Determination of Microbial Activities and Biomass in Biofilm Associated with Treatment Wetlands Compartments to Investigate Active Pollutant Processing Site
Received: 19-Oct-2017 / Accepted Date: 13-Nov-2017 / Published Date: 15-Nov-2017 DOI: 10.4172/2155-6199.1000419
Abstract
Although Floating treatment wetlands (FTWs) provide immense advantages over other natural treatment facilities, there is no information about biofilm functioning and microbial-based processes in FTW. Therefore, this study was aimed to evaluate the magnitude of microbial-based processes in the root, bottom and water column zones of the FTW by employing of macrophytes. For this experiment, primary domestic wastewater effluent was used in two pairs of FTWs (I. psuedacorus and P. stratiotes) and a pair of control. Total microbial activity was estimated using FDA hydrolytic activity and specific microbial activities were examined as denitrification and nitrification activities, whilst viable microbial number and distribution in the FTW compartments were determined using ATP assay. The average nitrification rates in the FTWs were 0.55, 0.81 and 2.75 μg/ml of water, gravel and root surface per hour respectively; and denitrification rates were 0.022, 0.053 and 0.132 μg/ml of water, gravel and roots surface respectively. The mean fluorescein concentration for the FTWs were 9.2, 1.1 and 0.06 μg/ml of root, gravel and freewater respectively, indicating that the highest total microbial activity in the FTW occurs in the biofilm associated with the root system. Mean viable microbial community 3.85 × 108, 3.7 × 107 and 1.3 × 107 cells/ml of root surface, water and gravel surface. Therefore, all the result suggested that active pollutant removal in all FTW stakes place in the root zone.
Keywords: Floating treatment wetland; Macrophytes; Microbial activity; Biofilm
Introduction
FTWs that incorporate common wetland plants growing in a hydroponic condition on floating rafts offer a potential solution to the major problems faced to ponds and conventional treatment wetlands [1]. FTW innovation can be practiced at all levels, with very low expense in all types of water body with ordinary engineering.
Despite the potential advantages of FTWs for the treatment of various wastewaters, there has been little information published to date about their design, construction and performance [2] and only few researchers assessed how the system functions [3,4].
Biofilms play the key roles in wastewater treatment systems including in conventional constructed wetlands and ponds. In FTWs, biofilm can effectively grow in the hanging roots, floating mat, sediment and in the free-water column between the sediment and the rhizosphere. Although, efficient removal of pollutants by FTW system is reported by few researchers, the location where the pollutants are actively processed and removed in the system has not been investigated yet.
Different zones of the system should be compared with respect to microbial parameters so that it gives clear information for the design and implementation of FTWs. Biofilm formations in the system need to be quantitatively explained. The active microbial cells distribution in the floating system should be also clearly identified so that the factors enhancing the microbial activity could be identified. This information is crucial in designing and implementing FTWs, which has no general design hitherto. Therefore, the objective of this study was to evaluate and compare microbial activities in different zones of the FTWs employing emergent macrophytes.
Materials and Methods
Experimental set up
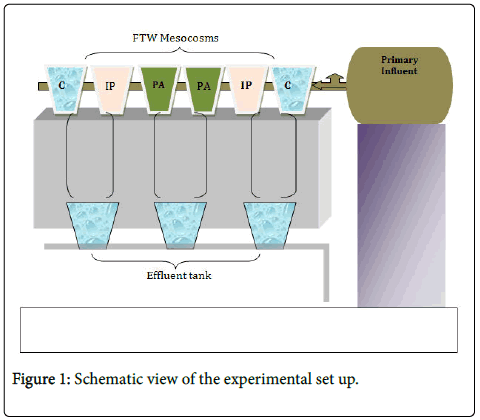
Six mesocosms were prepared from six buckets and one hundred liter influent tank was placed higher in the laboratory (Figure 1). The bottoms of every bucket were covered with gravels measuring about 2 Liters. Two pairs of suspending racks were prepared from white floater and several small holes were made to suspend the plants.
Two species of emergent macrophytes, Iris pseudacorus (IP) and Phragmites australis (PA), were selected. The macrophytes were placed on the suspending floater in such a way that the roots could grow suspended down to the water column. A pair of buckets was used as a control (without plant and floating mat). All of the mesocosms were prepared in duplicate. Twenty I. pseudacorus , twenty P. australis macrophytes were placed. The influent tank was filled with primary wastewater (taken from primary sedimentation pond). The system was adjusted to provide five days retention time for the influent. The set up was run for several months before biofilm sampling was done.
Nitrification and denitrification activity tests
The microbial activity tests were done as suggested by Halsey [5]. Known volume of gravel and root containing biofilm were sampled from each FTWs. For biofilms in the water column, about 10 ml of water sample was taken from each FTW. All the samples were placed in 250 ml flask; and 180 ml of phosphate buffer and 0.4 ml of 25 g/L ammonium sulphate were added. The flasks were placed on a rotary shaker for 5 minutes with the speed of 150 rpm and allowed to stand for approximately 5 minutes after removing from the shaker. Approximately 10 ml aliquot was filtered and half of the sample was used to measure initial NO3--N concentration. The other half of the filtrated samples were placed in a refrigerator at 4°C to be used as reference. The remnant gravel, root and water sample in the flask was incubated for 72 hours at room temperature. 10 ml samples were taken at different time intervals and NO3--N concentration was measured.
Potential Denitrification Activity test was done similarly but the substrate was 2 ml of 9 g/L sodium nitrate and 2 ml of 12 g/L glucose were added and the test was done under anoxic conditions.
Measurement of total microbial activity in the biofilm using FDA assay
Total microbial activity in the three compartments of the FTWs was estimated by Fluorescein diacetate (3',6'-diacetylfluorescein) hydrolysis [6,7].
For biofilm in the free-water column, 50-100 ml of water was filtered using 0.2 μm pore size polycarbonate membrane filter and carefully removed and placed into falcon tubes. Known volume of root and gravel were taken and placed in 50 ml of falcon tube in duplicate and then, 35 ml of 20 mM of phosphate buffer was added. Two control falcon tubes were prepared one without sample (C1) and the other without FDA (C2). To all tubes except C2, 0.35 ml of 1 mg/ml FDA was added. All tubes were incubated for 30-60 minutes at 38°C. Immediately after incubation, drops of acetic acid was added into the tubes and gently shaken with hand. The entire falcon tubes were span at 5000 rpm for 5 minutes and 5 ml at the top (aqueous) was filtered with 0.2 μm filter GF-C filter. The fluorescence was measured using spectrophotometer at a wave length of 490 nm. First, the absorbance was adjusted for the "C1" and "C2" and then, converted to concentration of fluorescein using calibration curve determined from known concentration of disodium fluorescein stock solution. The amount of fluorescein produced was determined per ml of water, gravel and root biofilm.
Estimation of physiologically active microbial community using ATP assay
Root and gravel samples were placed in 10 ml Milli-Q water whereas 10 ml of water sample was for water column biofilm. The samples were prepared in duplicate and handled carefully to avoid ATP contamination and biofilm disturbances. The biofilm was separated from the gravel and roots by treating with Bransonic sonicator for 3 minutes and this sample was used for extracellular and total ATP determination. Total ATP was measured from ultrasonicated sample where as extracellular ATP was measured after removal of viable cells by high speed centrifugation followed by filtering through 0.1 μm pore size filter.
1 ml of the supernatant and Milli-Q water (for control) were placed in eppendorf tubes and preheated 50 μL of Bac-Titer GloTM reagent / luciferase (Promega Co., WI, USA) added and shaken and incubated for 1 minute at 38°C. The photon produced was measured as relative light unit (RLU) by luminometer (GloMax 20/20R, USA). RLU was converted to ATP using standard calibration curve of known ATP concentration. ATP concentration was then transformed into carbon equivalents using a conversion factor (i) 1 ng/ml ATP=250 ng/ml cell carbon [8] (ii) 2.95 × 10-9 nmol ATP=1 μm3 biovolume [9]. The number of the living cells was then calculated from the living biomass with the assumption that one cell contains 20 fgC [8,9].
Results And Discussion
Nitrification activity: In all of the FTWs, nitrification by the root associated biofilm started immediately after incubation and increased rapidly (Table 1). The concentration increased from 14.68 μg/ml at the beginning to 212.8 μg/ml of root surface within 72 hours of incubation. This shows that the root biofilm contains enough nitrifiers to start nitrification process immediately after incubation, unlike the other biofilms which was delayed by several hours to start. Nitrification occurs only under aerobic conditions at dissolved oxygen level of 1 mg/L or more; and below 0.5 mg/L of dissolved oxygen, growth of microbes is low [10] and hence, nitrification rate is slow. Therefore, the sharply increasing nitrate concentration associated was probably due to the presence of the plants as they provide oxygenated zone around the rhizosphere and this virtually favored nitrifying bacteria.
| Incubation Time (hr) | Nitrate production by compartments | ||
|---|---|---|---|
| Nitrate (µg/ml root) | Nitrate (µg/ml ravel) | Nitrate (µg/ml water) | |
| 0 | 14.68 | 13.02 | 13.45 |
| 2 | 43.13 | 15.46 | 17.26 |
| 6 | 68.59 | 26.58 | 19.49 |
| 24 | 109.84 | 36.49 | 30.67 |
| 48 | 131.51 | 43.73 | 34.9 |
| 72 | 212.8 | 46.54 | 36.04 |
Table 1: Nitrate production through nitrification processes in the biofilms.
There were considerable variations in nitrification efficiency among FTWs, which can be related to several determinant factors such as oxygen production capacity and the quantity and quality of biofilm on the surface of the root. Oxygen evolution from roots depends on plant species, root type, location and age, light and temperature. For example, reports showed that highest oxygen release occurs in T. latifolia, P. australis and I. pseudacorus [11]. This fact is supported by maximum FDA hydrolysis test that showed maximum microbial activity in the root associated biofilms in all of the wetlands.
The slow nitrification rate and lesser nitrate accumulation is an indication that physiologically active nitrifying bacterial number in the water column was not enough to start nitirification rapidly in all of the wetlands and could nitrify. The delayed nitrification process in the gravel biofilm is an indication that the gravel contains less physiologically active microbes than the other compartments.
Denitrification activity: Average denitrification rate in the root, gravel and water for the FTWs over 72 hours of incubation varied between 0.09 and 0.19; 0.03 and 0.1; 0.02 and 0.24 μg NO3--N/ml/hour respectively. The variation among the compartments was tested by One-way ANOVA (unstacked) with post hoc comparison. The analysis showed that denitrification associated with the root biofilm was significantly varied (P< 0.05) from the other two compartments (Table 2).
| Incubation Time (hr) | Nitrate by compartment | ||
|---|---|---|---|
| Nitrate (µg/ml root) | Nitrate (µg/ml gravel) | Nitrate (µg/ml water) | |
| 0 | 9.56 | 8.75 | 8.65 |
| 2 | 8.51 | 8.74 | 8.59 |
| 17 | 7.81 | 6.72 | 6.58 |
| 24 | 4.87 | 5.7 | 5.55 |
| 33 | 3.16 | 2.12 | 2.82 |
Table 2: Loss of nitrate by denitrification process.
Nitrate concentration declined from 9.56 μg/ml to 0.05 μg/ml root surface within 72 hours of incubation (Table 2). The high denitirification rate and nitrate removal in the root zone of the FTW scan be associated with plant attributes to denitrifiers. Although denitrification predominantly takes place in the sediments of wetlands [12], recent studies showed significant contributions of denitrification taking place on periphytic communities attached to submerged macrophytes [13]. Decaying parts of the macrophytes provide suitable condition for denitrifying bacterial growth [13]. Although macrophytes oxygen supply through the roots can be inhibitory, the net effect depends on plant species, growth rate and total biomass.
Nitrate concentration declined from 8.75 μg/ml to 1.04 μg/ml gravel surface within 72 hours of incubation. The nitrate removal in gravel within 24 hours of incubation was very slow which was similar to denitrification in the free-water biofilm, but unlike the denitrification by free-water biofilm, the concentration rapidly declined and reached limiting concentration in 9 more hours of incubation. Compared to denitrification due to free-water biofilm, it was rapid and much better in terms of nitrate transformation capacity.
The low denitrification rate in the water column biofilm shows that physiologically active denitrifying bacteria suspended in the free-water column was low at the beginning and hence, until the denitrifiers multiply and become physiologically active, denitrification rate was low. This could be due to the influence of continuous aeration from the atmosphere into the water column, which may inhibit denitrifiers. The water column may not give diversified microhabitats like the rhizosphere so that the denitrification could be inhibited.
Total microbial activity
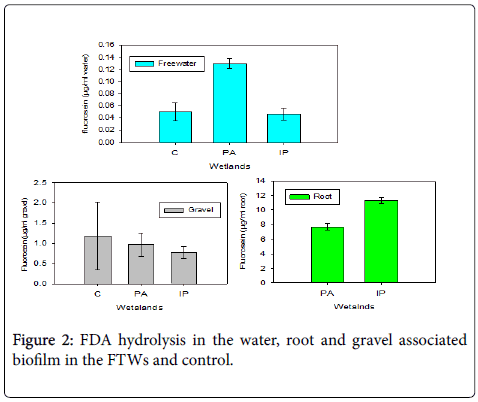
Total microbial activity in different zones of FTWs was measured as FDA hydrolysis. The mean hydrolysis product of the FDA, fluorescein concentration, varied from 7.7-11.3 μg/ml, 0.78-0.97 μg/ml and 7.5-12.7 μg/ml of root, gravel surfaces and water respectively (Figure 2). One-way ANOVA with Tukey's post hoc test showed that the FDA hydrolysis in the root zone was significantly varied from the gravel and free-water compartments in all of the FTWs (P< 0.05) but there were no statistically significant differences among the FTWs (P=0.36).
The higher FDA hydrolysis associated with root biofilm was due to the fact that the roots of plants can create conducive microhabitat for a wide array of microbial communities by providing oxygen, optimized pH media and surface area for attachment. The variation in the microbial activity among different FTWs could be attributable to the specific plant species used because of their differences in the quality and quantity of metabolites they produce, capacity to release oxygen and other associated conditions.
Fluorescein concentration in the gravel was higher than the freewater. Since FDA can be hydrolyzed by wide array of both extracellular and intracellular enzymes including esterase, lipases and proteases [7], the diversified composition of the gravel biofilm may cause higher FDA hydrolysis. This condition may not be true in FTWs employing emergent macrophytes since the floating mat hampers light entrance and this enhances the growth of heterotrophic and anaerobic microbial-dominated biofilm community. There was higher fluorocein hydrolysis in the control than the FTWs and this may be due to the fact that light can reach to the surface so that it gives favorable conditions for diversified microbial community growth.
Microbial activity in the bulk water was low compared to the gravel and the root zone may be due to the rarity of suitable attachment site in the free-water zone.
Viable microbial biomass
Viable microbial biomass in the biofilm taken from different FTW compartments was measured as a concentration of ATP (Table 3). Viable (physiologically active) microbial community in the three compartments varied significantly (P < 0.05). Viable microbial community varied between 3.2 × 108(ATP=9.66 ng/ml) (PA) and 4.5 × 108(ATP=35.99 ng/ml) (IP) cells/ml of root surface, 2.5 × 107 (ATP=2.5 ng/ml) (IP) and 4.9 × 107(ATP=3.9 ng/ml) (PA) cells/ml of water; and 1 × 107(ATP=0.8 ng/ml) (IP) and 1.6 × 107(ATP=1.3 ng/ml) (PA) cells per ml of gravel (Table 3). Physiologically active microbial number in the control was much lower than both FTWs.
| water Biofilm | Gravel Biofilm | Root Biofilm | ||||
|---|---|---|---|---|---|---|
| Wetlands | Cells/mL (107) | ATP (ng/ml) | Cells/mL (107) | ATP (ng/ml) | Cells/mL (108) | ATP (ng/ml) |
| C | 0.3 | 0.27 | 0.3 | 0.24. | ----- | ----- |
| PA | 4.9 | 3.9 | 1.6 | 1.3 | 3.2 | 9.66 |
| IP | 2.5 | 2 | 1 | 0.8 | 4.5 | 35.99 |
Table 3: Physiologically active microbial community number and distribution in FTWs Compartment.
The higher ATP molecule in the root zone (Table 3) is an indication of the presence of higher active bacterial biomass in the root biofilm. The dominant physiologically active microbial community associated with the root biofilm is possibly due to the fact that the root zone provides conducive microhabitats for biofilm formation, growth and its optimum activity. Plant species vary in the quality and quantity of organic substances they provide, surface area for microbial attachment and the capacity to oxygenate the medium [14].
The low number of active microbial community associated with gravel biofilm could be due to the influence of the surface cover by floating rack and the short time for the gravel biofilm to establish itself at the bottom. Active microbial community in the FTWs was much higher than the control, in fact this indicates the presence of vegetation, not only affect the root zone but also it influences the whole system in the FTWs.
In the present study, although the proportion of the root in each FTW was low (9%), it accounted more than half (59%) of the total physiologically active microbial cells in the system. Highest active microbial cell numbers (high intracellular ATP) is an indication of enhanced bacterial activity in the system.
Conclusion
• Evidences from the FDA hydrolysis for all of the FTWs showed that total microbial activity associated with the root biofilm was much higher than the other two compartments.
• Denitrification and nitrification activity tests conducted for all of the FTWs also confirmed that the rate of nitrogen transformation in the root associated biofilm was higher than other compartments.
• ATP assay confirmed that intracellular ATP concentration and estimated number of physiologically active bacterial cells was much higher in the root biofilm than the other compartments. Therefore, it is reasonable to conclude that in FTWs active pollutant transformation takes place primarily associated with the root biofilm.
References
- Headley TR, Tanner CC (2006) Application of floating wetlands for enhanced stormwater treatment: a review. Auckland Regional Council, Technical Publication, New Zealand, p: 324
- Headley T, Tanner C (2008) FTWs: an Innovative Option for Stormwater Quality Applications. In: 11th International Conference on Wetland Systems for Water Pollution Control, Indore, India.
- Faulwetter JL, Burr MD, Cunningham AB, Stewart FG, Camper AK, et al. (2011) FTWs for domestic wastewater treatment. Water Science and Technology 64: 2089-2095.
- Van de Moortel A, Meers E, De Pauw N, Tack F (2010) Effects of Vegetation, Season and Temperature on the Removal of Pollutants in Experimental FTWs. Water, Air & Soil Pollution 212: 281-297.
- Halsey K, Arp D, Sayavedra L, Beedlow C (2008) Nitrification Potential in Soils. Workshop organized by Oregon State University and Corvallis High School District 509J, USA.
- Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33: 943-951.
- Schnurer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43: 1256-1261.
- Karl DM (1993) Total microbial biomass estimation derived from the measurement of particulate adenosine-5´-triphosphate. In: Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, USA, pp: 359-367.
- Hammes F, Goldschmid F, Vital M, Wang Y, Egli T (2010) Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Research 44: 3915-3923.
- Paredes D, Kuschk P, Mbwette TSA, Muller RA, Koser H (2007) New aspects of microbial nitrogen transformation in the context of wastewater treatment – a review. Engineering in Life Sciences 7: 13-25.
- Stottmeister U, WieBner A, Kusck P, Kappelmeyer U, Kastner M, et al. (2003) Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnology Advances 22: 97-117.
- Seytzinger PS (1988) Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnol Oceanogr 33: 702-724.
- Toet S (2003) A treatment wetland used for polishing tertiary effluent from sewage treatment  plant: performance and processes. PhD thesis, University of Utrecht, Netherlands.
- Bastviken SK, Eriksson PG, Martins I, Neto JM, Leonardson L, et al. (2003) Potential nitrification and denitrification on different surfaces in a constructed treatment wetland. Journal of Environmental Quality 32: 2414-2420.
Citation: Ali AS (2017) Determination of Microbial Activities and Biomass in Biofilm Associated with Treatment Wetlands Compartments to Investigate Active Pollutant Processing Site. J Bioremediat Biodegrad 8: 419. DOI: 10.4172/2155-6199.1000419
Copyright: © 2017 Ali AS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4965
- [From(publication date): 0-2017 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 4025
- PDF downloads: 940