Depletion of BTAF1 Inhibits Epithelial-to-Mesenchymal Transition via the TGF-β Signal Pathway in the Osteosarcoma
Received: 10-Jul-2023 / Manuscript No. DPO-23-105382 / Editor assigned: 12-Jul-2023 / PreQC No. DPO-23-105382 (PQ) / Reviewed: 26-Jul-2023 / QC No. DPO-23-105382 / Revised: 15-Jan-2025 / Manuscript No. DPO-23-105382 (R) / Published Date: 23-Jan-2025
Abstract
Osteosarcoma (OS) is one of the most malignant tumors of bone with a poor outcome in children and young adolescents and has a poor response to radiotherapy and chemotherapy. At present, it is urgent to identify the effective biomarkers and to prevent and treat osteosarcoma. The BTAF1 (formerly known as TAFII170/TAF-172 and the human ortholog of Saccharomyces cerevisiae Mot1p), are evolutionarily conserved members of the SNF2-like family of ATPase proteins, and it has never been studied in OS. In this study, we first revealed BTAF1 is significantly upregulated in OS, and its expression level is highly correlated with clinicopathological parameters of OS patients. Our survival curve analysis demonstrated that BTAF1 is a candidate predictor for predicting patient prognosis. Functional experiment results showed BTAF1 promotes the proliferation of OS cells in vitro. Silencing of BTAF1 reduces the colony-forming ability of U2OS cells in vitro and reduces tumor growth in vivo. Mechanism studies have shown that inhibition of BTAF1 reduces the Epithelial-to-Mesenchymal Transition (EMT) through the TGF-β signaling pathway to inhibit OS progression. In summary, BTAF1 plays a regulatory role in the progression of OS, and it may be a new OS diagnostic marker and prognostic factor, providing new ideas for the treatment of OS.
Keywords: BTAF1; Osteosarcoma; Epithelial-to-Mesenchymal Transition (EMT); Prognosis
Introduction
Osteosarcoma (OS), one of the most prevailing primary bones malignancies originating from bone-forming mesenchymal cells, is a kind of invasive tumor which mainly occurred in adolescents and youngsters [1]. Although the annual incidence of osteosarcoma worldwide is only about 30 parts per million, local tumors can be removed by surgery, but metastasis caused by osteosarcoma can lead to death in most cases [2,3]. Currently, the survival rate of patients with metastatic osteosarcoma is very low, including a 5-years survival rate of only 11%-20% [4,5]. The current treatment for OS is surgical resection plus chemotherapy [6]. Although adjuvant chemotherapy has played a key role in the treatment of osteosarcoma and improved the clinical prognosis of some patients with OS, the overall prognosis is still disappointing, which is mainly affected by chemotherapy response and metastasis station [7]. Thus, an appropriate treatment method is urgently needed as the primary treatment strategy. Therefore, it is crucial to explore osteosarcoma markers and the mechanisms underlying the occurrence, progression, invasion and metastasis of OS to determine its biological characteristics and prognosis, and to define innovative clinical therapeutic objectives for effective molecular therapy.
The BTAF1 (formerly known as TAFII170/TAF-172 and the human ortholog of Saccharomyces cerevisiae Mot1p), are evolutionarily conserved members of the SNF2-like family of ATPase proteins. The function of BTAF1 has been studied mainly in yeast and human cells, which has revealed that it binds directly to TBP (TATA Binding Protein), forming the B-TFIID complex. This complex binds to core promoters of RNA polymerase II-transcribed genes and, of crucial importance, BTAF1-TBP interactions have been shown to affect the kinetics of TBP-promoter interactions. Understanding the regulation of gene expression is arguably the most crucial basic question of biology. In regulation of gene expression, transcription of protein-encoding genes by RNA polymerase II receives most attention [8-11]. However, the role of BTAF1 in OS progression is still unknown.
The unique properties of osteosarcoma may be related to either the cell of origin or components in the bone marrow microenvironment, such as the large amount of Transforming Growth Factor β1 (TGF-β1) [12]. Transforming Growth Factor-β1 (TGF-β1) is one of the strongest stimuli and is overexpressed in many types of human tumors, including OS [13]. TGF-β1 is also a pleiotropic cytokine that acts as a mediator upon the tumor to promote further tumor expansion, metastasis, and cytokine production [14]. Transforming Growth Factor-β (TGF-β) signaling is one of the most important EMT inducers, which is a crucial tumor-promoting process in cancer development at later stages, leading to tumor cell migration, invasion and metastasis. The epithelial-mesenchymal transition [15].
Numerous studies have shown that TGF-β can induce EMT and accelerate tumor metastasis by regulating the components of extracellular matrix [16,17]. EMT has been reported as an essential event in the malignant transformation of cancer, especially during tumor invasion and metastasis, by promoting the conversion of early-stage tumors into invasive malignancies [18]. EMT is a developmental process in which epithelial cells lose polarity and acquire the migration characteristics of mesenchymal cells [19]. TGF-β1 signaling is one of the most important EMT inducers, which is a crucial tumor-promoting process in cancer development at later stages, leading to tumor cell migration, invasion, and metastasis [15].
By analysing GSE database, we found that BTAF1 mRNA expression is higher in OS than in normal tissues. Additionally, functional experiment results indicated that overexpression of BTAF1 significantly stimulates the proliferation of OS cells. In this study, we focused on the effect of BTAF1 on osteosarcoma and unravel the potential mechanisms. We report for the first time that BTAF1 facilitates the Epithelial-to-Mesenchymal Transition (EMT) through the TGF-β signaling pathway to inhibit OS progression. Overall, this study reveals a new diagnostic marker and prognostic factor for OS, and it may provide new ideas and targets for the treatment of OS.
Materials and Methods
Clinical osteosarcoma samples
Clinical osteosarcoma samples were acquired from patients who received partial or radical tumorectomy in the department of orthopedics, Putuo people’s hospital, Tongji university, Shang Hai, China. 50 osteosarcoma samples were collected, and they all were paired with adjacent normal tissues. Fresh osteosarcoma tissue samples (n=50) were obtained from 28 patients with primary osteosarcoma without metastases and 22 patients with metastases. Of 28 patients with primary osteosarcoma, 19 were recurrence-free and 9 were recurrent patients. Part of the matched tissues was frozen in liquid nitrogen or -80°C freezer for RNA extraction or Western Blot. The remaining tissues were fixed in 10% formalin at room temperature for 24 hours, and then embedded in paraffin for immunohistochemistry. These patients had not received preoperative assisted anti-cancer treatment. This study and experimental procedures were approved by the Human Research Ethics Committee of Tongji university (Shanghai, China) and written informed consent was obtained from all the patients involved.
Cell lines and cell culture
Human osteosarcoma cell lines (U2OS) and the normal osteoblastic cell lines, hFOB, were purchased from the Shanghai institute for biological sciences, Chinese academy of sciences. All cells were cultured with Eagle's minimum essential medium (cat.no.30-2003; ATCC) containing 10% heat-inactivated fetal bovine serum in an incubator (37°C, 5% CO2).
Immunohistochemistry (IHC) and evaluation of the results
The collected OS tissues and paired adjacent normal tissues were fixed in formalin and then embedded in paraffin. For IHC, paraffinembedded sections were dewaxed in xylene and rehydrated in ethanol. Subsequently, after deparaffinization, rehydration, blocking with hydrogen peroxide and antigen retrieval, these sections were incubated with primary rabbit anti-BTAF1 polyclonal antibodies (A12251, ABclonal; Wuhan, China) overnight at 4°C. After washing three times with PBS, they were incubated with HRP-conjugated anti-rabbit secondary antibodies for 2 hours at room temperature. DAB was used for coloration, and dark brown was regarded as positive. Appropriate positive and negative controls were included in each run, and the absence of a primary antibody was used as a negative control. Two pathologists, blinded to clinical information, independently assessed BTAF1 staining under an Olympus CX31 microscope (Olympus, Centre Valley, PA). The positive rate of tumor cells ranged from 0% to 100%. Staining intensity was defined as: negative, 0; weak, 1; mild, 2; and intense, 3. Finally each case was given a weighted score (% × staining intensity) on a scale of 0 (0 × 0) to 3 (100% × 3).
Transient transfection assays
The BTAF1-short hairpin RNA (shRNA1, shRNA2) plasmids was constructed and purchased in Shandong Vigene Biosciences Company, China. Cell lines U2OS were seeded in 6-well plates at 50%-70% confluence when being transfected. 3 μg BTAF1-short hairpin RNA (shRNA) plasmids per well was added to the cells using LipofectamineTM 2000 reagents (Thermo Fisher Scientific; Waltham, USA). 24-48 hours after transfection, the cells were collected for efficiency determination and subsequent assays. All steps are carried out in accordance with the manufacturer's instructions.
Cell proliferation assays
The BTAF1-short hairpin RNA (shRNA) plasmids were purchased from GECEM biotechnology, Shanghai, China. 1 × 105 U2OS cells were inoculated in six-well plates and transfected with 5 ng BTAF1- shRNA1, BTAF1-shRNA2 and vector plasmid per well using Lipofectamine™ 2000 reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. 48 h after transfection, U2OS cells were added to 5 96-well plates at a density of 2000 cells per well, and cell viability was measured at 0, 24, 48, 72 and 96 h, respectively. Cell proliferation rate was measured by using Cell Count Kit-8 (CCK-8) according to the manufacturer’s protocol. Specifically, 10 μL CCK-8 solutions and 100 μL of the above cell culture medium were added to each well, and the optical density of each well was measured at 450 nm after 4 h. Each experiment was repeated three times independently.
Quantitative Real-Time-Polymerase Chain Reaction (qRTPCR)
According to the manufacturer’s agreement, use TRizol reagent (Invitrogen, Carlsbad, CA) to extract total RNA from OS tissues and cell lines. 1 μg enriched tissue or cell RNA was used to synthesize cDNA through reverse transcription, which was accomplished by TaqMan® Reverse Transcription Reagents (Applied Biosystems Inc.; Thermo Fisher Scientifc, Inc.). The procedure was based on the protocol provided by Invitrogen. The HieffTM qPCR SYBR Green Master Mix (Thermo; Massachusetts, USA) was used for qPCR analysis by ABI ViiA7 qPCR System (Applied Biosystems; Foster, CA). Specific primers were purchased from RiboBio (Guangzhou, China), they were listed as follows:
Forward primer 5`-CGCTCAGCTCTCTGGAAACT-3`, and reverse primer 5`-AAGGCGATCTAGCCTGGAGA-3`.
Western blotting
Whole-cell lysates (P0013B, Beyotime, Shanghai, China) were collected and prepared from BTAF1-shRNA or empty vector transfected cells. All the proteins of the processed tissues or cells were lysed on ice in RIPA protein lysis buffer with 1 mM PMSF (phenylmethylsulfonyl fluoride). Then the impurities were removed after centrifugation at 15,000 rpm at 4°C. Subsequently, the protein concentration of the solution was measured with PierceTM BCA protein assay kit (Thermo; Massachusetts, USA). The calculated 30 μg protein per well was separated in a 10% SDS-PAGE gel and transferred to a Polyvinylidene Fluoride (PVDF) membrane at 100 V for 1-2 hours. The PVDF membranes were washed thrice for 5 min each, and then blocked in PBST for 2 hours at room temperature. The blocked membranes were washed, and cut into different strips according to the molecular weight of target proteins, then incubated with primary antibodies against target proteins overnight at 4°C. The next day, the membranes were incubated with secondary antibodies (1:1000, Invitrogen) for 1 hour at room temperature. Blots were developed using the enhanced chemiluminescence method (Pierce™ ECL Western Blotting Substrate; Thermo Fisher Scientifc, Inc.). The primary antibodies used included anti-BTAF1, anti-E-cadherin, anti-vimentin, anti-α-actin, anti-Ncadherin and anti-snail.
Tumor xenografts in Nude mice
BALB/C-nu/nu nude mice were purchased from the National resource center for rodent laboratory animal of China. Each group included 5 nude mice, each of which was inoculated subcutaneously with 5.0 × 106 cells (BTAF1 knockdown and control tumor cells). Mice were kept in sterile animal facilities and their tumor growth was monitored daily. The tumor volume was measured weekly with Vernier calipers and calculated according to the formula, V=length × width2 × 0.25.2 months later the mice were sacrificed, tumors were dissected for histological examination. All data are expressed as mean ± SE. The animal experiments were approved by the institutional animal care and use committee of Tongji university and met all regulatory guidelines.
Statistical analysis
Significant differences between the experimental groups and controls were assessed using Student's t test. Survival information was assessed by Kaplan-Meier analysis, and differences were assessed using log-rank tests. χ2 tests were used to assess the significant correlation between BTAF1 expression and clinicopathological parameters of OS patients. Survival curve analysis was used to evaluate the diagnostic value of BTAF1. A cox proportional hazard regression model was used for univariate and multivariate analysis. All values are reported as means ± the Standard Deviation (SD), P<0.05 was statistically significant. The asterisks indicate that the data are significantly different from the controls, P<0.05, *; p<0.01, **; p<0.001, ***; p<0.0001, ****. GraphPad Prism 5.0 and SPSS 18.0 software were used for all statistical analysis.
Results
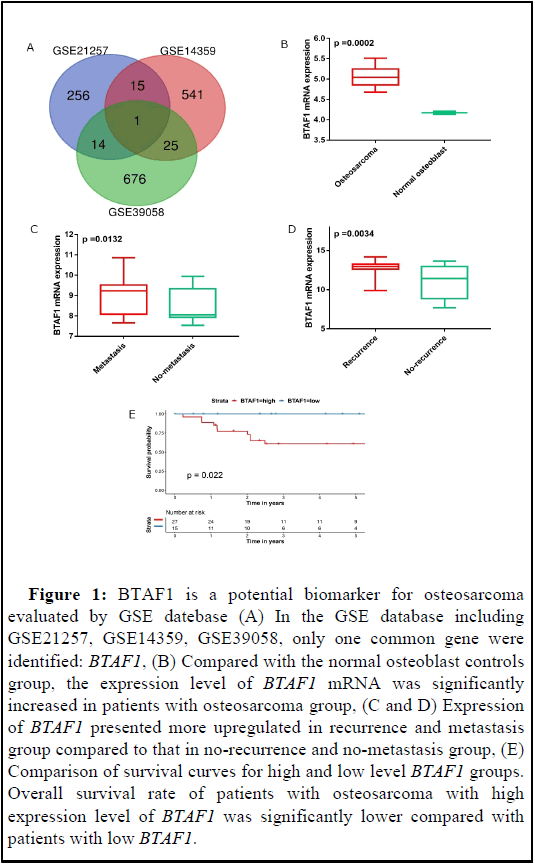
BTAF1 is a potential biomarker for osteosarcoma according to GSE database
In the GSE database including GSE21257, GSE14359, GSE39058, only one common gene were identified: BTAF1 (Figure 1A). We first utilized GSE database to access BTAF1 mRNA expression and its association with osteosarcoma prognosis. As shown in Figure 1B, BTAF1 was remarkably overexpressed in osteosarcoma compared to normal tissue at transcriptional level (p=0.002). Then we further evaluated its association with the clinical characteristics of osteosarcoma such as metastasis and recurrence. As shown in Figures 1C and 1D, BTAF1 was correlated to metastasis and recurrence; compared with the group without recurrence and metastasis, the expression of BTAF1 mRNA was higher in the group with metastasis and recurrence. Kaplan-Meier curves were used to analyze the correlation between gene expression and survival probability of patients. The results revealed that higher BTAF1 mRNA expression predicted lower survival probability. Lower expression of BTAF1 was associated with longer survival (Figure 1E).
Figure 1: BTAF1 is a potential biomarker for osteosarcoma evaluated by GSE datebase (A) In the GSE database including GSE21257, GSE14359, GSE39058, only one common gene were identified: BTAF1, (B) Compared with the normal osteoblast controls group, the expression level of BTAF1 mRNA was significantly increased in patients with osteosarcoma group, (C and D) Expression of BTAF1 presented more upregulated in recurrence and metastasis group compared to that in no-recurrence and no-metastasis group, (E) Comparison of survival curves for high and low level BTAF1 groups. Overall survival rate of patients with osteosarcoma with high expression level of BTAF1 was significantly lower compared with patients with low BTAF1.
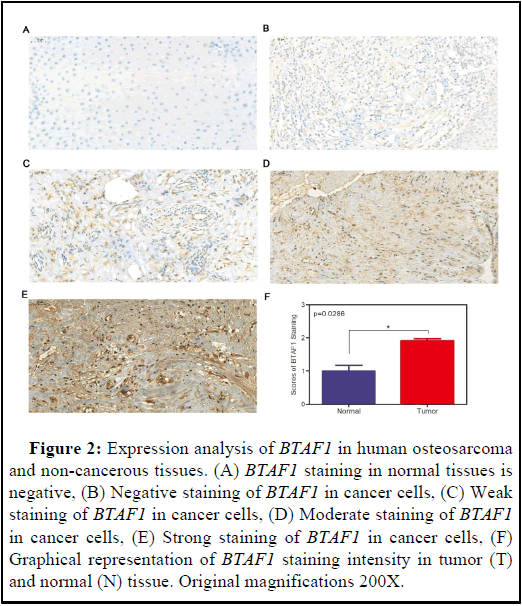
BTAF1 is up-regulated in OS specimens.
We performed immunohistochemical staining on 50 human osteosarcoma tissues and their corresponding non-cancerous tissue controls. Representative examples of IHC for BTAF1 in the OS and non-cancerous tissues are shown in Figures 2A-2F. BTAF1 staining in the non-cancerous tissues is negative (Figure 2A). However, by Student t test, BTAF1 expression is up-regulated in tumor cells (Figures 2B-2F). In addition, 62% (31/50) of the matched cases showed high BTAF1 expression in the OS tissues compared to that observed in the non-cancerous tissues. Taken together, these results indicate that BTAF1 is up-regulated in OS tissues.
Figure 2: Expression analysis of BTAF1 in human osteosarcoma and non-cancerous tissues. (A) BTAF1 staining in normal tissues is negative, (B) Negative staining of BTAF1 in cancer cells, (C) Weak staining of BTAF1 in cancer cells, (D) Moderate staining of BTAF1 in cancer cells, (E) Strong staining of BTAF1 in cancer cells, (F) Graphical representation of BTAF1 staining intensity in tumor (T) and normal (N) tissue. Original magnifications 200X.
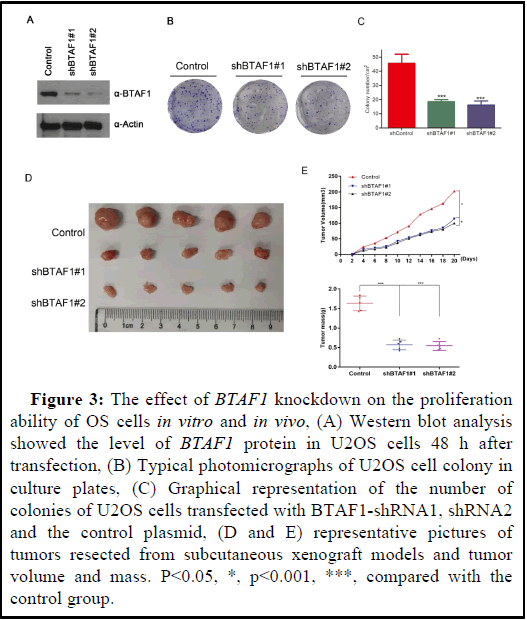
Silencing BTAF1 inhibits proliferation of OS cells.
Silencing of BTAF1 reduces the colony-forming ability of U2OS cells. The functional significance of U2OS cells was further investigated by knocking down BTAF1 using shRNAs. Compared with the control group, knocking down BTAF1 resulted in decreased U2OS cell viability (Figure 3A). To further clarify the potential role of BTAF1 in tumorigenesis, colony formation assay was used to determine whether the knockdown of BTAF1 expression affects the colony formation ability of U2OS cells. As a result, both the size and the number of colonies were markedly reduced in the shBTAF1#1 and shBTAF1#2 group compared with these parameters in the control group, shBTAF1#2 group had the lowest number and smallest size of cell clones (Figures 3B and 3C). In addition, BTAF1 down significantly reduced tumor growth as compared with the control groups (Figure 3D). Taken together, these results demonstrated that BTAF1 is important for OS cell growth.
Figure 3: The effect of BTAF1 knockdown on the proliferation ability of OS cells in vitro and in vivo, (A) Western blot analysis showed the level of BTAF1 protein in U2OS cells 48 h after transfection, (B) Typical photomicrographs of U2OS cell colony in culture plates, (C) Graphical representation of the number of colonies of U2OS cells transfected with BTAF1-shRNA1, shRNA2 and the control plasmid, (D and E) representative pictures of tumors resected from subcutaneous xenograft models and tumor volume and mass. P<0.05, *, p<0.001, ***, compared with the control group.
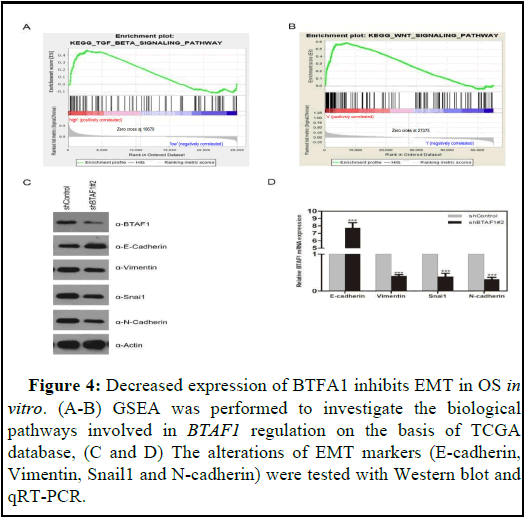
BTAF1 regulates EMT via the TGF-β signaling pathway
In order to explore how BTAF1 is involved in the occurrence and progression of OS, Gene Set Enrichment Analysis (GSEA) was performed to figure out the biological pathways regulated by BTAF1 based on the TCGA database. The results showed that PPT2 is highly correlated with TGF-β signal and WNT-β signal signatures (Figures 4A and 4B). Epider Growth Factor (EGF), Transforming Growth Factor-β (TGF-β), Fibroblast Growth Factor (FGF), Wnt and Notch and other factors have initiated EMT Process and is mediated by many EMT-related transcription factors such as Snail, Twist, Slug, ZEB, etc. [21,22]. EMT is a vital factor in tumorigenesis and progression, and is closely related to enhanced cell migration and invasion, tumor metastasis and anti-apoptosis capacity [23,24]. Therefore, we speculated that BTAF1 has an influence on the EMT process. To test this hypothesis, the alterations of several EMT markers were evaluated in shBTAF1#2 and shControl cells. The results showed E-cadherin expression increased, while Vimentin, Snail1 and N-cadherin expression decreased in OS cells transfected with shBTAF1#2 plasmids at both protein and mRNA levels (Figures 4C and 4D). In brief, BTAF1 knockdown significantly inhibits EMT of OS in vitro.
Figure 4: Decreased expression of BTFA1 inhibits EMT in OS in vitro. (A-B) GSEA was performed to investigate the biological pathways involved in BTAF1 regulation on the basis of TCGA database, (C and D) The alterations of EMT markers (E-cadherin, Vimentin, Snail1 and N-cadherin) were tested with Western blot and qRT-PCR.
Discussion
Many signaling pathways are involved in tumor development and progression. At present, extensive gene expression and regulatory mechanisms in OS are still unclear. In this study, through literature reading and database screening, we discovered the differentially expressed molecule BTAF1 in OS, which is closely related to cell metabolism. Further studies revealed BTAF1 is upregulated in OS, and high BTAF1 expression is associated with various clinicopathological parameters of OS patients. Functional experiment results indicated that overexpression of BTAF1 remarkably stimulates the proliferation of OS in vitro. Mechanistic investigations demonstrated that BTAF1 promotes the OS progression by reducing EMT. Taken together, we ultimately concluded, BTAF1 plays a vital role in the development of osteosarcoma, BTAF1 may be considered a new diagnostic markers and prognostic factors, and provide treatment target for osteosarcoma. At present, lots of studies have revealed that EMT is an essential mark of tumor occurrence and development, and is closely related to cell migration, enhanced invasion ability, tumor metastasis and antiapoptosis [24]. Previous studies have reported that osteosarcoma cells express a large number of EMT-related genes, suggesting that EMT also plays an important role in mesenchymal cell-derived sarcoma [25]. According to our experimental results, we associate EMT with the expression of BTAF1 in OS, and we believe that BTAF1 plays an important role in the progression of OS. The activation of EMT promotes the dissociation and invasion of tumor cells which are the fundamentals for tumor metastasis to distant organs, leading to tumor spread and treatment failure [26,27]. Many factors initiate the EMT process, including Transforming Growth Factor-β (TGF-β), Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF) and Wnt. And it is mediated via many EMT-related transcription factors such as slug, Snail and Twist [21,28].
EMT is a pathological feature of tumors with abnormal developmental and morphogenetic signaling pathways, such as TGF-β and growth factor signaling. The elevation of TGF-β promotes the malignant progression of cancer [15,29]. TGF-β also promotes the stemness characteristics of osteosarcoma. TGF-β-induced EMT is tumor-propagating in transformed epithelial progenitor cells [30]. Three members of the TGF-β family -TGF-β1, -β2, and -β3 have been recognized in mammals [31]. TGF-βs are widely accepted as tumor suppressors and tumor promoters depending on the type of cancer and the time of tumor progression [32-34]. TGF-β plays a role as a major player in the vicious cycle between osteosarcoma cells and the bone tumor microenvironment, thereby promoting tumor development and lung metastatic spread [33]. TGF-βs signals exhibits the effects by binding to two heteromeric cell surface receptors, namely type I (TβRI) and type II (TβRII) receptors [35,36].
Conclusion
TGF-β signal usually produces biological effects through the TGF- β/SMAD/Snail signaling pathway, for example, TGF-β induces the EMT process via this signaling pathway, leading to tumor occurrence and metastasis. Our results show that BTAF1 is highly correlated with the signal characteristics of TGF-β signaling. Based on the results of the early stage of this study, we speculate that BTAF1 plays a regulatory role in the progression of OS. In summary, the lack of BTAF1 inhibits the epithelial-mesenchymal transition of osteosarcoma through the TGF-β signaling pathway. In addition, BTAF1 may be a new OS diagnostic marker and prognostic factor, providing new ideas for the treatment of OS.
Acknowledgements
This study was completely supported by the spinal degenerative diseases foundation (2020tszb04)
Conflict of Interest
None.
Ethical Approval
This study and experimental procedures were approved by the Human Research Ethics Committee of Tongji University (ShangHai, China), and written informed consent was obtained from all the patients involved.
Competing Interests
No conflict of interest exits in the submission of this manuscript and manuscript is approved by all authors for publication.
Authors' Contributions
I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed. Hailong Zhang contributed to the conception of the study; Tao Xia performed the experiment; Yuanqing Yang contributed significantly to analysis and manuscript preparation; Zhengran Ying performed the data analyses and wrote the manuscript; Zhizhou Wang helped perform the analysis with constructive discussions.
Funding
Spinal degenerative diseases Foundation (ID:2020tszb04).
Availability of Data and Materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
- Mirabello L, Troisi RJ, Savage SA (2009) osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer 115: 1531-1543.
[Crossref] [Google Scholar] [PubMed]
- He X, Gao Z, Xu H, Zhang Z, Fu P (2017) A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J Orthop Surg Re 12: 1-6.
[Crossref] [Google Scholar] [PubMed]
- Bielack SS, Hecker-Nolting S, Blattmann C, Kager L (2016) Advances in the management of osteosarcoma. F1000Res 5: 2767.
[Crossref] [Google Scholar] [PubMed]
- Mercatelli D, Bortolotti M, Bazzocchi A, Bolognesi A, Polito L (2018) Immunoconjugates for osteosarcoma therapy: Preclinical experiences and future perspectives. Biomedicines 6: 19.
[Crossref] [Google Scholar] [PubMed]
- Tsiambas E, Fotiades PP, Sioka C, Kotrotsios D, Gkika E, et al. (2017) Novel molecular and metabolic aspects in osteosarcoma. J BUON 22: 1595-1598.
[Google Scholar] [PubMed]
- Wang S, Zhang D, Han S, Gao P, Liu C, et al. (2017) Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci Rep 7: 6215.
[Crossref] [Google Scholar] [PubMed]
- Geller DS, Gorlick R (2010) Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 8: 705-718.
[Google Scholar] [PubMed]
- Klejman MP, Zhao X, van Schaik FM, Herr W, Timmers HT (2005) Mutational analysis of BTAF1–TBP interaction: BTAF1 can rescue DNA-binding defective TBP mutants. Nucleic Acids Res 33: 5426-5436.
[Crossref] [Google Scholar] [PubMed]
- Pereira LA, Klejman MP, Timmers HT (2003) Roles for BTAF1 and Mot1p in dynamics of TATA-binding protein and regulation of RNA polymerase II transcription. Gene 315: 1-3.
[Crossref] [Google Scholar] [PubMed]
- Wansleeben C, van Gurp L, de Graaf P, Mousson F, Timmers HT, et al. (2011) An ENU-induced point mutation in the mouse BTAF1 gene causes post-gastrulation embryonic lethality and protein instability. Mech Dev 128: 279-288.
[Crossref] [Google Scholar] [PubMed]
- Klejman MP, Pereira LA, van Zeeburg HJ, Gilfillan S, Meisterernst M, et al. (2004) NC2α interacts with BTAF1 and stimulates its ATP-dependent association with TATA-binding protein. Mol Cell Biol 24: 10072-10082.
[Crossref] [Google Scholar] [PubMed]
- Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, et al. (1987) Osteoblasts synthesize and respond to transforming Growth Factor-type beta (TGF-beta) in vitro. J Cell Biol 105: 457-463.
[Crossref] [Google Scholar] [PubMed]
- Yang RS, Wu CT, Lin KH, Hong RL, Liu TK, et al. (1998) Relation between histological intensity of transforming growth factor-β isoforms in human osteosarcoma and the rate of lung metastasis. Tohoku J Exp Med 184: 133-1342.
[Crossref] [Google Scholar] [PubMed]
- Mandal S, Johnson KR, Wheelock MJ (2008) TGF-β induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp Cell Res 314: 3478-3493.
[Crossref] [Google Scholar] [PubMed]
- Sun L (2004) Tumor-suppressive and promoting function of transforming growth factor beta. Front Biosci 9: 1925-1935.
[Crossref] [Google Scholar] [PubMed]
- Raymond AK, Jaffe N (2010) Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat Res152: 63-84.
[Crossref] [Google Scholar] [PubMed]
- Katsuno Y, Lamouille S, Derynck R (2013) TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 25: 76-84.
[Crossref] [Google Scholar] [PubMed]
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. The J Clin Invest 119: 1438-1449.
[Crossref] [Google Scholar] [PubMed]
- Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139: 871-890.
[Crossref] [Google Scholar] [PubMed]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, et al. (2004) Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431: 707-712.
[Crossref] [Google Scholar] [PubMed]
- Voon DC, Huang RY, Jackson RA, Thiery JP (2017) The EMT spectrum and therapeutic opportunities. Mol Oncol 11: 878-891.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Lu X, Huang L, Wang W, Jiang G, et al. (2014) Different thresholds of ZEB1 are required for Ras-mediated tumour initiation and metastasis. Nat Commun 5: 5660.
[Crossref] [Google Scholar] [PubMed]
- Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, et al. (2005) Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res 11: 8070-8078.
[Crossref] [Google Scholar] [PubMed]
- Miyahara S, Hamasaki M, Hamatake D, Yamashita SI, Shiraishi T, et al. (2015) Clinicopathological analysis of pleomorphic carcinoma of the lung: Diffuse ZEB1 expression predicts poor survival. Lung Cancer 87: 39-44.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Liu S, Guo W, Zhang C, Zhang B, et al. (2014) hTERT promoter activity identifies osteosarcoma cells with increased EMT characteristics. Oncol Lett 7: 239-244.
[Crossref] [Google Scholar] [PubMed]
- Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease: Old views and new perspectives. Int J Dev Biol 53:1541-1547.
[Crossref] [Google Scholar] [PubMed]
- Creighton CJ, Gibbons DL, Kurie JM (2013) The role of epithelial–mesenchymal transition programming in invasion and metastasis: A clinical perspective. Cancer Manag Res 31: 187-195.
[Crossref] [Google Scholar] [PubMed]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, et al (2004). Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia 6: 603-610.
[Crossref] [Google Scholar] [PubMed]
- Thiery JP (2003) Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15: 740-746.
[Crossref] [Google Scholar] [PubMed]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704-715.
[Crossref] [Google Scholar] [PubMed]
- Lamora A, Talbot J, Mullard M, Brounais-Le Royer B, Redini F, et al. (2016). TGF-β signaling in bone remodeling and osteosarcoma progression. J Clin Med 5: 96.
[Crossref] [Google Scholar] [PubMed]
- Sheng J, Chen W, Zhu HJ (2015) The immune suppressive function of Transforming Growth Factor-β (TGF-β) in human diseases. Growth Factors 33: 92-101.
[Crossref] [Google Scholar] [PubMed]
- Han J, Alvarez-Breckenridge CA, Wang QE, Yu J (2015) TGF-β signaling and its targeting for glioma treatment. Am J Cancer Res 5: 945.
[Google Scholar] [PubMed]
- Colak S, Dijke P (2017) Targeting TGF-β signaling in cancer. Trends Cancer 3: 56-71.
[Crossref] [Google Scholar] [PubMed]
- Meulmeester E, Dijke P (2011) The dynamic roles of TGF-β in cancer. The J Pathol 223: 206-219.
[Crossref] [Google Scholar] [PubMed]
- Sisto M, Lorusso L, Ingravallo G, Tamma R, Ribatti D (2018) The TGF-β1 signaling pathway as an attractive target in the fibrosis pathogenesis of Sjogren’s syndrome. Mediators Inflamm 2018: 1-11.
[Crossref] [Google Scholar] [PubMed]
Citation: Xia T, Yang Y, Ying Z, Wang Z, Zhang H (2025) Depletion of BTAF1 Inhibits Epithelial-to-Mesenchymal Transition via the TGF-β Signal Pathway in the Osteosarcoma. Diagnos Pathol Open 10: 245.
Copyright: © 2025 Xia T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 415
- [From(publication date): 0-0 - Nov 05, 2025]
- Breakdown by view type
- HTML page views: 304
- PDF downloads: 111