Development and Validation of the Indirect Whole Cell ELISA for Diagnosis of Bovine Brucellosis
Received: 04-Feb-2021 / Accepted Date: 18-Feb-2021 / Published Date: 25-Feb-2021
Abstract
This study was carried out at autumn to diagnose Bovine Brucellosis using intact Brucella antigen (whole cell). Fifty blood samples were collected from Friesian cattle at Kuku Scheme (Khartoum North) from females above the age of puberty and haven’t been vaccinated against brucellosis before. The samples were tested with Rose Bengal to detect negative and positive sera then indirect ELISA tests using intact Brucella bacteria were carried out. Four known positive sera were used to choose optimum concentration of intact Brucella which will be used as an antigen. There was a great deal of variation amongst the tested sera in antibodies titer in which 30 samples were positive (40%) and 20 were negative with Rose Bengal and 4 were negative and 46 were positive (60%) with indirect ELISA. The result showed that the upper limit of the control sera is taken as the lower limit of positivity which called cut off point and took the mean and standard deviation and considered that negative sera were under 0.2 OD and the above of this were positive. In this study, Chi square test in SPSS program was used and showed the variation in detection of positive and negative samples between Rose Bengal and ELISA which used with intact bacteria with probability of >0.05 and this showed the significance of ELISA. The intact Brucella ELISA test showed high sensitivity and economic impact and detect all epitopes. This can be useful for diagnosis and epidemiological surveys, and may reduce the dependence on imported, expensive commercial materials.
Keywords: Brucellosis; Rose bengal; Intact whole Ag; ELISA
Introduction
Brucella are facultative intracellular coccobacilli belonging to the order Rhizobiales of the α-2 subgroup of Proteobacteria, Brucella abortus, B.melitensis and B.suis are highly pathogenic for humans. Infected tissues, cultures and potentially contaminated materials must be handled under appropriate containment conditions and precaution of biosafety level3 and the pathogen is classified by the CDC as a category (B) pathogen that has potential for development as a bio- weapon. Brucella spp. is considered as the most common laboratory- acquired pathogens [1]. Animal brucellosis poses barrier to trade in animals and animal products and could seriously impair socio- economic development, especially for livestock owners [2]. In cattle it was known by many names: infectious abortion which was also referred to as Bang’s disease, contagious abortion and slinking of the calf (1897) and undulant fever until the 1940s became brucellosis [3]. Also higher culling rate and longer inter-calving intervals [4].
Literature Review
Brucellosis
Brucellosis is a one of the highly contagious and most important zoonotic diseases in tropical area and a significant cause of reproductive losses in animals OIE (2009).
The first isolation of Brucella organism from animal was by Bang, et al., [5]. Generally, the disease is found in Africa, where it remains one of the most important zoonotic diseases [6]. In Sudan the disease was suspected as early as Simpson, et al., who reported 20 clinically diagnosed cases in the Blue Nile and Kassala provinces. Bennet, et al., isolated Brucella abortus for the first time from a Dairy herd in Khartoum. Hasseb was the first to report a case of human brucellosis. Hasseb, et al., and Dafalla, et al., stated that the disease was diagnosed in all provinces except Bahr El Gazal, in the southern Sudan up to 1955. Recent study found that Khartoum State only loses 7, 293,084.6 $US annually as a result of the disease [7-11].
In Sudan, nomadism renders detection illusory, even by surveys and the presence of wildlife reservoirs raises the problems.
In Sudan the disease is wide spread and cause huge losses in economics due to exportation cessation, reproduction losses, animal production losses, and misdiagnosis and treatment trials.
Laboratory diagnosis
Diagnosis and control of the disease in animals must be carried out on a herd basis. There may be a very long incubation period in some infected animals and individuals may remain serologically negative for a considerable period following infection. The identification of one or more infected animals is sufficient evidence that infection is present in the herd, and that other serologically negative animals may be incubating the disease and present a risk. Agglutination tests was first serological test for brucellosis. The use of serological tests is recommended as a mean of diagnosing the disease however many current serological tests have proved to be either too sensitive giving false positive result or too specific giving false negative results [12,13]. The isolation of Brucella is a definitive proof that the animal is infected, but not all infected animals give a positive culture and the methods and facilities are always time consuming.
Bacteriological methods: The isolation and identification of Brucella offers a definitive diagnosis of brucellosis and may be useful for epidemiological purposes and to monitor the progress of a vaccination programme. It should be noted that all infected materials present a serious hazard, and they must be handled with adequate precautions during collection, transport and processing and recommended precaution of biosafety level 3 [1].
Serological methods: The detection of specific antibody in serum or milk remains the most practical means for diagnosis of brucellosis [14]. The most efficient and cost-effective method is usually screening all samples using a cheap and rapid test which is sensitive enough to detect a high proportion of infected animals.
Serological results must be interpreted against the background of disease incidence, use of vaccination and the occurrence of false positive reactions due to infection with other organisms such as Yersinia enterocolotica may cross react with smooth Brucella spp. [15].
Rose Bengal Plate Test (RBPT): The RBPT is one of a group of tests known as the buffered Brucella antigen tests which rely on the principle that the ability of IgM antibodies to bind to antigen is markedly reduced at a low pH. The RBT and other tests such as the buffered plate agglutination tests and the card test play a major role in the serological diagnosis of brucellosis worldwide. The RBPT is a simple spot agglutination test where drops of stained antigen and serum are mixed on a plate and any resulting agglutination signifies a positive reaction. The test is good screening test but may be over sensitive for diagnosis in individual animals, particularly vaccinated ones. The OIE considers these tests “prescribed tests for trade (OIE, 2009).
Serum Agglutination Test (SAT): The SAT has been used extensively for brucellosis diagnosis and, although simple and cheap to perform, its lack of sensitivity and specificity mean that it should only be used in the absence of alternative techniques [16]. The SAT, RB, and BAT are commonly used as screening tests for the diagnosis of bovine brucellosis. However, the OIE and the EU have recently decided not to recommend use of the SAT because they consider it inferior to the other standard tests [17].
Complement Fixation Test (CFT): The complement fixation test is technically challenging because a large number of reagents must be titrated daily and a large number of controls of all the reagents is required.
According to some literature this test is not highly sensitive but shows an excellent specificity because the test is difficult to standardize, it is progressively being replaced by ELISAs [18,19].
This test is a “prescribed test for trade” by the OIE [20]. Other problems include the subjectivity of the interpretation of results, occasional direct activation of complement by serum (competent activity) and the inability of the test for use with haemolysed serum samples. However, the test has also some of the disadvantages presented for the diagnosis of bovine brucellosis such as complexity, necessity for serum heat inactivation, and competent activity of some sera, difficulty in performing with hemolized sera and the prozone phenomena [21].
ELISA tests: The ELISA tests offer excellent sensitivity and specificity whilst being robust, fairly simple to perform with a minimum of equipment and readily available from a number of commercial sources in kit form. They are more suitable than the CFT for use in smaller laboratories and ELISA technology is now used for diagnosis of a wide range of animal and human diseases.
Although in principle ELISAs can be used for the tests of serum from all species of animal and man, results may vary between laboratories depending on the exact methodology used. For screening, the test is generally carried out at a single dilution.
It should be noted, however, that although the ELISAs are more specific than the RBT, sometimes they do not detect infected animals which are RBT positive. The ELISA was first developed by Carlsson, et al., for the diagnosis of human brucellosis .since then, a large number of variations have been described, however ,the most common format uses SLPS antigen coated passively onto a polystyrene matrix [22].
One disadvantage of the ELISA is its inability to differentiate vaccinal antibody resulting from B.abortus S19 or B.melitensis Rev1 vaccination from antibody induced by pathogenic strains [23].
Serum antibodies based tests was found to be better than those using milk, for example, Milk ring test (MRT) is a simple and effective method, but can only be used with cow’s milk because it is not sensitive enough to detect Brucella in goats [24]. This test is not considered
Objectives
General objective: To diagnose bovine brucellosis using serological method.
Specific objective: To use specific sensitive and economical Brucella intact antigen ELISA.
Materials and Methods
Samples collection
Approximately 7-10 ml of blood was down from Jugular vein of fifty aborted animals from Khartoum state (Hilt-kuku) farms using plain vacutainer tubes and needles.
Samples were transported to Microbiology Laboratory in the Faculty of Veterinary Medicine, University of Khartoum and kept overnight at 4°C to allow the separation of serum then centrifuged at 3000 rpm for 10 minutes. The collected sera were coded and kept at -20°C up to the time of the test.
Serologial examination of the samples: The collected sera were screened for the presence of antibodies against Brucella antigens Alton, et al., by using the Rose Bengal plate test “RBPT” and further using an indirect enzyme linked immunosorbent assay (iELISA) [25].
Transportation of samples
The serum samples were labelled and placed in container with ice then quickly transported to Microbiology Laboratory in Ministry of science and technology central laboratory for further processing.
Preparation of RBPT antigen
Rose bengal test
Antigen production: Antigen for the RBT was provided by Central Veterinary Research Laboratory (CVRL) (Soba) department of Brucella according to OIE. using killed B. abortus S99 or S1119-3 cells. Then the antigen was stored as recommended by the manufacturer without freezing.
Examination of the serum samples by RBPT
Test procedure: The serum samples and antigen were brought to room temperature (22 ± 4°C) and only sufficient antigen for the day’s tests was removed from the refrigerator. 25-30 μl of each serum sample were placed on a white tile, enamel or plastic plate, or in a WHO haemagglutination plate.
The antigen bottle was shake well, but gently, and placed an equal volume of antigen was placed near each serum spot. Immediately after the last drop of antigen has been added to the plate, the serum and antigen were mixed thoroughly (using a clean glass or plastic rod for each test) to produce a circular or oval zone approximately 2 cm in diameter.
The mixture was agitated gently for 4 minutes at ambient temperature on a rocker or three-directional agitator (if the reaction zone is oval or round, respectively).
Reading for agglutination was immediately after the 4-minute period was completed. Any visible reaction is considered to be positive. A control serum that gives a minimum positive reaction was tested before each day’s tests are began to verify the sensitivity of test conditions.
Plate coating procedure (preliminary coating) and confirmatory examination
Trial (1): Serial tenfold dilution of Intact Brucella Antigen, (4 x ) (culture of Br. abortus strain-19) obtained from Central Veterinary Research Laboratory (Soba) CVRL, serial dilutions were made (,,,, until. Then 100 μl from each dilution tube was took onto ELISA plate and put overnight at 37°c. After that culture was made from ELISA plate to 6 Petri dishes and divided into two parts 1\2 -3\4 … and 6th plate with N.S\ was empty.
Trial (2): The plate was coated with the same Intact Brucella antigen (4 x) and Sealed then left overnight at 4°C Then washed and from discard smear was made and stained with Gram stain, Few or non-Gram negative cocobacilli were seen in the smear, which indicate coating of the plate with Brucella bacteria.
Standardization of the ELISA coating conditions
Nine ml of Sodium bicarbonate (coating buffer) were added to 4 tubes then 1 ml of the culture (4 x) were added to make serial dilution. Then from each dilution 100 μl were dispensed in 2 rows.
One microtiter plate was coated with serial Brucella concentration 4 x, 4 x, 4 x, 4 xin duplicate, The ELISA plate was sealed and incubated at 4°C overnight.
°Washing procedure: The plate was washed 3 times with (PBS +Tween 20 “0.05%”) by automatic washing machine. Plate blocking was made with PBSTM coating buffer 200 μl (PBS+Skimmed milk “1%” +Tween 20 “0.05%“). After blocking the plate was incubated for 1 hour at 37°C. Then the plate was washed 3 times.
Adding the known positive and negative sera (kits): The wells in column 1 from A to H were left empty, this would be the blank. column 2 wells from A to H were filled with 180 μl of PBSTM buffer and the rest of the plate (column 3-11) were filled with 100 μl of the same solution , then 20 μl of –ve and +ve control sera were applied in duplicate with different serial dilution to choose optimum intact antigen concentration .
Using a Finn pipette, 100 μl were set and slowly mixed in column 2 well of row A thoroughly (10x), without air bubbles. 100 μl of the solution were transfer to column 3 well and mixed again 10x and 100 μl were transferred to column 3 well. This procedure was repeated up to well 11 and after mixing this was discard 100 μl. well 12 was left empty as shown in Table 1.
| Well column | Dilution |
|---|---|
| 2 | 1/50 |
| 3 | 1/100 |
| 4 | 1/200 |
| 5 | 1/400 |
| 6 | 1/800 |
| 7 | 1/1600 |
| 8 | 1/3200 |
| 9 | 1/6400 |
| 10 | 1/12800 |
| 11 | 1/25600 |
Table 1: Representation of well columns and its serial dilutions.
The serial dilutions were repeated form rows B to H. The plate was incubated for 1 hour at 37°C, and then washed 3 times. 100 μl of conjugate (anti-bovine IgG with horseradish peroxidase) was added (1:5000) to each well. The plate was incubated for one hour at 37ºCc, and then washed for 3 times. 100 μl of TMB substrate were added. The plate was incubated for 20 minutes at dark place, finally 50 μl of stop solution 30% H2SO4 were added. The optical densities were read at 450 nm. Conventional ELISA was carried and the optimum antigen concentration was found to be (4 x).
Testing the samples: The antigen was made by adding 1ml of Intact Brucella (4 x) (culture of Br. abortus strain-19) to 9 ml of Na bicarbonate (coating buffer) .The plate was coated with antigen and Sealed then left overnight at 4°C.
Washing procedure: The plate was washed 3 times with washing buffer .Then plate was blocked with PBSTM buffer 200 μl (coating buffer). The plate was incubated for 1 hour at 37°C. Then plate was washed x3.
Testing sera: The well (1) of rows A to H were left empty, this would be the blank. The well (2) of rows A to H were filled with 245 μl of PBSTM coating buffer and the rest of the plate (3-11) were filled with 100 μl of PBSTM coating buffer. Then 5 μl of –ve serum in row (A) were added and 5 μl of +ve sera to other row (B). Using a Finn pipette 100 μl were set and slowly mixed well 2 of row A thoroughly (10x), without air bubbles. 100 μl of the solution were transfer to well 3 and mixed again 10x and 100 μl were transferred to well 4. This procedure was repeated up to well 11 and after mixing this was discard 100 μl. well 12 was left empty.
The dilutions were repeated for rows B to H and the plate was incubated for 1 hour at 37°c. The plate was washed x3. Then 100 μl of conjugate (anti-bovine IgG with horseradish peroxidase) was added and the plate was incubated for 1 hour at 37°C. Then plate was washed 3 times. 100 μl of TMB substrate were added to each well. The plate was incubated for 20 minutes at RT in a dark place. 50 μl of stop solution were added. The optical densities were read at 450 nm.
Statistical analysis
Association between intact ELISA and Rose Bengal test was analyzed using Chi-square test. The sequence optical densities of intact ELISA were analyzed using IBM SPSS program for windows V. 20 (Armonk NY.IBM corp).
The null hypothesis: H0 Result of ELISA is independent of the result of Rose Bengal. P>0.05 suggest that null hypothesis should not be rejected we don’t reject that the results of the two tests are independent.
Results
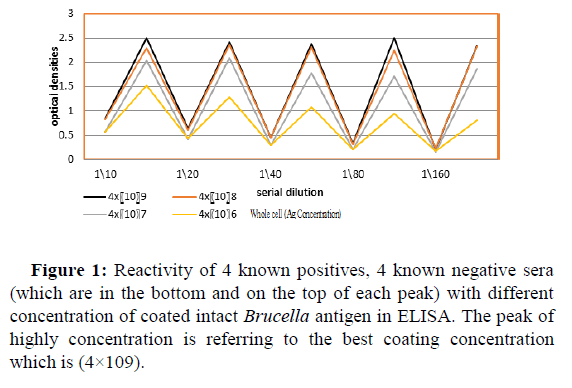
Table 3 along with Figure 1 represent the reactivity of the average of optical densities of 4 known positives (2 positive commercial kit control and 2 of strongly Rose Bengal positives (++++) “obtained from known brucellosis infected cows” were taken and titrated against four different concentration of coat intact brucella antigen, the four negative produced low reading . It can be seen that there was little difference in the ability of binding with 4 x and 4 x per well ,the concentration of antigen that gave higher titer of the average of 4 positive with consistency was taken as an optimum antigen concentration and found to be 4 x bacteria per well as shown in Table 2.
| Well column | Dilution |
|---|---|
| 2 | 1/10 |
| 3 | 1/20 |
| 4 | 1/40 |
| 5 | 1/80 |
| 6 | 1/160 |
| 7 | 1/320 |
| 8 | 1/640 |
| 9 | 1/1280 |
| 10 | 1/2560 |
| 11 | 1/5120 |
Table 2: Representation of well columns and its dilutions.
| Serum dilution | Antigen concentration | 1\10 | 1\20 | 1\40 | 1\80 | 1\160 |
|---|---|---|---|---|---|---|
| A | 4 × 109 | 0.842 | 0.638 | 0.459 | 0.327 | 0.211 |
| B | 4 × 109 | 2.504 | 2.427 | 2.394 | 2.516 | 2.352 |
| C | 4 × 108 | 0.829 | 0.602 | 0.447 | 0.31 | 0.236 |
| D | 4 × 108 | 2.296 | 2.371 | 2.329 | 2.257 | 2.336 |
| E | 4 × 107 | 0.579 | 0.424 | 0.292 | 0.208 | 0.167 |
| F | 4 × 107 | 2.043 | 2.09 | 1.786 | 1.714 | 1.887 |
| G | 4 × 106 | 0.571 | 0.425 | 0.288 | 0.208 | 0.169 |
| H | 4 × 106 | 1.525 | 1.299 | 1.08 | 0.942 | 0.811 |
Rows : B,D,F, and H were the reactivity of the 4 positives
Rows : A,C,E and G showed the optical densities of the 4 negatives
Table 3: Reactivity of known positive and control sera , negative and control sera among different concentration of coated intact Brucella antigen in ELISA.
Detection of positive and negative sera
Table 4 represent the reactivity of the 50 sample sera in Rose Bengal and showed the ability of intact Brucella ELISA to distinguish between positive and negative samples. Thirty sera were found to be positive (60%) and showed variation in their reactivity from + to +++ + .The remaining 20 sera were found to be negative (40%) [28, 30-37]. Then the samples were tested with intact Brucella ELISA (whole cell) where 46 sera were found to be positives and only 4 sera were negatives.
| Test | Positive | Negative |
|---|---|---|
| Rose Bengal | 30 (60%) | 20 (40%) |
| ELISA | 46 (92%) | 4 (8%) |
Table 4: Represent the reactivity of the 50 sample sera in Rose Bengal and intact Brucella ELISA.
In Table 5 the results of intact Brucella ELISA was compared with Rose Bengal test reactivity of the 50 sample sera using chi test Chi square test was performed to calculate P value and it was <0.05 which considered statistically significant.
π2=0.181, P>0.05
| ELISA | ||||
|---|---|---|---|---|
| +ve | -ve | Total | ||
| Rose Bengal | +ve | 28 | 2 | 30 |
| 60.90% | 50% | 60% | ||
| -ve | 18 | 2 | 20 | |
| 29.10% | 50% | 40% | ||
| Total | 46 | 4 | 50 | |
| 100% | 100% | 100% | ||
Table 5: Comparison between intact Brucella ELISA and rose Bengal test reacyivity of 50sample sera using chi test.
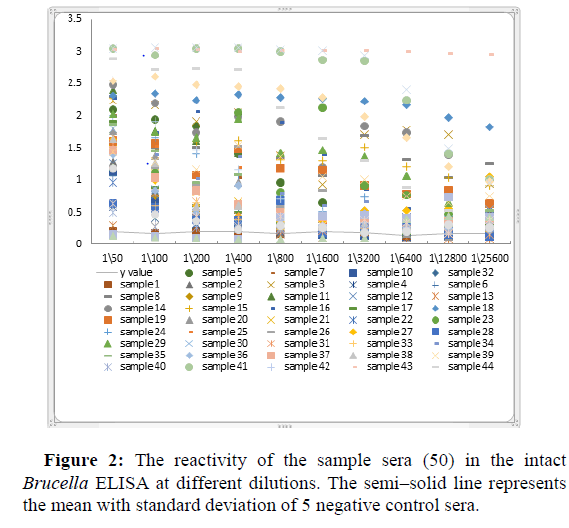
Figure 2 showed the reactivity of the sample sera (50 sera) in intact Brucella ELISA at different dilutions. the semi-solid line represent the mean with standard deviation of 5 negative control sera which were 2 commercial kit negative control and 3 strongly Rose Bengal negatives when the upper limit of the negative control sera is taken as the lower limit of positivity [26].
Discussion
Most confirmatory tests for bovine brucellosis are more complicated and more expensive to perform. In this study, developed indirect ELISA using intact antigen for the first time, can reduce the cost of import ELISA kits. In addition to increase the sensitivity of the test; while whole Brucella obtained from vitro culture can provide a relatively stable source of a wide range of antigenic determinants, perhaps including some which are not available in the soluble antigen preparation [27, 38-45].
In this study, reactivity of 50 serum samples collected from lactating cows in Shriq Al-neel locality (Kuku scheme), Khartoum State were tested by Rose Bengal Plate Test (RBPT); and the seroprevalence was 60%. It was higher than that reported in the previous study about 4 years ago, where the overall seroprevalence of bovine brucellosis within the milking cows was 32% and 38.8% using RBPT and sELISA, respectively. It was also higher than the seroprevalence of 27% by RBPT and 24.4% by sELISA reported by Salman, et al.,[27].
The prevalence was also higher than that reported in the previous study among all localities of Khartoum State that estimate the average rate for the State was found to be 27.5% and the weighted average was 25.1% prevalence of Brucellosis using Rose Bengal test; suggesting that the prevalence of the disease is growing and the indirect ELISA and RBT of this study is growing. In 2009 the prevalence in Kuku scheme was 30.1% [28, 46-57].
However using modified ELISA the detecting levels of Brucella was higher (46) than the detecting level using Rose Bengal. The obtained result also cope with the truth that although the ELISAs are more specific than the RBT, sometimes they do not detect infected animals which are RBPT positive [58-61].
Rose Bengal false negative may be due to prozoning or because the antibody produced is only IgG isotype in acute infection cases, bearing in mind that IgG is less agglutinator than IgM. ELISA used to detect more positive sera since it can detect all isotypes.
Thus by intact Brucella ELISA we can show the sensitivity of the ELISA to detect the positives and negatives samples. The cut-off point of intact Brucella ELISA was determined using 5 negative controls and sera and it was 0.2 OD which is the cutoff point according to the kit from veterinary research laboratory; that means those samples results higher than 0.2 OD value will be considered as positives [62-64].
We have thus demonstrated that the use of intact Brucella in an ELISA to detect anti Brucella antibodies would be practical method and useful in epidemiological studies or for early diagnosis and we recommend to introduce and validate this method in massive bovine herds diagnosis programs in the field to establish its limits.
This study showed the ability of ELISA test using intact Brucella abortus to detect the titer of Antibodies on the serum of animals suspected with Brucellosis. We agree with Chachra, et al., that in order to get a fool proof diagnosis of Brucellosis, a combination of RBPT and Dot ELISA should be used, especially in case of samples found negative by either RBPT or STAT used alone or in combination [29].
Conclusion
This is the first study in which intact Brucella (whole cell) antigen to be used in ELISA. Intact ELISA technique is sensitive and specific which expected to detect only antibody of B.abortus so cross reaction with other bacteria can be eliminated.
Recommendations
Sera from cattle infected with related microorganism (E.coli,Yersinia…) should be tested in this type of ELISA to investigate the cross reaction. Local production of antibovine conjugate is needed for more minimizing ELISA cost and application. This type of ELISA is recommended to be used for immunodiagnosis and epidemiological survey. Study the stability of intact Brucella antigen in the plate upon storage on -20°C, 4°C and at room temperature for long time and under different conditions.
References
- Fiori P, Mastrandrea S, Rappelli P, Cappuccinelli P (2005) Brucella abortus infection acquired in microbiology laboratories. J Clin Microbial 38(5): 2005-2006.
- Corbel MJ (2006) Brucellosis in Humans and Animals. Pp: 12-79.
- Craig CF (1903) Malta Fever: Its occurrence in the United States Army, with a review of the literature. Amer J Med Sci 125(1): 105-115.
- Blood DC, Radostits M, Henderson JA (1983) Veterinary Medicine. Pp: 677-696.
- Bang B (1897) The etiology of epizootic abortion. J Comp Path Therap 10: 125-149.
- Gameel SE, Mohammed SO, Mustafa AA, Azwai SM (1993) Prevalence of camel brucellosis in Libya. Trop Anim Health Prod 25(2): 91-93.
- Simpson RJS (1908) Malta fever from the Blue Nile. Journal of Roy Army Med Corps 11: 593-596.
- Bennett SGJ (1943) Annual Reported of the Sudan Veterinary Service. Pp:29-32.
- Hasseb MA (1950) Undulant fever in the Sudan. Sud J Med 53(12): 241-244.
- Dafalla EN, Khan AQ (1962) The occurrence, epidemiology and control of animal brucellosis in the Sudan. Bull Epizootic Dis Afr 6: 243-247.
- Angara TE, Ismail AAA, Ibrahim AM, Osman SZ (2016) Assessment of the economic losses due to bovine brucellosis in khartoum state, Sudan. Int J Tech Res Appl 4(2): 85-90.
- Morgan WJ, Mackinnon DJ (1979) Brucellosis. In: Fertility in Domestic Animals. Laing JA (Ed) ELBS, London: BailliereTindall Pp: 98-171.
- Farina R (1985) Current serological methods in B. melitensis diagnosis. Brucella melitensis 32: 139-146.
- Whicher K (1981) Brucella In: Principle of immunological diagnosis in medicine. Milgrom F, Abeyounis C, Kano K (Eds), Philadelphia: Lea and Febiger, P: 97-101.
- Corbel MJ, Gill KPW, Thomas EL, Hendry D (1983) Methods for the identification of Brucella. Ministry of Agriculture, Fisheries and Food, London.
- Baum M, Zamir O, Bergman-Rios R, Katz E, Beider Z, et al. (1995) Comparative evaluation of microagglutination test and serum agglutination test as supplementary diagnostic methods for brucellosis. J Clin Microbiol 33(8): 2166-2170.
- Greiner M, Verloo D, Massis FD (2009) Meta-analytical equivalence studies on diagnostic tests for bovine brucellosis allowing assessment of a test against a group of comparative tests. Prev Vet Med 92(4): 373-381.
- Emmerzaal A, Wit JJD, Dijkstra T, Bakker D, Zijderveld FGV (2002) The dutch brucella abortus monitoring programme for cattle: The impact of false-positive serological reactions and comparison of serological tests. Vet Q 24(1): 40-46.
- McGiven JA,Tucker JD, Perrett LL, Stack JA, Brew SD, et al. (2003) Validation of FPA and cELISA for the detection of antibodies to brucella abortus in cattle sera and comparison to SAT CFT, and iELISA. J immunol Met 278(1-2): 171-178.
- OIE Terrestrial Manual (2009) Bovine Brucellosis: Brucella abortus. Institute for international cooperation in animal biology and OIE collaborating center, Paris, France.
- Blasco JM (1990) In: Nielsen KH, Duncan JR, Eds. Brucellaovis. Boca Raton: CRC Press. Pp: 351-78.
- Carlsson HE, Hurvell B, Lindberg AA (1976) Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitic. Acta Pathol Microbiol Scand 84(3): 168-176.
- Nielsen K, Gall D (1994) Advances in the diagnosis of bovine brucellosis use of enzyme immunoassay. Gen Eng Biotech 14: 25-39.
- Shimi A, Tabatabayi AH (1981) Pathological, bacteriological and serological responses of ewes experimentally infected with Brucella melitensis. Bull Off Int Epizoot 93(11-12): 1411 -1422.
- Alton GG, Jones LM, Angus RD, Verger JM (1988) Techniques for the brucellosis laboratory. Paris: INRA (Institut National de la Recherche Agronomique).
- Voller A, Bidwell DE, Bartlett A (1980) Enzyme Linked immunosorbent assay on manual of clinical immunology. Rose N and Friedman H (Eds), Washington, Am soc of Micro Pp359-371.
- Salman A, Nasri HAE (2012) Evaluation of four serological tests to detect prevalence of bovine brucellosis in Khartoum State. J Cell Anim Biol 6(15): 140-143.
- Angara TEE, Ismail AAA, Agab H, Saeed NM (2009) Seroprevalence of bovine brucellosis in kuku dairy scheme Sudan. J Vet Sci Animal Husb 48(2): 27-35.
- Chachra D, Saxena HM, Kaur G, Chandra M (2009) Comparative efficacy of Rose Bengal plate test, standard tube agglutination test and Dot ELISA in immunological detection of antibodies to Brucella abortus in sera. J Bacteriol Res 1(3): 030-033.
- Abdalla A, Hamid ME (2012) Comparison of conventional and non-conventional techniques for the diagnosis of bovine brucellosis in Sudan. Trop Anim Health Prod 44:1151-1155.
- Adil S, Elniema M, Medard A, Lmyaa H (2014) Application of Different Serological Tests for the Detection of the Prevalence of Bovine Brucellosis in Lactating Cows in Khartoum State, Sudan. J App Indust Sci 2(5): 213-218.
- Agab H, Abbas B, Ahmed HEJ, Maoun IE (1995) First report on the isolation of Br.abortus biovar 3 from camel (camelus dromedary) in Sudan. Rev Elev Med Vet Pays Trop 47(4): 361-363.
- Baum M, Zamir O, Bergman-Rios R, Katz E, Beider Z, et al. (1995) Comparative evaluation of microagglutination test and serum agglutination test as supplementary diagnostic methods for brucellosis. J Clin Microbiol 33(8): 2166-2170.
- Bruce D (1887) Note on the discovery of a microorganism in Malta fever. Practicioner 39: 161-170.
- Greiner M, Verloo D, Massis FD (2009) Meta-analytical equivalence studies on diagnostic tests for bovine brucellosis allowing assessment of a test against a group of comparative tests. Prev Vet Med 92(4): 373-381.
- Young EJ, Corbel MJ (1989) Laboratory techniques in the diagnosis of human brucellosis. In: Brucellosis: Clinical and Laboratory Aspects. Taylor and Francis Group, CRC Press, Boca Raton, USA, Pp. 73-83.
- ElAnsary EH, Mohammed BA, Hamad AR, Karan AG (2001) Brucellosis in eastern Sudan. Vet Rec 72: 1230-1236.
- Emmerzaal A, Wit JJD, Dijkstra T, Bakker D, Zijderveld FGV (2002) The dutch brucella abortus monitoring programme for cattle: The impact of false-positive serological reactions and comparison of serological tests. Vet Q 24(1): 40-46.
- Fadul ED ( 2006) A study on brucellosis in Kosti area, White Nile state, Sudan.
- McGiven JA,Tucker JD, Perrett LL, Stack JA, Brew SD, et al. (2003) Validation of FPA and cELISA for the detection of antibodies to brucella abortus in cattle sera and comparison to SAT CFT, and iELISA. J immunol Met 278(1-2): 171-178.
- Carlsson HE, Hurvell B, Lindberg AA (1976) Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitic. Acta Pathol Microbiol Scand 84(3): 168-176.
- Salman A, Nasri HAE (2012) Evaluation of four serological tests to detect prevalence of bovine brucellosis in Khartoum State. J Cell Anim Biol 6(15): 140-143.
- Khalid HA (2006) Serodiagnosis of brucellosis and the differentiation between vaccinated animals from infected one.
- Angara TEE, Ismail AAA, Agab H, Saeed NM (2009) Seroprevalence of bovine brucellosis in kuku dairy scheme Sudan. J Vet Sci Animal Husb 48(2): 27-35.
- Chachra D, Saxena HM, Kaur G, Chandra M (2009) Comparative efficacy of Rose Bengal plate test, standard tube agglutination test and Dot ELISA in immunological detection of antibodies to Brucella abortus in sera. J Bacteriol Res 1(3): 030-033.
- MacKinnon D (1963) The complement fixation test in brucellosis. Bull OIE 60: 383-400.
- Abdalla A, Hamid ME (2012) Comparison of conventional and non-conventional techniques for the diagnosis of bovine brucellosis in Sudan. Trop Anim Health Prod 44:1151-1155.
- Mohammed (2004) Studies on animals brucellosis in Red sea state.
- Musa MT (2004) Epidemiology of brucellosis in animals and man. The national training workshop. In: Surveillance, diagnosis and control of brucellosis. Khartoum, the Sudan.
- Musa MT (1995) Brucellosis in Darfur .The magnitude of the problem and problem and methods of control. Thesis, University of Khartoum pages 5-65.
- Angara TEE, Shuaib YA (2015) Evolution of bovine brucellosis over 11-years period in the Sudan. Inter J Vet Sci 4(1): 33-38.
- Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, et al. (2010) Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int J Infect Dis 14: 469-478.
- Euzeby JP (1997) List of Bacterial names with standing in Nomenclature: A folder available on the Internet. Int J Syst Bacteriol 47(2): 590-592.
- Omer RI (2000) Studies on Brucellosis in Camels and cattle in Darfour states, University of Khartoum.
- Farrell ID (1974) The development of new selective medium for the isolation of Brucella abortus from contaminated sources. Res Vet Sci 16(3): 280-286.
- Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A (2007) Brucella ceti sp. nov. and brucella pinnipedialis sp. nov. for brucella strains with cetaceans and seals as their preferred hosts. Int J Syst Evol microbiol 57: 2688-2693.
- Hosie BD, Al-Bakre OM, Futter RJ (1985) Survey of brucellosis In goat and sheep in Yemen Arab Republic: Comparison of tests for Brucella melitensis infection in sheep. Trop Anim Health Prod 17(2): 93-99.
- Kuzdas CD, Morse EV (1953) A selective medium for the isolation of brucellae from contaminated materials. J Bacteriol 66(4): 502-504.
- Langoni H, Ichihara SM, Silva AVD, Pardo R, Tonin F, et al. (2000) Isolation of Brucella species from milk of brucellosis positive cows in São Paulo and Minas Gerais states. J Vet Res Anim Sci 3796: 1413-9596.
- Mantur BG, Amarnath SK, Shinde RS (2007) Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol 25(3): 188-202.
- Shawgi DG (2009) Brucellosis in Yemen and Sudan seroprevalence, causative agent and evaluation of different methods of diagnosis, University of Khartoum.
- Shigidi MA, Razig SA (1971) Isolation of Brucella abortus from Aknee hygroma in abull Sudan. J Vet Sci Anim Husb 14: 33-35.
- Nielsen K (2002) diagnosis of brucellosis by serology. Vet Microbiol 90(1-4): 447-459.
- World organisation for animal health OIE (2009) Bovine brucellosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. paris OIE.
Citation: Khogali A, Mohamed AEO, Hussien M, Elrufai H (2021) Development and Validation of the Indirect Whole Cell ELISA for Diagnosis of Bovine Brucellosis . ECR 11:S3:002.
Copyright: © 2021 Khogali A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1419
- [From(publication date): 0-2021 - May 17, 2025]
- Breakdown by view type
- HTML page views: 723
- PDF downloads: 696