node involvement leads to think of a hematogenous spread. Hematogenous spread would suggest that the circulating cancer cells used the blood to migrate from the primary site, then, hibernated for eight years before they began growing in the scalp to give rise to a cancer. Lymphatic spread would seem even less likely with the cancer cells passing through the regional lymph nodes to then enter the bloodstream to spread to the scalp. Even if these implausible events were to occur, metastases do not typically reproduce the entire spectrum of cancer subpopulations within the primary cancer. In fact, if it were not for the similarity in immunohistochemical staining as the primary tumor, this metastasis would have been mistaken for a second primary tumor. What triggered the development of cancer eight years after the primary remains speculative; however, recent studies in cancer biology have linked Cancer Stem Cells (CSC) to tumor recurrence and metastatic spread in head and neck squamous cell carcinoma [12]. These recent discoveries make cancer stem cells the most plausible and promising etiologic factor in our case.
Occult metastases could originate from cancer stem cells. Cancer Stem Cells (CSC) can either arise from normal stem cells or from mutated progenitor cells; and thus they possess characteristics similar to normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample [13,14]. Therefore, due to their ability to generate tumors through the stem cell processes of selfrenewal and differentiation into multiple cell types, these cancer stem cells have been proposed to persist in tumors as a distinct population and to cause relapse and metastasis by giving rise to new tumors [14,15]. Normal adult stem cells are usually dormant undifferentiated cells that reside among differentiated cells in an organ or tissue. When the need arises; they divide and differentiate to replace the surrounding differentiated cells. During the differentiation process, they are not immune from mutation and could well give rise to a mutated cell that becomes cancerous, a stem cell cancer. A stem cell cancer arising within a stem cell population can lead to the appearance of tumor cells at any time. Uniquely, CSC can give rise to all the malignant subpopulations within a cancer, truly reproducing the entire primary cancer.
A stem cell whether be normal or cancerous, has to have the builtin ability to better withstand pressure (chemical or physical) from the environment than its counterpart differentiated cells if its primary purpose is to regenerate cells that have been destroyed, otherwise there will be no regeneration if these stem cells were easily negatively affected by environmental factors. This ability to withstand pressure from the environment can make cancer stem cells less vulnerable or more resistant even to therapies used against tumors. Conventional therapies have been successful at targeting differentiated, rapid growing cells, but not so much for slow growing cells such as CSC. Because of their slow growing nature, CSC could evade these therapies and regenerate tumors or metastases with time. This approach could explain recurrence and delayed metastases after therapy and/or loco regional control have been achieved. Furthermore, some markers like CD133 found on CSC have been showed to give these cells the ability to resist radiotherapy further allowing CSC to persist despite therapy [14].
A critical step in metastasis is the ability for tumor cells to migrate away from the primary tumor; normal stem cells have been known to facilitate that process in order to generate or replace cells. Similarly, cancer stem cells are capable of using the same mechanism to generate metastases [12]. Studies are currently underway focusing on targeting this process in order to better understand it and provide potential therapies. One potential target has been the EMT (epithelial- mesenchymal transition) molecule, a key molecule during embryogenesis that allows epithelial cells to break down cell-cell and cell-extracellular matrix connections and migrate to different locations in the body [16]. It has been found that this molecule provide cancer cells the ability to infiltrate surrounding tissues and ultimately metastasize [17].
Upon evasion from therapy and migration from the primary tumor, cancer stem cells remain in circulation or find a niche where they can remain dormant. Our current methods of detection and follow up for head and neck cancer lack the ability of tracking or detecting cancer stem cells since they have no markers to rely on. Recent studies have proposed three markers that when found in head and neck squamous cell carcinomas give the functional definition ability of a CSC to a subpopulation of cells in these tumors. These genetic markers, CD44, CD133 and ALDH have been implicated in head and neck CSC ability for self-renewal, tumor progression and aggressiveness, chemo resistance, increased migration and invasiveness, and poor cancer prognosis. Having specific markers that are unique to cancer stem cells would be a great tool in the fight against cancer and will enable us to identify and track cancer stem cells among tumor cells and to predict the likelihood of recurrence and metastases [18].
Despite recent advances in cancer stem cell biology, the understanding of cancer stem cells role in head and neck squamous cell carcinoma is still in its infancy. Several mechanisms may play a role in cancer stem cells ability to metastasize and to resist current therapies. However until a means of detecting and preventing occult distant metastases becomes available, the likelihood of translating loco regional control into free of cancer will remain difficult.
Our case highlights the need for a method to identify and track tumor cell lineage, the importance of thorough examination to be performed during routine oncologic follow-up visits and the need for better understanding of cancer stem cells role in head and neck squamous cell carcinoma in order to further develop cancer-stem-cellbased therapies that will help improve the survival of patients with head and neck cancer in the future and prevent recurrence or occurrence of delayed distant metastases. Testing isolated distal recurrences more than five years from the index case to identify possible stem cell metastases may provide insight into future treatment therapies.
References
- Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, et al. (2001) Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope 111: 1079-1087.
- Okamoto M, Nishimine M, Kishi M, Kirita T, Sugimura M, et al. (2002) Prediction of delayed neck metastasis in patients with stage I/II squamous cell carcinoma of the tongue. J Oral Pathol Med 31: 227-233.
- Brug���¨re JM, Mosseri VF, Mamelle G, David JM, Buisset E, et al. (1996) Nodal failures in patients with NO N+ oral squamous cell carcinoma without capsular rupture. Head Neck 18: 133-137.
- Carvalho AL, Kowalski LP, Borges JA, Aguiar S Jr, Magrin J (2000) Ipsilateral neck cancer recurrences after elective supraomohyoid neck dissection. Arch Otolaryngol Head Neck Surg 126: 410-412.
- Hoch S, Fasunla J, Eivazi B, Werner JA, Teymoortash A (2012) Delayed lymph node metastases after elective neck dissection in patients with oral and oropharyngeal cancer and pN0 neck. Am J Otolaryngol 33: 505-509.
- Shingaki S, Suzuki I, Kobayashi T, Nakajima T (1996) Predicting factors for distant metastases in head and neck carcinomas: an analysis of 103 patients with locoregional control. J Oral MaxillofacSurg 54: 853-857.
- Papac RJ (1984) Distant metastases from head and neck cancer. Cancer 53: 342-345.
- Schultz BM, Schwartz RA (1985) Hypopharyngeal squamous cell carcinoma metastatic to skin. J Am AcadDermatol 12: 169-172.
- Alvi A, Johnson JT (1997) Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck 19: 500-505.
- Pitman KT, Johnson JT (1999) Skin metastases from head and neck squamous cell carcinoma: incidence and impact. Head Neck 21: 560-565.
- Le���³n X, Quer M, Or���ºs C, del Prado Venegas M, L���³pez M (2000) Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck 22: 680-686.
- Shiozawa Y, Nie B, Pienta KJ, Morgan TM, Taichman RS (2013) Cancer stem cells and their role in metastasis. PharmacolTher 138: 285-293.
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, et al. (2006) Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442: 818-822.
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, et al. (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. ProcNatlAcadSci U S A 104: 973-978.
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730-737.
- Radisky DC, LaBarge MA (2008) Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell 2: 511-512.
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442-454.
- Horst D, Kriegl L, Engel J, Kirchner T, Jung A (2009) Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorect al cancer. Cancer Invest 27: 844-850.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17469
- [From(publication date):
August-2014 - May 01, 2025] - Breakdown by view type
- HTML page views : 12864
- PDF downloads : 4605