Determining Risk Factors of a Non-Point Source Outbreak of Campylobacter Cases Using Case-Case and Case-Control Studies
Received: 19-Sep-2015 / Accepted Date: 23-Oct-2015 / Published Date: 28-Oct-2015 DOI: 10.4172/2161-1165.1000203
Abstract
Background: Investigating foodborne outbreaks is a resource and time-intensive process using traditional casecontrol methodology. The use of case-case studies in outbreak investigations is not well studied, although they require fewer resources to conduct and limit selection and recall bias. In this study we investigated a cluster of Campylobacter infections using almost simultaneous case-control and case-case studies to compare results from the two methodologies.
Methods: In 2011 a significant increase in Campylobacter cases was detected in Pima County, AZ through routine surveillance. To determine potential sources of the outbreak we conducted two studies. The case-control study used randomly selected non-ill controls. The case-case study used historical surveillance data. Logistic regression analysis was used to determine risk factors for infection.
Results: Statistically significant risk factors associated with disease differed by design with travel (OR=4.1), Hispanic (OR=4.5), and youth (OR=3.6) in the case-control and untreated water (OR=3.4) and fresh eggs (OR=2.5) in the case-case. Effect modification by travel was found for untreated water (OR=14.0 for travelers vs. OR=undefined for non-travelers) and eggs (OR=11.5 for travelers vs. OR=1.5 for non-travelers).
Conclusions: Travel history, a commonly reported risk factor, is a distal part of the exposure pathway. These studies exposed the more proximal cause to be largely attributed to travelers who had exposure to untreated water and fresh eggs. Case-case methods were found to be useful in outbreak investigations of a foodborne illness. This outbreak is also an example where a student response team response with a local public health department.
Keywords: Case-case studies; Case-control studies; Foodborne outbreaks; Campylobacter
163515Introduction
Case-control studies are the primary design used in foodborne outbreak investigations. Yet the resources required for conducting frequent case-control studies are often beyond the capacity of local health departments. Developing rapid, minimal resource methods to identify factors associated with an outbreak could lead to more consistent and timely response. Case-case studies are a potential alternative for some outbreak investigations [1-5]. Case-case designs have been utilized in studies of other diseases such as cancer [6] and more recently been used for enteric diseases [4,7-9]. In this study, historical cases from surveillance data were compared to outbreak cases to identify the possible source contributing to an increase in cases.
Campylobacter is the leading cause of bacterial gastroenteritis globally [10]. In the United States 45,000 laboratory confirmed cases are reported each year [11], but are estimated to cause 2.4 million cases annually [12]. Most of these cases are sporadic in nature [13-19], however, for outbreaks that do occur, most are linked to unpasteurized dairy [20,21] and untreated water [22]. While these studies help to determine disease risk factors associated, they are costly and time consuming. This often makes them prohibitive to conduct for health departments outside of large, point source outbreaks. In Arizona, the annual rate of reported campylobacteriosis has been on average 1.23 times higher than the national average over the past ten years [23,24] with an average of 900 laboratory confirmed cases reported each year [25].
In mid-September of 2011, the Pima County Health Department (PCHD) in southern Arizona noted a sharp increase in the number of Campylobacter spp. infections reported through their routine surveillance system. Collaboration between PCHD and the University of Arizona’s student outbreak response team (SAFER) [26] sought to identify the possible source for this outbreak and to evaluate the efficiency of two parallel study designs; a case-case study and a casecontrol study.
Methods
Aberration Detection: A standard aberration detection algorithm was used to calculate the running mean of historical cases for the time period 2006-2010 [27-29]. Current cases were grouped in 4 week intervals to be compared to five years of historical data for the same 4 week intervals. These weeks represent the Center for Disease Control’s Morbidity and Mortality Weekly Report (MMWR) reporting week (1-52) and not necessarily the onset date of the case. Average yearly case counts were determined for successive time intervals (the current time period, the 4 weeks before and the 4 weeks after) [27,30]. Outbreaks are suspected when the number of current cases is above two standard deviations of the running mean [30].
Case-control study
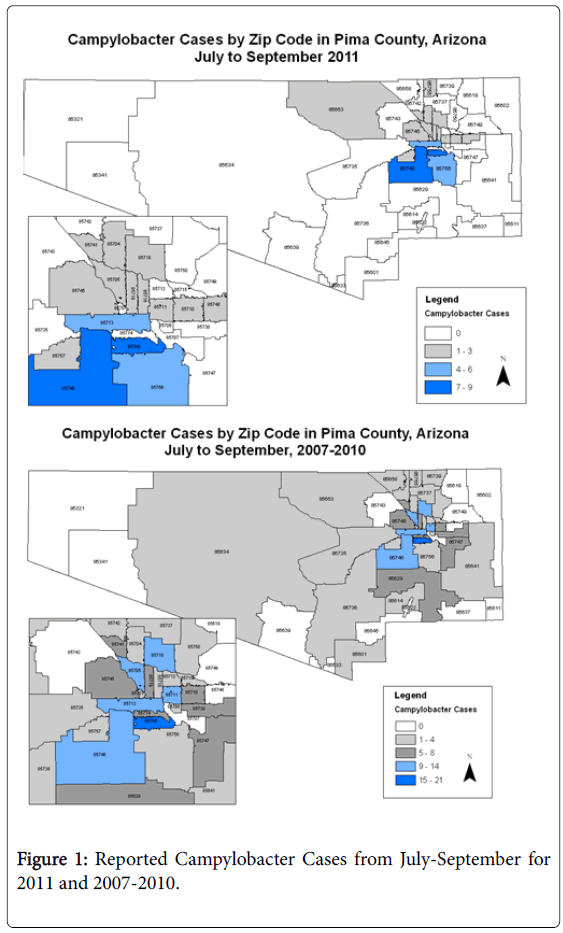
Case definition and interviews: ‘Outbreak’ cases were defined as laboratory confirmed Campylobacter spp. cases identified through the county health department’s routine surveillance system from July to September 2011 shown in Figure 1.
Pulse Field Gel Electrophoresis (PFGE) was not being conducted on Campylobacter cases in Arizona at the time, so it was not possible to identify genetically linked cases. Outbreak cases were interviewed by county epidemiologists using a standard investigation form. Deidentified data were provided to the University team on demographics symptoms, travel, and exposure history to common risk factors for Campylobacter infection [13,15-17,31,32].
Control definition, recruitment, and interviews: Controls were identified through random digit dial by generating phone numbers in Excel (Microsoft Corp. Seattle, WA) using county pre-fixes. Ineligible households were those where residents spoke a language other than English, or no one met the needed age and gender criteria. Controls were matched to cases by gender and age group to be consistent with other published studies [13,15,19,33]. The goal was a 2:1 frequency match for controls to cases. Controls were excluded if they reported a diagnosed infection due to Campylobacter in the last month. For minors, the available guardian was either interviewed or consented to interview the child.
The control questionnaire mirrored the case interview for most potential risk factors. As there was a three month delay between case and control interviews due to lags in surveillance and IRB approval for University participation, exposures that were potentially less subject to recall bias (i.e. untreated water exposures and social gathering attendance) were asked for the period of time of the identified outbreak (July-September). Food consumption of specific items for the two weeks prior to the interview was asked to more closely match the recall period required of cases. Surveys were administered over the telephone by members of the University’s student outbreak response team using the survey program Illume (DatStat Corp, Seattle, WA).
Case-case study
Cases: The same cases were used for the case-case study as the casecontrol study.
Historical cases: Historical comparison cases were identified from surveillance data on campylobacteriosis cases reported to PCHD from January 2007 to September 2010. Of the 681 potential cases from this time period, 105 without risk factor data were excluded. Comparison cases were restricted to those cases occurring during the same season as the outbreak cases: July-August of 2007-2010 (n=186). The same questionnaire was used for both the historical and outbreak cases as shown in Figure 1.
Statistical analyses
While the spike in cases was noted in August (weeks 33-36), cases with onset dates from July-September were included as the outbreak cases to account for any earlier or later exposures or reporting. When the months of July (N=10), August (N=26) and September (N=12) were combined, a total of 48 suspect outbreak cases were included in the analyses.
Counts and frequencies were determined for demographics, symptoms and risk factors for controls and both sets of cases. Fisher’s exact tests and Student’s t-tests were performed to determine differences in demographic characteristics between the outbreak cases, phone controls and historical cases. Logistic regression analysis was used to estimate odds ratios with 95% confidence intervals, although interpretation of odds ratios differed by study design. Multivariate models were used to evaluate ethnicity and travel history as either potential confounders or effect modifiers. All analyses were conducted in Stata version 11.0 (Stata Corp, College Station, TX).
Results
Aberration detection
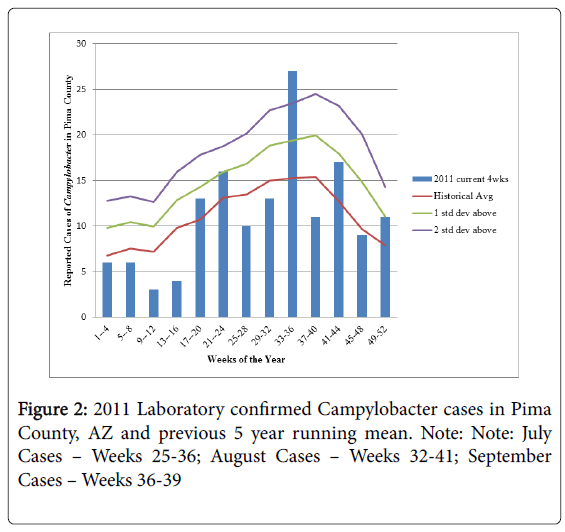
Figure 2 shows the running means by week for 2011 cases compared to the 2007-2010 historical cases. These cases were largely sporadic in nature with no reported outbreaks in the county during this time period. The reported cases in weeks 33-36 (N=27) of 2011 were 2 standard deviations above what was expected given historical data and were almost double the historical average of 15 cases during the same time period indicating a potential outbreak of Campylobacter infection had occurred.
Case-control and case-case studies
Response rate: During the 22 days of data collection, 2,016 randomly selected phone numbers were called; 624 (31%) numbers were never answered, 784 (39%) were disconnected and 156 (7.7%) were businesses. Of the 452 households contacted, 31 either spoke only Spanish (N=17) or did not meet the age and gender criteria for the frequency match (N=14). Sixty-eight (N=68) completed interviews (15% response rate from eligible households). On average, these interviews required 2.8 hours of student time for each successful interview, though each interview took approximately 20-25 minutes to complete.
Demographics of study populations: Table 1 compares demographic characteristics of the outbreak cases (N=48), historical cases (N=186) and phone controls (N=68). When comparing the outbreak cases to the phone controls, there were no statistically significant differences by gender or age, indicating the frequency matching was successful. However, 44% of cases were Hispanic compared to only 28% of phone controls (p=0.002). The percentage of Hispanic residents in the county as a whole was 34.6% [34].
| Outbreak CasesJuly-September 2011N (%) | Historical CasesJuly-September 2007-2010Mean, (N4yr) (%) | Controls (%)N % | |
|---|---|---|---|
| Total | 48 | 46.5 (186-by year 43, 53, 42, 48) | 68 |
| Male | 21 (44%) | 25.75 (103) (55%) | 24 (35%) |
| Female | 27 (56%) | 20.75 (83) (45%) | 44 (65%) |
| Age | |||
| 0-11mo | 1 (2%) | 0.25 (1) (0.5%) | 0 (0%) |
| 1yr-9yr | 5 (10.4%) | 4.5 (18) (9.7%) | 2 (2.9%) |
| 10yr-19yr | 11 (23%) | 4.5 (18) (9.7%) | 7 (10.3%) |
| 20-29yr | 5 (10.4%) | 7.75 (31) (16%) | 7 (10.3%) |
| 30-59yr | 14 (29%) | 17 (68) (37%) | 29 (43%) |
| 60+ | 12 (25%) | 7.75 (31) (16%) | 23 (34%) |
| Hispanic | 21 (44%) | 20 (80) (43%) | 19 (28%) |
| Non-Hispanic | 8 (8%) | 15 (60) (32%) | 41 (60%) |
| County location | |||
| Zip Code 1* | 9 (20%) | 21(12%) | 0 |
| Zip Code 2† | 7 (15%) | 11 (6.3%) | 0 |
| Zip Code 3† | 5 (11%) | 4 (2.3%) | 0 |
*p<0.05 for Fishers’ exact test for difference between 2011 cases and phone controls.
†p<0.05 for Fishers’ exact test for difference between both historical cases and 2011 cases.
Table 1: Demographic characteristics of Campylobacter outbreak cases, historical cases, and controls
Those under the age of 20 made up 35% of the outbreak cases, with the majority between 10-19 years old. There were more boys reported in the outbreak cases (60% of the 1-9 year olds were boys and 64% of the 10-19 year olds) (data not shown). For the phone controls, women accounted for a higher proportion than outbreak cases (56% versus 65% - no statistical difference).
Spatial distribution: Outbreak cases were found to occur disproportionately in three zip codes that accounted for 46% of all cases in July-September 2011. These zip codes were geographically adjacent and include 15.2% [34] of the total population for the county. During these three months, 20% of cases came from zip code 1, 15% from zip code 2 and 11% from zip code 3, compared to the historical cases residing in zip code 1 (12%), zip code 2 (6.3%) and zip code 3 (2.3%). None of the phone controls resided within these three zip codes. Within these zip codes, Hispanics made up the majority of cases, however they also accounted for the majority of residents according to the 2010 U.S. Census [34]. Analyses of common risk factors were completed for these three areas alone with no statistically significant differences identified compared to all other outbreak cases (data not shown).
Symptomology: Symptoms were compared for the outbreak cases and the respective historical cases (Table 2). Most symptoms were reported consistently between the two sets of cases. However, hospitalizations were reported more commonly among cases from July-September 2011 as compared to cases for those same months in the previous four years (25% vs. 15%, p=0.1 using Fisher’s exact test).
| Outbreak cases July-September 2011N (%) | Annual mean of historical casesJuly-September 2007-2010N (N4yr) (%) | |
|---|---|---|
| Total | 48 | 46.5 (186) |
| Diarrhea | 44 (92%) | 45 (180) (97%) |
| Bloody stools | 19 (40%) | 18 (72) (39%) |
| Nausea | 30 (63%) | 23 (92) (49%) |
| Vomiting | 18 (38%) | 18 (72) (39%) |
| Fever | 29 (61%) | 31.75 (127) (68%) |
| Cramps | 36 (75%) | 38.5 (154) (83%) |
| Chills | 22 (46%) | 20.75 (83) (45%) |
| Headache | 18 (38%) | 17.5 (70) (38%) |
| Muscle Aches | 26 (54%) | 18 (72) (39%) |
| Fatigue | 27 (56%) | 24.5 (98) (53%) |
| Hospitalized | 12 (25%) | 7 (28) (15%) |
*No statistically significant differences between any symptoms were found using Fisher’s exact test.
Table 2: Comparison of symptoms between outbreak cases and historical cases
Risk factor comparisons
Case-control analysis: For consumption of various food items and lifestyle behaviors, cases were more likely to report consuming unpasteurized dairy (15% vs. 10%), and have a travel history (29% vs. 10%), particularly outside of the U.S. (25% vs. 10%) than controls (Table 3). The statistically significant crude risk factors were any travel history in the last 30 days (OR=4.07; 95% CI 1.49-11.13), travel to Mexico (OR=3.24; 95% CI 1.01-10.43), travel anywhere outside of the U.S., including Mexico (OR=3.88; 95% CI 1.33-11.28) and being Hispanic (OR=4.53; 95% CI 1.79-11.5). Eating poultry was found to be ‘protective’ but not statistically significant and no distinction was made between consumption in the home or away from home in the questionnaire. Exposure to untreated water had no association for all three months combined, but when exposure for controls was limited to only the month of August, the odds ratio increased from 1.01 to 1.87 although neither was statistically significant. Fresh eggs could not be analyzed because the question was asked differently for the cases and controls. When the models were adjusted for age and gender, the odds ratios for each decreased with travel history in the last 30 days (OR=3.3; 95% CI 1.2-9.4), travel to Mexico (OR=2.6; 95% CI .8-8.7), and being Hispanic (OR=3.6; 95% CI 1.2-10.5) remaining significant.
| Outbreak casesN=48N (%) | Phone controlsN=68N (%) | July-Sept 2007-2010 historic casesN=186N (%) | Outbreak cases vs.July-Sept 2007-2010 historic casesOR (CI) [p-value] | Outbreak cases vs.phone controlsOR (CI) [p-value] | Outbreak cases vs.phone controlsaOR (CI) [p-value]* | |
|---|---|---|---|---|---|---|
| Exposure to untreated water† | 8(17%) | 15 (22%) | 2.75 (11) (5.9%) | 3.4 (1.26-9.3) [0.016] | 1.01 (0.38-2.67) [0.99] | 0.77 (0.27-2.15) [0.62] |
| Raw dairy consumption | 7 (15%) | 7 (10%) | 6.5 (26) (14%) | 1.01 (0.40-2.56) [0.98] | 1.73 (0.55-5.42) [0.34] | 1.79 (0.56-5.78) [0.33] |
| Fresh Eggs‡ | 15 (31%) | 57 (83%) | 7.75 (31) (17%) | 2.5 (1.14-5.44) [0.02] | ----------- (0.14 p=000) | ---------(0.15 p=0.000) |
| Runny or uncooked eggs | 4 (8.3%) | 11 (16%) | 4.5 (18) (9.7%) | 0.94 (0.30-3.02) [0.93] | 0.62 (0.18-2.14) [0.45] | 0.69 (0.19-2.46) [0.57] |
| Attend a gathering | 10 (21%) | 24 (35%) | 8 (32) (17%) | 1.25 (0.55-2.83) [0.59] | 0.62 (0.26-1.48) [0.28] | 0.58 (0.23-1.42) [0.23] |
| Poultry | 25 (52%) | 59 (85%) | 28.8 (115) (62%) | 0.70 (0.30-1.66) [0.42] | 0.38 (0.13-1.09) [0.07] | 0.37 (0.13-1.09) [0.07] |
| Any travel | 14 (29%) | 7 (10%) | 15 (60) (32%) | 0.86 (0.43-1.75) [0.68] | 4.07 (1.49-11.13) [0.01] | 3.34 (1.19-9.43) [0.02] |
| Travel to Mexico | 9 (19%) | 5 (8.6%) | 6.5 (26) (14%) | 1.43 (0.62-3.33) [0.40] | 3.24 (1.01-10.43) [0.05] | 2.62 (0.79-8.72) [0.12] |
| Travel outside of US | 12 (25%) | 7 (10.3%) | 10.5 (42) (23%) | 1.15 (0.54-2.44) [0.71] | 3.88 (1.33-11.28) [0.01] | 3.2 (1.06-9.56) [0.04] |
| Contact-person w/diarrhea | 8 (17%) | Not asked | 8 (32) (17%) | 0.93 (0.39-2.21) [0.88] | --------- | --------- |
| Contact-ill animal | 1 (2.1%) | 4 (5.9%) | 2.5 (10) (5.4%) | 0.29 (0.04-2.36) [0.25] | 0.37 (0.04-3.48) [0.39] | Undefined |
| Youth (age 0-19) | 17 (35%) | 9 (13.2%) | 14 (56) (30%) | 1.27 (0.65-2.49) [0.48] | 3.59 (1.44-9.0) [0.01] | ---------- |
| Hispanic | 21 (44%) | 19 (28%) | 20 (80) (43%) | 1.58 (0.69-3.59) [0.28] | 4.53 (1.79-11.5) [0.00] | 3.20 (1.14-8.95) [0.03] |
*Adjusted for gender and age group for matched study.
†Exposure to untreated water includes getting face wet in river, lake or pond or drinking untreated water.
‡Eggs removed from analysis for case-control - question in control interview asked for eggs only and not fresh eggs
Table 3: Comparison of risk factors for outbreak cases, historical cases, and controls.
To test for confounding, ethnicity (non-Hispanic as the reference) was added to each regression model. In adjusted models, the odds ratio decreased from 4.07 to 1.48 for any travel and from 4.5 to .43 for travel to Mexico; neither relationship was statistically significant (data not shown). However, for other risk factors the inclusion of ethnicity into the model made little difference in the measure of effect. Due to this, the decision was made not to adjust for ethnicity in the final models and to stratify by travel.
Case-case analysis: Exposure to untreated water was statistically significant OR=3.4 (CI 1.3-9.3) as well as consumption of fresh eggs OR=2.5 (95% CI 1.1-5.4) (Table 3). The location in which eggs were purchased and consumed was asked but there were no significant differences between the outbreak cases and historical cases (data not shown).
Given the elevated odds ratio for travel observed in the case-control study, the role of travel history was further explored in the case-case study by stratification of cases by travel. Exposure to untreated water was a large and statistically significant risk factor for travelers (OR=14.0, CI 3.2-61.1) as was consumption of eggs (OR=11.2, CI 2.0-62.8) (Table 4). For non-travelers, these relationships were either not observed or reduced greatly. However, the stratified results did not differ for all risk factors including poultry consumption and contact with a person with diarrhea. When analyses were limited to only weeks 33-36, odds ratios for untreated water and eggs were both higher in the full and stratified analyses, but were not statistically significant (data not shown).
| Case-CaseTravelersOR (CI) [p-value] | Case-CaseNon-TravelersOR (CI) [p-value] | |
|---|---|---|
| Exposure to untreated water | 14.0 (3.2-61.1) [0.00] | Null (0 cases w/ exposure) |
| Raw dairy consumption | 0.96 (0.23-3.9) [0.96] | 1.34 (0.33-5.5) [0.68] |
| Eggs | 11.2 (2.0-62.8) [0.01] | 1.5 (0.57-3.9) [0.41] |
| Runny or raw eggs | 2.5 (0.2-31) [0.48] | 0.74 (0.19-2.8) [0.66] |
| Attend a gathering | 2.2 (0.63-7.7) [0.22] | 0.86 (0.26-2.8) [0.8] |
| Poultry | 0.6 (0.13-2.7) [0.51] | 0.75 (0.26-2.1) [0.6] |
| Hispanic | 4.58 (0.5-42.3) [0.18] | 1.1 (0.41-3.0) [0.8] |
| Contact w/ someone with diarrhea | 0.93 (0.39-2.2) [0.88] | 0.73 (0.25-2.1) [0.56] |
| Contact with ill animal | Undefined | 0.29 (0.03-2.4) [0.25] |
| Youth | 2.33 (0.7-7.6) [0.16] | 0.72 (0.28-1.84) [0.49] |
| Hospitalized | 0.88 (0.09-8.3) [0.9] | 1.8 (0.74-4.6) [0.19] |
Table 4: Case-case stratification by travel history.
Discussion
Conducting case-control and case-case studies within the same populations and general time frame allowed for a comparison of these methods and evaluation of the utility of the case-case design. This method is commonly used when PFGE data is available, but this data is not always available to delineate the ‘outbreak’ cases from other routine cases. This meant that not all ‘outbreak cases’ were in fact, tied to an outbreak and were part of the normal endemic cases seen in the county. However, this type of misclassification would bias the results towards the null because these cases would be more like the historical cases used as a comparison. Use of historical cases drawn from the same surveillance system as the outbreak cases reduces selection bias [35]. Recall bias is also minimized because all cases had symptoms and were interviewed within a similar time period following their laboratory confirmation. These studies can be conducted with existing case data, eliminating the staff time and effort needed to find and interview controls. While direct cost comparisons were not possible for this study, we identified a significant amount of time was necessary to recruit each control relative to pulling historical case data. Furthermore, the use of a student response team to recruit and interview controls expanded the health department’s capacity to further investigate this outbreak.
Travel history had the strongest measure of effect in the case-control study. Foreign travel has been associated with Campylobacter infection in a number of case-control studies [13,15,17,36] and, while including all foreign travel had a stronger strength of association, given the proximity to the U.S. Mexico border, the association particularly with Mexico was a finding that has relevance to public health planning in this region. Interestingly, when this association was adjusted for Hispanic ethnicity, it was clear that being Hispanic was a confounder in relationship between travel history and development of disease but not an effect modifier (ethnicity did not change the measure of association when stratified by travel history).
One limitation was in the recruitment of phone controls. While the recruitment goal was a 2:1 match of controls to cases, this did not occur in all the age groups, particularly among boys (age 1-19). This group represented 33% of all cases reported from outbreak cases but only 13% of the phone controls. This difference may have led to a larger effect size being attributed to youth in the case-control analyses. In retrospect, we would not choose to match on gender for children to help the recruitment efforts of controls. In addition, since almost 58% of the outbreak cases resided in three adjoining zip codes, it may have been a better strategy to match based on residence of the cases. However, based on prior experiences, matching based on location greatly increases recruitment time and resources. Finally, there were no bilingual interviewers available for control interviews, however only 17 (0.8%) of the 2,017 control calls required a Spanish speaking interviewer and given the response rate, would have only resulted in another 2-3 completed control interviews.
Unlike reported studies where the case-case and case-control studies resulted in similar findings [4,37,38], identified risk factors differed somewhat between study designs. This may be explained by slight differences in questionnaire design between the two studies. The case questionnaire asked about ‘unpasteurized eggs’ while controls were asked only about ‘eggs’ with results being a higher percentage of controls, than cases reported eating eggs. While this may have in fact been the case, it is possible that the cases were confused with the term ‘unpasteurized eggs’. For untreated water, cases were asked about exposures that took place in the 2 weeks prior to their illness which was during the summer months. Since control interviews were conducted in November, information on exposures during the months July-September was gathered, instead of October/November when recreational water exposures would be lower. This decision resulted in controls reporting for a 12 week period of time, rather than the 2 weeks for the cases, moving the association towards the null. Given the longer exposure period, it was not surprising that the proportion of controls that reported a water exposure was higher than that of cases. However, when the case-control analysis was limited to only exposures reported for the 4 weeks of August, the odds ratio for exposure to untreated water increased to 1.87 (95% CI .65-5.4).
Given that travel was such a significant risk factor in the casecontrol results, it was examined for interaction within the case-case study. When the case-case study was stratified by travelers and nontravelers, travel history was a clear effect modifier of certain risk factors. Exposure to untreated water and fresh egg consumption, in particular, was higher among travelers. The strong association between travel and campylobacteriosis in the case-control study was consistent with previous studies [13,15,31,36,38,39]. The lack of an increase in odds ratio in the case-case analyses was not surprising, given rates of travel among cases have been reported to be as high as 20% [11] to 42% [39]. Surprisingly, given the high odds of travel associated with disease, the specific exposures related to travel have not been explored in the literature. The case-case study identified the likely exposures that were being overwhelmed by prevalence of travel among the cases in the case-control study. This method could be very useful to further explore the true exposure leading to disease among travelers.
Conclusion
Risk factors were identified by both studies and provided valuable information on exposures associated with Campylobacter infections. More importantly, the use of concurrent study methods helped to identify more relevant exposures related to travel, since travel in itself is not a true pathogen exposure. These results show that case-case studies can be used not only as an efficient precursor to a case-control study, but can provide additional information about the transmission of disease. Given the limited resources available in many state and local health departments, new and innovative ways of comparison study designs should be considered and investigated.
References
- Gaulin C, Levac E, Ramsay D, Dion R, Ismail J, et al.(2012) Escherichia coli O157:H7 outbreak linked to raw milk cheese in Quebec, Canada: use of exact probability calculation and casecase study approaches to foodborne outbreak investigation. J Food Prot 75(5): 812-8
- Giraudon I, Cathcart S, Blomqvist S, Littleton A, Surman-Lee S, et al. (2009) Large outbreak of salmonella phage type 1 infection with high infection rate and severe illness associated with fast food premises.Public Health 123: 444-447.
- Gobin M, Launders N, Lane C, Kafatos G, Adak B (2011) National outbreak of Salmonella Java phage type 3b variant 9 infection using parallel case-control and case-case study designs, United Kingdom, July to October 2010.Euro Surveill 16: 20023.
- Krumkamp R, Reintjes R, Dirksen-Fischer M (2008) Case-case study of a Salmonella outbreak: an epidemiologic method to analyse surveillance data.Int J Hyg Environ Health 211: 163-167.
- Pogreba-Brown K, Ernst K, Harris RB (2014) Case-case methods for studying enteric diseases: A review and approach for standardization. OA Epidemiology. 2: 1-9.
- Martnez M, Cruz GI, Brewster AM, Bondy ML, Thompson PA (2010) What Can We Learn about Disease Etiology from Case-Case Analyses? Lessons from Breast Cancer. Cancer Epidemiology Biomarkers & Prevention 19:2710-2714.
- Gillespie IA, O'Brien SJ, Frost JA, Adak GK, Horby P, et al. (2002) A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses.Emerg Infect Dis 8: 937-942.
- Wilson N, Baker M, Edwards R, Simmons G (2008) Case-case analysis of enteric diseases with routine surveillance data: Potential use and example results.EpidemiolPerspectInnov 5: 6.
- Aiken AM, Lane C, Adak GK (2010) Risk of Salmonella infection with exposure to reptiles in England, 2004-2007.Euro Surveill 15: 19581.
- (2009) FAO/WHO Risk assessment of Campylobacter spp. in broiler chickens: Interpretative Summary in Microbiological Risk Assessment Series. 2009: Geneva.
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, et al. (2011) Foodborne illness acquired in the United States--major pathogens.Emerg Infect Dis 17: 7-15.
- Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (1999) Campylobacter jejuni--an emerging foodborne pathogen.Emerg Infect Dis 5: 28-35.
- Eberhart-Phillips J, Walker N, Garrett N, Bell D, Sinclair D, et al. (1997) Campylobacteriosis in New Zealand: results of a case-control study.J Epidemiol Community Health 51: 686-691.
- Domingues AR, Piers SM, Halasa T, Hald T (2012) Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiology and Infectection 140: 970-81.
- Friedman CR, Hoekstra RM,Samuel M, Marcus R,Bender, et al. (2004) Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clinical Infectious Diseases 38:S285-S296.
- Kapperud G, Skjerve E, Bean NH,Ostroff SM, Lassen J (1992) Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. Journal of Clinical Microbiology 30:3117-21.
- Neimann J, Engberg J, Mølbak K, Wegener HC (2003) A case-control study of risk factors for sporadic campylobacter infections in Denmark.Epidemiol Infect 130: 353-366.
- Potter RC, Kaneene JB, Hall WN (2003) Risk factors for sporadic Campylobacter jejuni infections in rural michigan: a prospective case-control study.Am J Public Health 93: 2118-2123.
- Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, et al. (2004) Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999.Clin Infect Dis 38 Suppl 3: S165-174.
- Batz MB, Hoffmann S, Morris JG Jr (2012) Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation.J Food Prot 75: 1278-1291.
- Headrick ML, Korangy S, Bean NH, Angulo FJ, Altekruse SF, et al. (1998) The epidemiology of raw milk-associated foodborne disease outbreaks reported in the United States, 1973 through 1992.Am J Public Health 88: 1219-1221.
- Taylor EV, Herman KM, Ailes EC, Fitzgerald C, Yoder JS, et al. (2013) Common source outbreaks of Campylobacter infection in the USA, 1997-2008.Epidemiol Infect 141: 987-996.
- (2011) CDC. Incidence of laboratory-confirmed bacterial and parasitic infections, and postdiarrheal hemolytic uremic syndrome (HUS), by year and pathogen, Foodborne Diseases Active Surveillance Network (FoodNet), United States, 1996-2011. Foodborne Diseases Active Surveillance Network (FoodNet).
- Rates of Reported Cases of Notifiable Diseases by Year for Arizona, 2000-2010, per 100,000 population. 2010, Arizona Department of Health Services, Office of Infectious Diseases: Phoenix, AZ, USA.
- (2010) ADHS, Summary of Selected Reportable Diseases January- December.Arizona Department of Health Services Phoenix.
- Pogreba-Brown K, Harris RB, Stewart J, Anderson S, Erhart LM, et al. (2010) Outbreak investigation partnerships: utilizing a student response team in public health responses. Public Health Rep 125: 916-22.
- Centers for Disease Control (CDC) (1989) Proposed changes in format for presentation of notifiable disease report data.MMWR Morb Mortal Wkly Rep 38: 805-809.
- Stroup DF, Williamson GD, Herndon JL, Karon JM (1989) Detection of aberrations in the occurrence of notifiable diseases surveillance data.Stat Med 8: 323-329.
- Stroup DF, Wharton M, Kafadar K, Dean AG (1993) Evaluation of a method for detecting aberrations in public health surveillance data.Am J Epidemiol 137: 373-380.
- Teutsch S, Churchill RE (2000) Principles and Practice of Public Health Surveillance. (2ndedn) Oxford University Press: New York. 389.
- Schorr D, Schmid H, Rieder HL, Baumgartner A, Vorkauf H, et al. (1994) Risk factors for Campylobacter enteritis in Switzerland.ZentralblHygUmweltmed 196: 327-337.
- Tenkate TD, Stafford RJ (2001) Risk factors for campylobacter infection in infants and young children: a matched case-control study.Epidemiol Infect 127: 399-404.
- Adak GK, Cowden JM, Nicholas S, Evan HS (1995) The Public-Health Laboratory Service National Case-Control Study of Primary Indigenous Sporadic Cases of Campylobacter Infection. Epidemiology and Infection 115:15-22
- Rates of Reported Cases of Selected Notifiable Diseases, by 5-Year Age Group and Gender, per 100,000 population, Arizona, 2010, O.o.I.D. Arizona Department of Health Services, Editor. 2010: Phoenix, AZ, USA.
- McCarthy N, Giesecke J (1999) Case-case comparisons to study causation of common infectious diseases.Int J Epidemiol 28: 764-768.
- Neal KR, Slack RC (1997) Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study.Epidemiol Infect 119: 307-311.
- Wingstrand A, Neimann J, Engberg J, Nielsen EM, Gerner-Smidt P, et al. (2006) Fresh chicken as main risk factor for campylobacteriosis, Denmark.Emerg Infect Dis 12: 280-285.
- Kassenborg HD, Smith KE, Vugia DJ, Rabatsky-Her T, Bates MR, etal. (2004) Fluoroquinolone-resistant Campylobacter infections: eating poultry outside of the home and foreign travel are risk factors. ClinInfect Dis 38 3: S279-84.
- Kendall ME, Crim S, Fullerton K, Han PV, Cronquist AB, et al. (2012) Travel-associated enteric infections diagnosed after return to the United States, Foodborne Diseases Active Surveillance Network (FoodNet), 2004-2009. Clinical Infectious Diseases. 54 5:S480-7.
Citation: Pogreba-Brown K, Ernst K, Woodson L, Harris RB (2015) Determining Risk Factors of a Non-Point Source Outbreak of Campylobacter Cases Using Case-Case and Case-Control Studies. Epidemiology (sunnyvale) 5:203. DOI: 10.4172/2161-1165.1000203
Copyright: © 2015 Pogreba-Brown K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11941
- [From(publication date): 12-2015 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 11022
- PDF downloads: 919