Distribution Pattern of Babesia and Theileria Species in Sheep in Qena Province, Upper Egypt
Received: 24-Apr-2017 / Accepted Date: 09-May-2017 / Published Date: 15-May-2017
Abstract
Sheep is often infected by various protozoan parasites that induce considerable losses of livestock resources. Scarce data have been reported to identify major parasites in sheep in Qena, Upper Egypt. Accordingly, the study aimed to estimate the prevalence of blood parasites infecting sheep in Qena. A field survey was conducted during the period between February 2014 and January 2015. Blood samples were collected from sheep and Giemsa-stained smears were prepared to identify blood parasites under microscopy. Out of 130 examined samples, 15 (11.53%) were infected with Babesia motasi (B. motasi); 13 (10.0%) infected with Babesia ovis (B. ovis) and 10 (7.69%) infected with Theileria ovis (Th. ovis) (single infection). Six (4.61%) hosts had mixed infections with B. motasi and Th. ovis; 3 (2.3%) with B. motasi and B. Ovis; 2 (1.53%) with B. Motasi, B. Ovis and Th. ovis and one (0.76%) was infected with Toxoplasma sp. and Th. Ovis. The total prevalence of infections with B. motasi, B. Ovis, Th. ovis and Toxoplasma sp. Were 20.0, 13.83, 14.6 and 0.76%, respectively. This study is the first report on the prevalence of blood parasites infecting sheep in Qena province. The infection with B. motasi was the most prevalent. Further investigations with more sensitive and applicable methods such as molecular methods to identify more blood protozoan parasites in sheep and other ruminants are needed.
Keywords: Babesia motasi; Babesia ovis; Theileria ovis; Toxoplasma gondii; Sheep; Egypt
66214Introduction
Sheep is one of the most important domesticated animals in Egypt. The total population is exceeded 4 million heads and are raised mainly for meat production [1,2]. Their productivity is thought to be greatly reduced by haemoparasitic diseases that are the most destructive diseases affecting animal health and therefore obstacle the successful production.
Babesiosis and theileriosis are the most blood parasitic disease belonging to the complex of several tick-transmitted diseases with different causative agents. Indeed, such parasites are responsible for the high economic losses in sheep industry worldwide [3].
In sheep, toxoplasmosis is a common cause of infectious abortion resulting in significant economic losses. Therefore, the knowledge about the prevalence of such diseases in sheep is of interest to implement future strategies on public health programs. The present study was performed for the purpose of surveying blood parasite in sheep in Qena province, Upper Egypt, as a one of the key steps for control and health management of sheep through using successful treatment for certain parasites [4].
Materials and Methods
Blood samples were taken from the jugular vein of 130 (37males and 93 females) sheep in Qena, Upper Egypt and were untreated before with antiprotozoal drugs. Thin blood films were made on dry and clean slides, air dried in a clean place, and then they were fixed with absolute methyl alcohol for 1-3 minutes, stained with Giemsa [5]. Stain for 20 minutes and then examined under light microscopy at x100 oil immersion for protozoan blood parasites. Photographs were taken by using a digital photograph unit (Leica Microsystems, CH-9435 Heebrugg, Ec3, Singapore).
Results
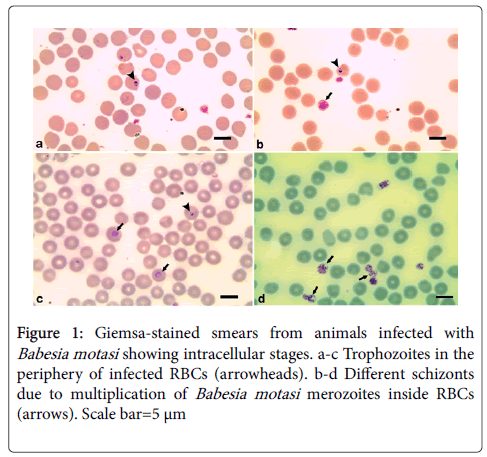
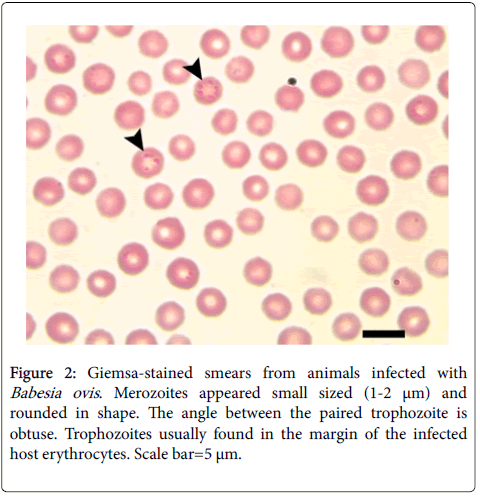
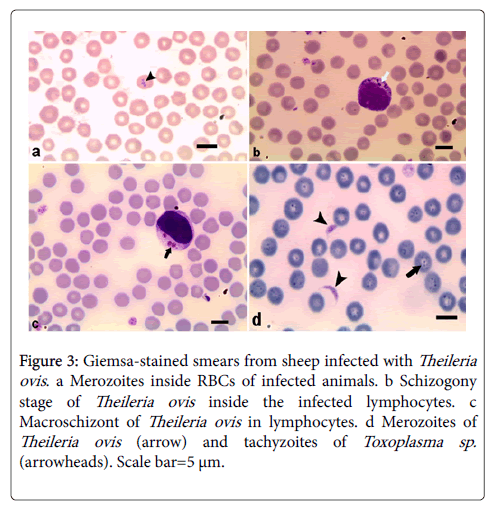
Examination of 130 blood samples revealed that 50 (38.46%) animals were infected with one or more blood parasites: 15 (11.53%) were infected with merozoites of Babesia motasi in their red blood cells. The trophozoite is single, pyriform-shaped and measured 2.2-4 x 2 μm (Figure 1). Thirteen (10.0%) animals were infected with Babesia ovis (Figure 2). Merozoites appeared single, small sized (1-2 μm) and rounded in shape they are single or paired where the angle between the paired trophozoite are obtuse. Trophozoites usually found in the margin of the infected host erythrocytes. Meanwhile, 10 (7.69%) sheep were infected with Theileria ovis (Figure 3). Merozoites appeared within infected erythrocytes in different fission phases (arrows) (Figure 3a). The schizogony stage, Kock’s blue bodies, appeared in the infected lymphocytes (Figure 3b). Macroschizonts contained chromatin granules (0.5-1.5 μm in diameter) (Figure 3c). They are the precursors of micomerons. Moreover, 6 (4.61%) have a mixed infection with Babesia motasi and Theileria ovis; 3 (2.3%) were infected with both Babesia motasi and Babesia ovis; 2 (1.53%) have a mixed infection with Babesia motasi , Babesia ovis and Theileria ovi. Furthermore, one (0.76%) was infected with Toxoplasma sp. (Figure 3d). It is worthy to mention that the total infection rates were 30.0 (n=39), 14.6 (n=19) and 0.76% (n=one) for Babesia spp., Theileria sp. and Toxoplasma sp., respectively.
Figure 3: Giemsa-stained smears from sheep infected with Theileria ovis. a Merozoites inside RBCs of infected animals. b Schizogony stage of Theileria ovis inside the infected lymphocytes. c Macroschizont of Theileria ovis in lymphocytes. d Merozoites of Theileria ovis (arrow) and tachyzoites of Toxoplasma sp. (arrowheads). Scale bar=5 μm.
Regarding sex, it has been found that the infection rates were as follows: single infection rates of females were 10.75% (n=10), 8.6% (n=8) and 8.6% (n=8) with Babesia motasi, Babesia ovis and Theileria ovis, respectively. In males, infection rates were 13.5% (n=5), 13.5% (n=5) and 25.4% (n=2), respectively. Moreover, in females, mixed infection with Babesia motasi and Theileria ovis was 5.37% (n=5), Babesia motasi and Babesia ovis; 1.07% (n=one), Babesia motasi, Babesia ovis and Theileria ovis 2.15% (n=2) and Theileria ovis and Toxoplasma sp. 1.07% (n=one), respectively. Mixed infections in males with Babesia motasi and Theileria ovis was 2.7% (n=one), Babesia motasi and Babesia ovis 5.4% (n=2).There were no mixed infection with Babesia motasi, Babesia ovis and Theileria ovis or with Theileria ovis and Toxoplasma sp. in male sheep.
Discussion
Blood parasites are one of the major bands of sheep productivity in Egypt. In the current study, the overall of high prevalence of blood protozoan parasite in sheep was (38.46%) which indicated a very intense transmission within the sheep population. These results may reflect the actual pathway of blood protozoan parasites prevalence in the whole Upper Egypt region because of the close similarity of rearing systems. In the present investigation; the achieved results (38.46%) were nearly similar to the results obtained by Reda et al. in Egypt (39%) [6]. But on the contrary, Kandasamy et al. recorded a higher infection rate (87.0%) through using blood smear method [7]. Oppositely, Adamu et al. and Velusamy et al. revealed a lower prevalence rates (11.0% and 21.0%, respectively) [8,9]. The Variation in the prevalence rates of infection might be related to some factors as the climatic condition of the area of the study and lack of hygienic measure that enhances the life cycle of ticks and gives a higher chance to tick to infest animals as potential sources of infection and subsequently increasing the prevalence of blood parasites. Also, Variation may be due to the immunological status of animals and lack of the veterinary supervision who advice of giving medicine.
In Egypt, many researchers carry out studies on protozoan blood parasite of sheep. Our result recorded that 30% of examined sheep harbored Babesia. spp. Moreover it was not in the same line with other Egyptian studies were done by Mahmoud et al. and Ramadan et al. (8.5%) [10,11] and Reda et al. who recorded lower prevalence rate (23.1%, 8.5%, 17%) [6] respectively and in other countries by Rjeibi et al. (2.9%) in Tunisian also reported lower incidence of Babesia spp. [12]. Oppositely Ibrahim et al. (50.7%) in Egypt who recorded higher prevalence rate [13]. These difference in prevalence may be attributed to differences in climatic condition and sampling design as our study was not focused on flocks with known blood protozoan infection history but random selection were occurred.
Among the Babesia spp. observed in this study, B. motasi (20.0%) were predominant.The current finding was conflicted with previous studies which recorded a higher prevalenc rate of B. motasi such as Sisodia et al. (15-90%) in India [14]. Oppositely, Razmi et al. in Iran (14.0%) [15] and Al-Khalifa (4%) [16] in Saudi Arabia also recorded lower prevalence rate of B. motasi in sheep, followed by presence of B. ovis in (13.8%) of sheep examined and this result is in conformity with the findings from, Ziapour et al. and Fakhar et al. in Iran (16.03%, 15.4%) respectively [17,18], and contradicted with finding obtained by Rjeibi et al., Yeruham et al. and Savin et al. who recorded a lower rate (2.9%, 5.56%, 10.0%) respectively [12,19,20]. Oppositely Razmi et al. recorded a higher rate (23.5%) [15]. The diversity between studies might be due to differences in locality, the biology of the various tick species and control measure.
On the other hand the study revealed the presence of Theileria spp. which reported in 14.6% of sheep examined and this percentage was approximately similar to be those recorded by Fazly et al. (14.30%) [21] and Asmaa et al. (15.56%) [22]. On the other hand, it was lower than those recorded in Egypt by Reda et al., Ibrahim et al., Harfoush et al., Hala et al. and Radwan et al. (20.0%, 58.2%, 50.7%, 87.5%, and 33.75%, respectively) [6,13,23-25] also in other countries by Sadeghi-Dehkordi et al. (26.0%) in Iran [26], Ziapour et al. 87% in the Jaffna District [7]. The variation might be due to different in localities, the diversity and the distribution range of ticks species which act as potential vectors of theileriosis and directly related to distribution of a particular Theileria spp.
Concerning Theileria sp. , it was revealed that only T. ovis was present in our study agreeing with that obtained by M’ghirbi et al. and Rjeibi et al. in Northern Tunisia [27,28]. The same results were reported by Altay et al. in Turkey [29]. Previous studies from northern Ethiopia have shown that the infection rate in sheep was 92.0% Gebrekidan et al. [30]. Rjeibi et al. recorded a lower prevalence (4.8%) [12]. Such discrepancy of the prevalence is related to the presence and abundance of tick species, genetic variation among sheep breeds in Egypt, the climatic conditions for the survival and transmission of tick and immunological status of animal
The current research showed that sheep was equally infected by Th. Ovis (14.6%) and B. ovis (13.84%). This was in a conflict with findings given by Nagore et al. who found that the sheep was infected with Th. Ovis (18.0%) more than B. ovis (2.5%) [31] and Rjeibi et al. who revealed that sheep were more infected by Th. Ovis (16.3%) than B. ovis (7.8%) [12]. Oppositely, Rjeibi et al. showed that sheep were more infected with B. ovis (17.4%) than Th. Ovis (5.8%) [28]. the difference between the studies might be related to the similarity of tick density in the area of our study during the period of observation and the available population of sheep in the study region.
Furthermore, mixed infection of Babesia spp. and Theileria sp. appeared in 6.15% of examined sheep. It was higher than those recorded by Reda et al. (2%) [6], Ranjbar-Bahadori et al. (2.6%) [32], Nasir et al. (3.1%) [33] and García-Sanmartín et al. (3.9%) [34], but lower than that obtained by Mazyad et al. (24.0%) [35] in North Sinai, Egypt. Mixed infections might be attributed to the exposure of animals to several species of ticks, or due to the ability of ticks to transmit more than one parasite.
Currently, Toxoplasma sp. infection was nearly similar to those obtained by Gharbi et al. (1.8%) in the north Tunisia [36], and lower to those reported by Andrade et al. in Brazil (22.1%) [37], Sawadogo et al. in Marrakech (27.6%) [38], Alvarado-Esquivel et al. in Mexico (29.9%) [39], Chikweto et al. in West Indies (44.1%) [40] and Lahmar et al. in Southern Tunisia (37.0%) [41]. This discrepancy and low prevalence recorded in our study might be related to the rearing system in which sheep are not exposed to many potential sources of Toxoplasma parasitic infection in our study.
Concerning sex susceptibility to infection present work showed the highest prevalence of blood protozoan infection was observed in female sheep than in males, and it was similar to the data obtained by Rjeibi et al. [12]. Such finding contradicted with the study done in Northern Tunisian by Rjeibi et al. who found no difference in the prevalence between both sexes [28], and Iqbal et al. in Pakistan who found that males were more infected than females [42]. This could explain by the fact that the majority of females are grazing outside whereas the males are kept indoors.
Regarding the seasonal variation, we didn't consider the season because the protozoan blood parasite in Egypt spread all over the year which supports the fact that, the vector found to be active in all over the year Asmaa et al. [22].
Conclusion
It has been concluded that the high haemoparasitic infection in sheep in Qena province, Upper Egypt mainly by B. motasi followed by Th. ovis. The proper usage of drugs, developing vaccines and the implementation of tick control on farms will facilitate controlling parasitic infections and reduce the economic losses in sheep production.
Conflict of Interest
The authors declare no conflict of interests in relation to this work.
Acknowledgements
Our laboratory was supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (CHEMAL, TDR/WHO), the National Science and Technology Development Agency of Thailand (NSTDA Career Development Award), the Thailand Research Fund (TRF Basic Research), and Chulalongkorn University, Thailand.
References
- Ali BA (2003) Genetic similarity among four breeds of sheep in Egypt detected by random amplified polymorphic DNA markers. Afr J Biotechnol 2: 194-197.
- Ambak MA, Bolong AA, Ismail P, Tam BM (2006) Genetic variation of snakehead fish (Channastriata) populations using random amplified polymorphic DNA. Biotechnol 5: 104-110.
- Ahmed J, Yin H, Schnittger L, Jongejan F (2002) Ticks and tick-borne diseases in Asia with special emphasis on China. Parasitol Res 88: S51–S55.
- Freyre A, Bonino J, Falcon J, Castells D, Correa O, et al. (1999) The incidence and economic significance of ovine toxoplasmosis in Uruguay. Veterinary Parasitology, 81: 85-88.
- Saal JR (1964) Geimsa stain for the diagnosis of bovine babesiosis. II. Changes in erythrocytes infected with Babesiabigemina and B. argentina. J Protozool 11: 582–585.
- Fadly RS (2012) Prevalence of blood parasites of some farm animals at behera province. Assiut Vet Med J 58: 134.
- Kandasamy G, Rajapakse RPVJ, Rajakaruna RS (2013) Gastrointestinal and blood parasites of a free grazing flock of sheep in Kaithady farm in the Jaffna District. J Natn Sci Foundation Sri Lanka 41: 195-201.
- Adamu B, Samaila, Musa BL (2012) Prevalence of Haemoparasites of Sheep and Goats Slaughtered in Bauchi Abattoir. IJABR Vol. 4: 128-133.
- Velusamy R, Rani N, Ponnudurai G, Anbarasi P (2015) Prevalence of intestinal and haemoprotozoan parasites of small ruminants in Tamil Nadu, India. Vet World 8: 1205-1209.
- Mahmoud MS (1992) Some serological studies on Babesia species infecting sheep in Egypt. Fac Vet Med Cairo Univ, Egypt.
- Ramadan MY, El-Akabawy ML (2000) Studies on sheep babesiosis in Kalubyia governorate, Egypt. Minufiya Vet J 1: 147-155.
- Rjeibi MR, Darghouth MA, Gharbi M (2016) Prevalence of Theileria and Babesia species in Tunisian sheep. Onderstepoort J Vet Res 83: 1-6.
- Ibrahim MS, El Seify MA, Hafez AM, Deghidy NS, Harfoush MA (2000) Studies on blood parasites in sheep. The 1st scientific congress for provincial laboratories May15-17.
- Sisodia RS (1981) Present status of sheep theileriosis in India. A review. Livestock adviser 4: 15-19.
- Razmi GR, Hosseini M, Aslani MR (2003) Identification of tick vectors of ovine theileriosis in an endemic region of Iran. Vet Parasitol 116: 1-6.
- Al-Khalifa MS, Hussein HS, Diab FM, Khalil GM (2009) Blood parasites of livestock in certain Regions in Saudi Arabia. Saudi J Biol Sci 16: 63-67.
- Ziapour PS, Esfandiari B, Youssefi RM (2011) Study of the prevalence of babesiosis in domesticated animals with suspected signs in Mazandaran province, North of Iran, during 2008. J Ani and Vete Adv 10: 712-714.
- Fakhar M, Hajhasani A, Alizadeh HM, Shirzad H, Piri F, et al. (2012) An epidemiological survey on bovine and ovine babesiosis in kurdiston province western Iran. Trop Anim Health Prod 44: 319-322.
- Yeruham I, Hadani A, Gafker F, Rosen SH, Schlien J (1992) A field study of haemoparasites in two flocks of sheep in Israel. Israel J Vet Mede 47: 107-111.
- Savini IG, Conte A, Semproni G, Scaramozzino P (1999) Tick borne diseases in ruminants of central and southern Italy: epidemiology and case reports. Parassitologia 41: 95-100.
- Zainalabidin FA, Raimy N, Yaacob MH, Musbah A, Bathmanaban P (2015) The Prevalence of Parasitic Infestation of Small Ruminant Farms in Perak, Malaysia. Trop Life Sci Res 26: 1-8.
- Hegab AA, Fahmy MM, Mahdy OA, Wahba AA (2016) Parasitological and molecular identification of Theileria Species by PCR-RFLP Method in Sheep, Egypt. Int J Adv Res Biol Sci 3: 48-55.
- Harfoush MA (2001) Some studies on blood parasites in both cattle and tick vector 6th SCI. Cong, Egyptian society for cattle diseases, November 4-6, Egypt.
- Hala HW, El-Kelesh EA (2006) Haematological and biochemical changes in blood of sheep suffering from Theileria infection, Egypt. J Anim Poult Manag 1: 201-217.
- Radwan IGH, El–Kelesh EA (2009) Identification of Theileria species in sheep and goats by ploymerase chain reaction (PCR). Kafrelsheikh Vet Med J 460-473.
- Sadeghi-Dehkordi Z, Zakeri S, Nabian S, Bahonar A, Ghasemi F (2010). Molecular and biomorphometrical identification of ovine babesiosis in Iran. Iranian J Parasitol 5: 21-30.
- M’ghirbi Y, Ros-GarcÃa A, Iribar P, Rhaim A, Hurtado A, et al. (2013) A molecular study of tick-borne haemoprotozoan parasites (Theileria and Babesia) in small ruminants in Northern Tunisia. Vet Parasitol 198: 72-77.
- Rjeibi MR, Gharbi M, Mhadhbi M, Mabrouk W, Ayari B, et al. (2014) Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterisation of Babesia ovis in Africa. Parasite 21: 23.
- Altay K, Dumanli N, Aktas M (2007) Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet Parasitol 147: 161-165.
- Gebrekidan H, Hailu A, Kassahun A, Rohoušová I, Maia C, et al. (2014) Theileria infection in domestic ruminants in northern Ethiopia. Vet Parasitol 200: 31-38.
- Nagore D, GarcıaÌ-SanmartıÌn J, GarcıaÌ-Pérez AL, Juste RA, Hurtado A (2004) Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int J Parasitol 34: 1059-1067.
- Ranjbar-Bahadori S, Eckert B, Omidian Z, Shirazi NS, Shayan P (2012) Babesia ovis as the main causative agent of sheep babesiosis in Iran. Parasitol Res 110: 1531-1536.
- Nasir AA, Hashmi AH, Afzal M (2000) Prevalence of haemoparasites in exotic cattle. Int J Agri Biol 2: 402-403.
- GarcÃa-SanmartÃn J, Nagore D, GarcÃa-Pérez AL, Juste RA, Hurtado A (2006) Molecular diagnosis of Theileria and Babesia species infecting cattle in Northern Spain using reveres line blot macroarrays. BMC Vet Res 2: 1-7.
- Mazyad SA, Khalaf SA (2002) Studies on Theileria and Babesia infecting live and slaughtered animals in Al Arish and El Hasanah, North Sinai Governorate, Egypt. J Egypt Soc Parasitol 32: 601-610.
- Gharbi M, Zribi L, Jedidi M, Chakkhari H, Hamdi S, et al. (2013) Prevalence of Toxoplasma gondii infection in Tunisian sheep. Bull Soc Pathol Exot 106: 184-187.
- Andrade MM, Carneiro M, Medeiros AD, NetoA V, Vitor RW (2013) Seroprevalence and risk factors associated with ovine toxoplasmosis in Northeast Brazil. Parasite 20: 20.
- Sawadogo P, Hafid J, Bellete B, Sung RT, Chakdi M, et al. (2005) Seroprevalenceof T. gondii in sheep from Marrakech, Morocco. Vet Parasitol 130: 89-92.
- Alvarado-Esquivel C, Gayosso-Dominguez EA, Villena I, Dubey JP (2013) Sero prevalence of Toxoplasma gondii infection in captive mammals in three zoos in Mexico City, Mexico. J Zoo Wild Med 44: 803-806.
- Chikweto A, Kumthekar S, Tiwari K, Nyack B, Deokar MS, et al. (2011) Seroprevalence of Toxoplasma gondii in pigs, sheep, goats, and cattle from Grenada and Carriacou, West Indies. J Parasitol 97: 950-951.
- Lahmar I, Lachkhem A, Slama D, Sakly W, Haouas N, et al. (2015) Prevalence of toxoplasmosis in sheep, goats and cattle in Southern Tunisia. J Bacteriol Parasitol 6: 245.
- Iqbal F, Khattak RM, Ozubek S, Khattak MNK, Rasul A, (2013) Application of the reverse line blot assay for the molecular detection of Theileria and Babesia sp. in sheep and goat blood samples from Pakistan. Iranian J Parasitol 8: 289-295.
Citation: Hussein NM, Mohammed ES, Hassan AA, El-Dakhly KM (2017) Distribution Pattern of Babesia and Theileria Species in Sheep in Qena Province, Upper Egypt. Arch Parasitol 1: 102.
Copyright: © 2017 Hussein MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 7732
- [From(publication date): 0-2017 - Aug 24, 2025]
- Breakdown by view type
- HTML page views: 6525
- PDF downloads: 1207