Research Article Open Access
Eco-friendly Alternatives for the Removal of Heavy Metal Using Dry Biomass of Weeds and Study the Mechanism Involved
| Archana Dixit1*, Savita Dixit1and Goswami CS2 | |
| 1Department of Chemistry, Maulana Azad National Institute of Technology, Bhopal, India | |
| 2Department of Chemistry, Kamal Radha Girls College, Gwalior, India | |
| Corresponding Author : | Archana Dixit Research scholar Department of Chemistry Maulana Azad National Institute of Technology Bhopal, India Tel: 08871117510 E-mail: dixit.archana07@gmail.com |
| Received March 09, 2015; Accepted April 28, 2015; Published April 30, 2015 | |
| Citation: Dixit A, Dixit S, Goswami CS (2015) Eco-friendly Alternatives for the Removal of Heavy Metal Using Dry Biomass of Weeds and Study the Mechanism Involved. J Bioremed Biodeg 6:290. doi:10.4172/2155-6199.1000290 | |
| Copyright: ©2015 Dixit A, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Metal contamination issues are becoming increasing day by day common in India and elsewhere, with many documented cases of metal toxicity in mining industries, foundaries, smelters, coal-burning power plants and agriculture. Heavy metals, such as cadmium, copper, lead; chromium and mercury are major environmental pollutants, particularly in areas with high anthropogenic pressure causing increased depositions of heavy metals in the terrestrial and aquatic environment. The release of these pollutants without proper treatment poses a significant threat to both environment and public health, as they are non-biodegradable and continual. Thus, their treatment becomes inevitable and in this endeavor, absorption seems to be a promising alternative for treating metal contaminated waters. In the present study two plants, one macrophyte Eichhornia crassipes and other terrestrial Canna indica are used as adsorbents to study the process of biosorption. The studied plants have not gained much importance and are found in abundance in the Indian climate. Also the former has been declared as a weed and its management has become a great problem so with the study the plants can be better and further utilized for wastewater treatment for the removal of metal ions and other impurities. For the present study multi species solution of four heavy metals i.e. cadmium, chromium, lead and zinc are tested for the adsorption by the selected bio sorbent. This technology out-performs its predecessors not only due to its cost effectiveness but also in being eco-friendly i.e., where other alternatives fail.
| Keywords |
| Biosorption; Biomass; Eichhornia crassipes; Canna indica; Heavy metal; Desorption pretreatment |
| Introduction |
| Water bodies are being regularly forced to collect all types of waste matter deposition. Heavy metals are among the toxic substances reaching hazardous levels [1]. Heavy metals of concern include lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, silver, gold, and nickel [2]. Heavy metal pollution in the aquatic system has become a serious threat today and of great environmental concern as they are non-biodegradable and thus persistent. Metals are mobilized and carried into food web as a result of leaching from waste dumps, polluted soils and water. The metals increase in concentration at every level of food chain and are passed onto the next higher level by a phenomenon called bio-magnification [3]. Heavy metals even at low concentrations can cause toxicity to humans and other forms of life, its adverse effects on human health are quite evident. The toxicity of metal ion is owing to their ability to bind with protein molecules and prevent replication of DNA and thus the subsequent cell division [4]. To avoid health hazards it is essential to remove these toxic heavy metals from waste water before its disposal. |
| Techniques presently in existence for removal of heavy metals from contaminated waters include: reverse osmosis, electro dialysis, ultra filtration, ion-exchange, chemical precipitation, phytoremediation, etc. However, all these methods have disadvantages like incomplete metal removal, high reagent and energy requirements, generation of toxic sludge or other waste products that require careful disposal [2]. With increasing environmental awareness and legal constraints being imposed on the discharge of effluents, a need for cost-effective alternative technologies are essential. In this endeavor plant biomass can emerged as an option for developing economic and eco-friendly wastewater treatment through a process called biosorption. |
| ¨Biosorption can be defined as “a non-directed physicochemical interaction that may occur between metal/radionuclide species and microbial cells” [5]. It is a biological method of environmental control and can be an alternative to conventional contaminated water treatment facilities. It also offers several advantages over conventional treatment methods, including cost effectiveness, efficiency, minimization of chemical/biological sludge, the requirement of additional nutrients, and regeneration of bio sorbent with possibility of metal recovery. |
| The bio sorption process involves a solid phase (sorbent or bio sorbent; usually a biological material) and a liquid phase (solvent, normally water) containing a dissolved species to be sorbed (sorbate, a metal ion). Due to higher affinity of the sorbent for the sorbate species the latter is attracted and bound with different mechanisms. The process continues till equilibrium is established between the amount of solid-bound sorbate species and its portion remaining in the solution. The heavy metals are adsorbed on the surface of the biomass and thus, the bio sorbent becomes enriched with metal ions. |
| Mechanisms involved in biosorption can be classified taking into account various criteria that are, based on cell metabolism, they are classified as metabolism dependent and non- metabolism dependent while based on the location of the sorbate species it is classified as extra cellular accumulation/precipitation, cell surface sorption/precipitation and intra cellular accumulation. The adsorbed ions are transported cross the membrane in the same mechanism by which metabolically important ions such as potassium, magnesium, and sodium are conveyed. These mechanisms comprise (i) physical adsorption e.g., electrostatic interaction has been demonstrated to be responsible for copper biosorption by bacterium Zooglea ramigera and alga Chorella vulgaris [6], (ii) ion exchange e.g., biosorption of copper by fungi Ganoderma lucidium and Asperigillus niger [7], (iii) complexation e.g., biosorption of copper by C. vulgaris and Z. ramigera takes place through both adsorption and formation of co-ordinate bonds between metals and amino or carboxyl groups of cell walls [6]. Various bio sorption mechanisms mentioned above can take place simultaneously. A successful bio sorption process requires preparation of good bio sorbent. The process starts with selecting various types of biomass. Pretreatment and immobilization are done to increase the efficiency of the metal uptake. The adsorbed metal is removed by desorption process and the bio sorbent can be reused for further treatments. |
| Selection and types of biomass |
| While choosing the biomass for metal biosorption, its origin is a major factor to be taken into account. Biomass can come from, activated sludge or fermentation waste from industries like those of food, diary and starch. Also, organisms (e.g., bacteria, yeast, fungi and algae) coming from their natural habitats are good sources of biomass. Fast growing organisms that are specifically cultivated for biosorption purposes (e.g., crab shells, seaweeds) [1] can be used as biosorbents. Apart from the microbial sources even agricultural products such as wool, rice, straw, coconut husks, peat moss, exhausted coffee [8], waste tea [9], walnut skin, coconut fibre, cork biomass [10], seeds of Ocimum basilicum [11], defatted rice bran, rice hulls, soybean hulls and cotton seed hulls [12], wheat bran, hardwood (Dalbergia sissoo) sawdust, pea pod, cotton and mustard seed cakes, [13] are also proven as good biomass sources. However, sea weeds, molds, yeasts, bacteria have been tested for metal biosorption with encouraging results [1]. |
| In the present study dry biomass of two plants, one aquatic Eichhornia crassipes and other terrestrial Canna indicia are taken as adsorbents for analysis. |
| Eichhornia crassipes |
| Eichhornea crassipes is well studied as an aquatic plant that can improve effluent quality from oxidation ponds and as a main component of one integrated advanced system for treatment of municipal, agricultural and industrial wastewaters, [14-17]. To regret water hyacinth is often described in literature as serious invasive weed [14-18] and it is ranked on eight places in the list of world’s ten most serious weeds, [19]. It has high affinity for the metals and is used for phytoremediation. In present study whole plant dry biomass are taken for heavy metal adsorption/removal from aqueous solvent and also from waste water. The accumulation of metals by macrophytes is more intensive in the first hours and during the first day; then, the uptake rate decreases. The time required for saturation is directly related to the metal concentration in water, and the saturation degree is inversely related to it. The uptake rate varies for different chemical elements at similar concentrations. The metal accumulation by plants can be affected by the increased concentrations of other elements. Metals can enter plants directly from the water and by plants’ contact with suspended particles. The process involves two stages: the adsorption on the surface and the absorption followed by the metal fixation in plant tissues. And in case of dry biomass the main mechanism for the fixation of metals is the formation of complexes via the addition of ions to the functional groups of organic compounds (carboxyl, amino, imino, hydroxyl, sulfhydryl and keto groups). |
| Canna indica |
| Canna indica commonly called Indian shot, it is a perennial plant with rapid growth [20], height limits to 1.5-2 m [21,22]. Very less studies are done on the nutrient adsorption/ uptake by dry biomass of Canna indica. The present study will help in adding to the data on utilization of Canna indica biomass for waste water treatment. In this study an attempt has been made to use the dry biomass of whole plant parts of a terrestrial plant, Canna indica as bio sorbent to be used for the removal of metals from wastewater. |
| In the present context, it is an effort to make use of dry biomass of the plants, which till now are being used for wastewater treatment or water purification as living. The study assesses their ability to treat waste water in their dead form by utilizing their dead biomass as an adsorbent. |
| Materials and Methods |
| In the present study, the dry biomass of Echornia crassipes, common name, water hyazinth and Canna indica common name saka siri were utilized for the removal of heavy metal and nutrient ions from its aqueous solution. Both the plants were collected from Shahpura lake lower drainage basin near chunabhatti area Bhopal city, India. The plants were cut into small pieces and they were washed several times with water and then with Milli-Q water to eliminate the remains of lake sediments and particulate matter. Final washings were done with double distilled water, and the pieces were dried under the sun until all moisture was evaporated. i.e. a maximum for a week. The resulting product was cooled to room temperature and sieved to the desired particle sizes: 50, 100, 150, 200, 250, 300 mesh. This is finally preserved in polythene bags which were stored in vacuum desiccators until required for further adsorption experiments. |
| The Extract was prepared by Solid-liquid extraction using soxhlet apparatus in this process a solute or solutes are removed from solid using liquid solvent, in present study water is taken as non-polar solvent and ethanol as polar solvent. For ethanol extract 70 g of dry biomasses of both the plants was extracted with 150 ml ethanol and for preparation of water extract 70 g biomasses were extracted with 280 ml water. The diffused solvent dissolves the solutes i.e. transfers the solute to the liquid phase. In leaching by the soxhlet apparatus multiple contacts of solids with the fresh solvent is performed at each stage of operation. After the completion of the extraction time the sample is carefully transferred to evaporating dish and dry overnight. Soxhlet apparatus allows 100% active material recovery. |
| Results and Discussion |
| Heavy metal removal |
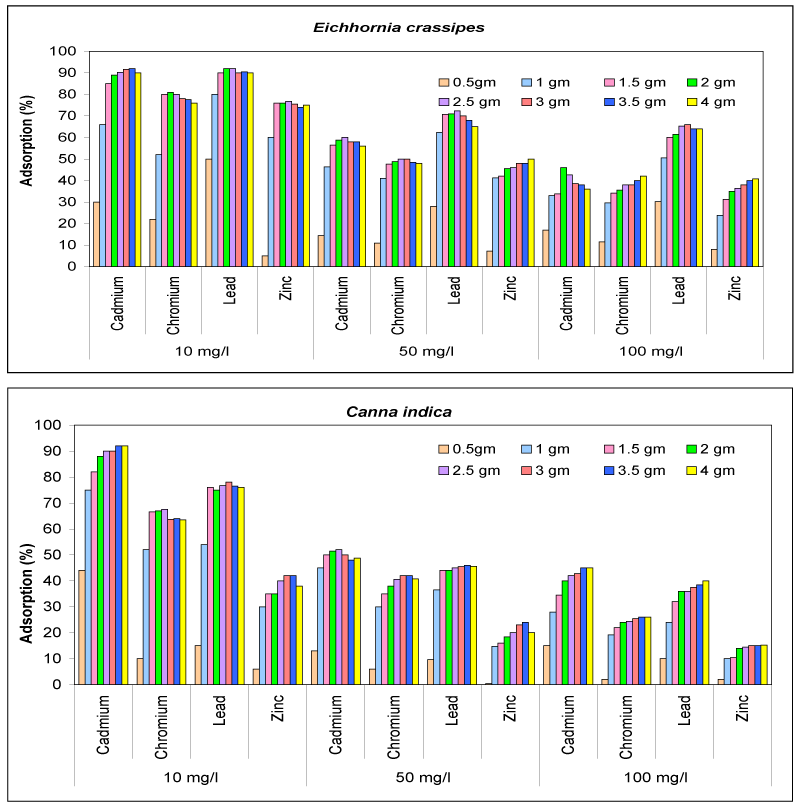
| The plot of percent adsorption versus amount of adsorbent at different initial concentration of metal ions present as multiple species in solution is shown in Figure 1 It is observed that at lower concentration i.e.10 mg/l of multiple species same as the case of E. crassipes the percent adsorption reaches a value of 93% using 2.5 g of adsorbent in the case of Pb2+, however in case of Cd2+ 3.0 g of adsorbent is required and for Cr6+ ion 1.5 g adsorbent is used for maximum adsorption of 82% and 4 g of adsorbent is required to reach to adsorption of 75% by Zn2+ ions. At higher concentrations (viz. 50 mg/l) of Cd2+, Cr6+, Pb2+and Zn2+ the percent adsorption is low when the quantity of adsorbent is low and it reaches a value of 98-99% only when 3.0-4.0 g of adsorbent is used. Whereas in case of C. indica the percent adsorption reaches a value of 84% using 2.5 g of adsorbent in the case of Pb2+, however, in case of Cd2+ with 3.5 g of adsorbent 92% adsorption is seen and for Cr6+ ion 1.5 g adsorbent is used for maximum adsorption of 70% and 3.0 g of adsorbent is required to reach to adsorption of 42% by Zn2+ ions. In case of 100 mg/l multiple species solution much higher quantity of adsorbent is required. |
| The purification of 100 ppm of cadmium, chromium, lead and zinc ion sample solution at various bio sorbent concentrations of E. crassipes and C. indica were studied. The experiments showed that ion uptake increases when biomass concentration [23] rises by both the type of adsorbents. But with further increase in bio-adsorbent after a defined/ specific quantity the adsorption becomes constant and then further decreases. This reduction is attributable to metal concentration shortage in the solution. Using the bio-Sorbents there was an increase in metal uptake up to 4 g biomass for selected metal ions. |
| The increase in percent adsorption with the increase in quantity of adsorbent at a particular concentration of metal ions in single as well as multiple species can be explained on the basis that with the increase in the quantity of dry biomass of E. crassipes and C. indica the availability of ionic sites increases, since the concentration of metal ions in solution is fixed, initially more ions get exchanged with the metal ions but after the saturation is reached adsorption decreases. |
| However, at higher initial concentration (50 and 100 mg/l) the percent adsorption is low when the quantity of adsorbent is low, the increase in the quantity of adsorbent results in the increase of percent adsorption, because the total sites of adsorption are increased. But the interference and competition between available binding sites at higher biomass densities caused a decrease in the specific adsorption capacity of the bio Sorbent [24] as in the case of the multiple species solution. |
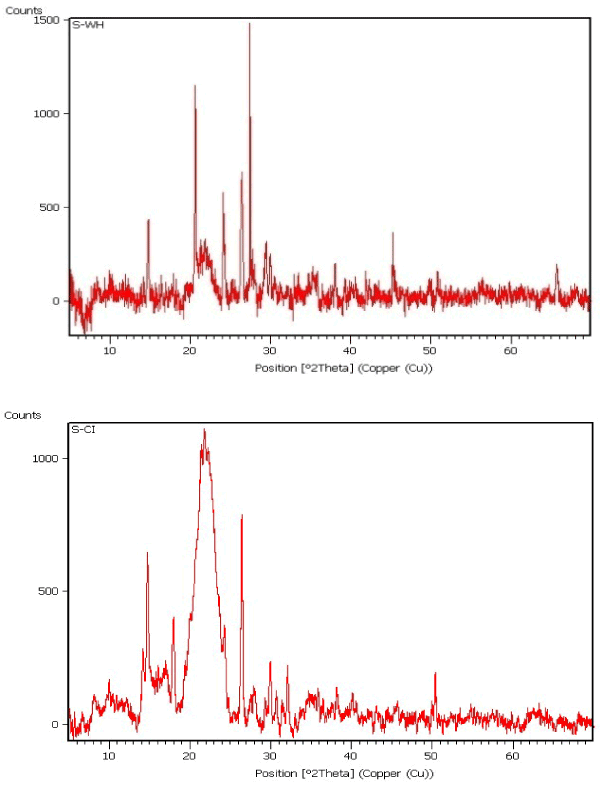
| In order to find the structural and chemical composition of the biomass, so as to clearly establish a protocol for the adsorption mechanism, various analyses such as FTIR, XRD, phytochemical tests have been carried out as described below: |
| FTIR Spectral and XRD analysis |
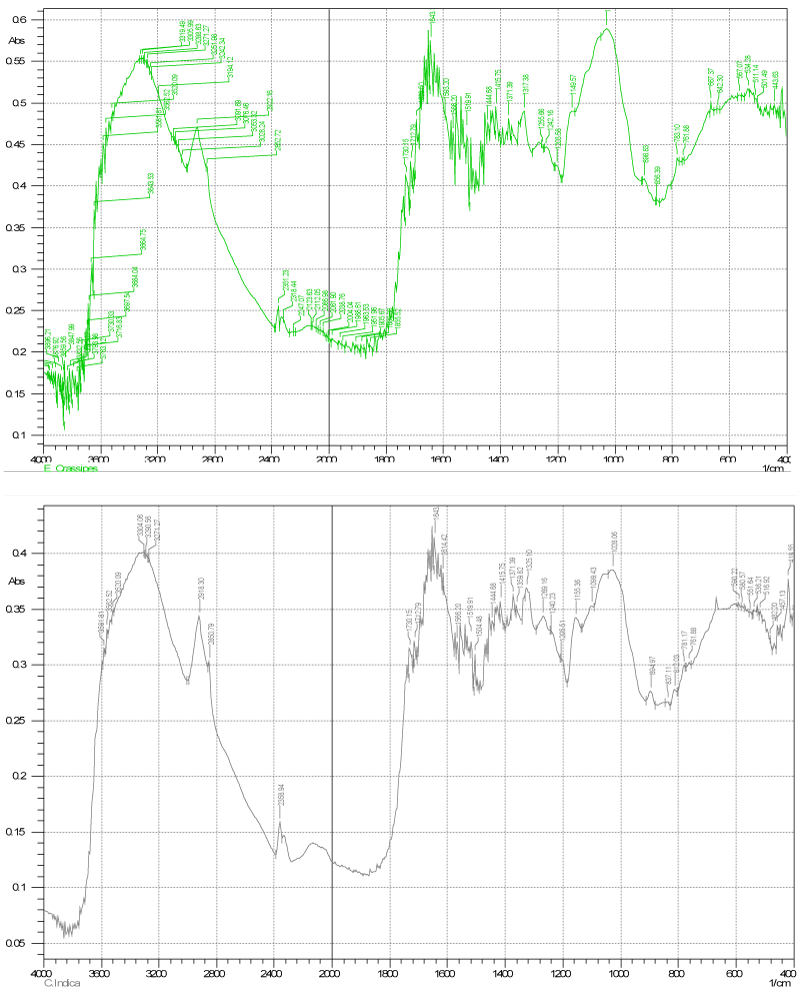
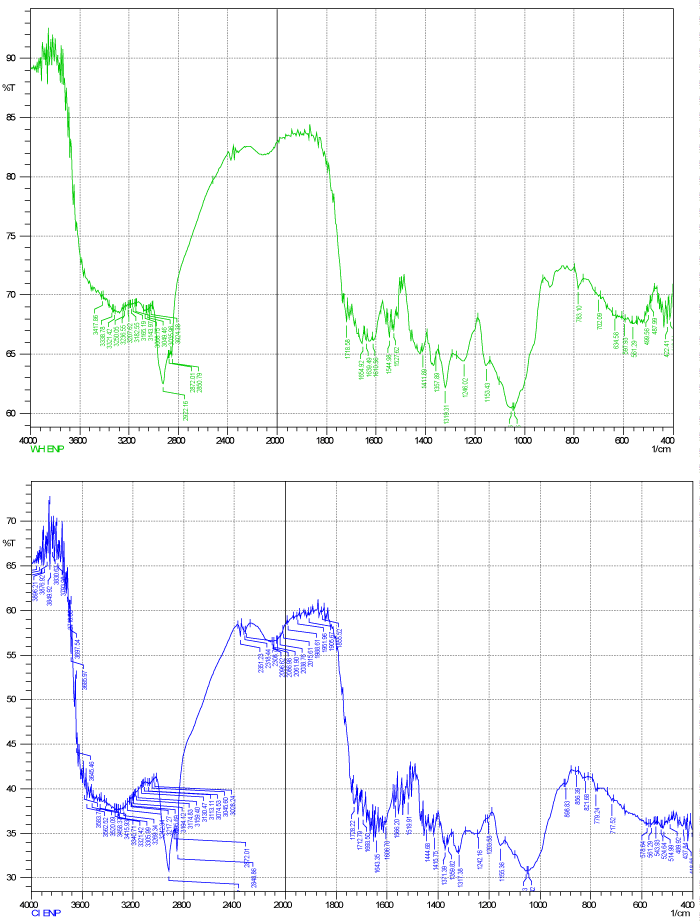
| strong adsorption behavior of certain biomass towards metal ions is a function of the chemical make-up of the biomass. The Fourier transform infrared (FT-IR) and XRD, for the studied dry biomass of the two plants E. crassipes and C. indica is carried out in order to know the chemical composition of the biomasses that played a role in the adsorption/biosorption of metals. Major characteristic bands recorded for dry biomass of E. crassipes (Figures 2 and 3) included: 3300-2900 ¥ cm-1 indicate O-H stretch which may be carboxylic group also O-H and N-H stretching, vibration band obtained around 3413 cm-1 for intermolecular hydrogen bonding (H-bonded OH groups) attributed to phenolic groups, peaks at 2400-1800 ¥ cm-1 show C≡C or C≡N , at 1190 ¥ cm-1 C-O stretch is obtained this all indicate carboxylic group which is responsible for metal binding. In case of C. indica peak at 3200-2850 shows C-H stretch (Figures 2 and 3) may be of benzene or hydrogen bonding, peak at 1550-1400 in bending may be nitro or of methylene group or of aromatic double bond stretch, 1150 peak show C-O-C stretch of ether, the IR spectrum of the dry-biomass of the two plants focuses only on a few groups such as carboxylic, amide, ether and hydro carbons supporting for the adsorption of heavy metals. |
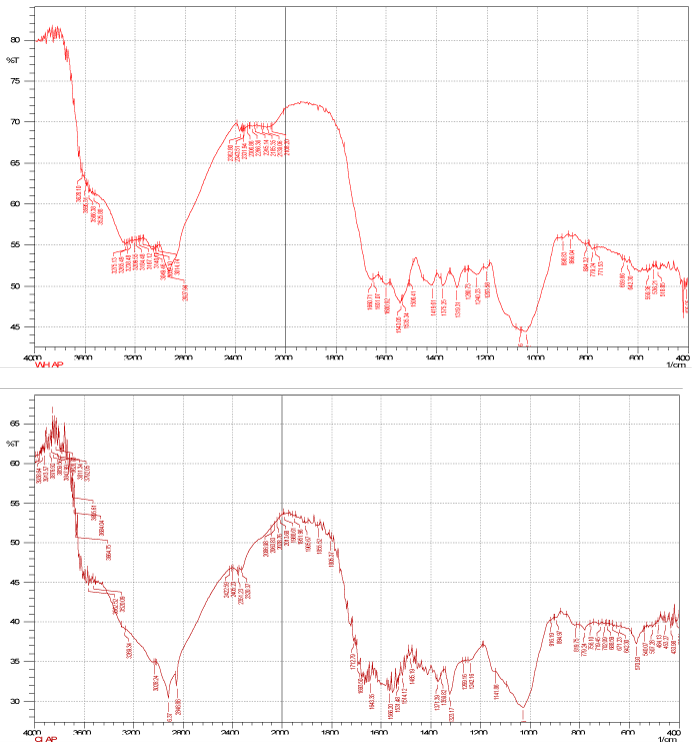
| IR spectra of the polar and non-polar extract of the dry biomass of the two plants the polar spectra of E. crassipes indicated that peaks at 3600-3200 showed broad O-H stretch (Figure 2) of carboxylic acid also C=O stretch at 1750-1700 also indicate presence of carboxylic acid group also C-O stretch at 1240 ¥ cm-1 shows carbony group, thus the main functional groups are mainly carboxylic acid and benzene and that of C. indica (Figure 4) shows the O-H stretch and triple bond stretch peaks which are hide peaks and not appearing as strong peaks, the groups may be C=O, C-O C, CH2, CH3, benzene. Similarly for the non-polar extract spectra for E. crassipes (Figure 5) depicted, hydroxyl or carboxylic groups as peaks obtained at 3600-3200 having a broad O-H stretch and C=O stretch was also obtained at 1750 ¥ cm-1 indicating carbonyl group may be carboxylic acid in addition stretch of C=C at 1650-1600 is the indication of benzene. The methyl asymmetric C-H bending was observed at 1350 cm-1 could be attributed to C-O stretching of alcohol, sulfoxides, carbohydrates or polysaccharides-like substances. Thus the major functional groups recognized are mainly carboxylic acid, and benzene. Similarly for the non-polar C. indica dry biomass extract the major groups further reported were COOH,OH, C=O,C-O C,CH2, CH3,benzene (Figure 5). The results concurred with [25] who found in T. harzianum the presence of OH, =CH and C=O and NH2 functional groups. It is reasonable to assume, that the peak values suggested metal chelating. The structure of the metal bound to carboxyl ligands on the biomasses is likely to take place. The most remarkable difference between the four spectra is at intensity of 3000- 3800 cm-1 representing hydroxyl (-OH) group stretching, and at intensity of 1600-1700 cm-1 representing carbonyl (-C=O) stretching. This may signify the involvement of hydroxyl groups in the binding of Cd and Cr. The presence of slight changes in the peak regions indicates the presence of bio sorption. Interestingly, the peaks get shifted with the presence of metal ions suggesting an interaction of metals with these functional groups. In addition, the main functional groups responsible for a bio sorption process are the hydroxyls, carbonyls, carboxyls, amides, phosphonates and phosphodiester groups as affirmed by Pradhan [26]. Some of these groups are present in the dry biomass of the selected plants and interact with the metal ions and it directly seems that these functional groups participate in metal binding process. Carboxylic and carboxylate groups have also been identified as the main metal-sequestering functional ionic groups [27-30]. Periasamy and Namasivayam reported that electrostatic forces as wells as specific chemical interaction play important role in metal adsorption. Carboxylate groups of alginate have been identified as the main metal binding site. The correlation between the metal uptake capacity and the amount of acidic groups is related to the number of carboxylic groups (weak acid) occurring in the biomass. These groups play the predominant role in metal binding [31-33]. |
| Desorption and metal recovery |
| The regeneration of the bio sorbent may be crucially important for keeping the process economical and in opening the possibility of recovering the metals extracted from the liquid phase. For this purpose it is desirable to desorbs the sorbed metals and to regenerate the bio sorbent material for another cycle of application. The desorption process should yield the metals in a concentrated form, restore the bio sorbent close to the original state for effective reuse with undiminished metal uptake and no physical changes or damages to the biomass. Dilute mineral acids (HCl, H2SO4, HNO3) have been used for the removal of metals from biomass [34-37] and also organic acids (Citric, acetic, lactic) and complexing agents (EDTA, thiosulphate, etc.) can be used for metal elution without affecting the biosorbent [38]. |
| The desorption for recovery of the sorbed heavy metal has been conducted based on the E. crassipes and C. indica biomass recovered after sorption tests have been performed on single ion and multi ion systems. Experiments were conducted for regenerating the dry biomass of E. crassipes and C. indica using various desorbing agents such as HNO3, HCl, KCl, NaCl and NaOH, desorption of Cadmium, Chromium, lead, and zinc from dry biomass of both the adsorbents was examined in batch reactors. In order to show the reusability of the bio sorbent, experiment on cyclic sorption of heavy metal was conducted in which 1 g of each dry biomass was contacted with 100 ml of a respective ion solution having a concentration of 100 mg/l for 1 h. The cycle was repeated for 4 times at pH-2 and temperature 28 ± 2ºC followed by a successive desorption after each biosorption cycle. At the end of every sorption cycle, metal solution was filtered and the metal concentration determined. The maximum amount of Cd(II), Cr(VI), Pb(II), and Zn(II) sorbed on dry biomass by respective desorbing agents was 100%, 80.0%, 99.0% by HNO3 and 98.5% by KCl after 1st, 2nd, 3rd and 4th cycle, respectively. |
| Conclusion |
| Despite the fact that the technology also suffers inherent disadvantages like early saturation of biomass, little biological control over the characteristics of biosorbents. It offers several advantages, including cost effectiveness, high efficiency, minimization of chemical/ biological sludge, and regeneration of biosorbent with possibility of metal recovery. In countries, with the rush for rapid industrial development coupled with lack of awareness about metal toxicity, there is an urgent need for developing an economical and eco-friendly technology which satisfies these demands where other conventional methods fail. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 18422
- [From(publication date):
May-2015 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 13363
- PDF downloads : 5059
